Review Article Open Access
Antiviral Phytochemicals: An Overview
Rita Kapoor, Bhupender Sharma and Shamsher Singh Kanwar*Department of Biotechnology, Himachal Pradesh University, Shimla, India
- Corresponding Author:
- Shamsher Singh Kanwar
Department of Biotechnology
Himachal Pradesh University, Shimla-171 005, India
Tel: 94180-85397; 94180- 85397
E-mail: kanwarss2000@yahoo.com
Received date: May 17, 2017; Accepted date: June 19, 2017; Published date: June 27, 2017
Citation: Kapoor R, Sharma B, Kanwar SS (2017) Antiviral Phytochemicals: An Overview . Biochem Physiol 6:220. doi:10.4172/2168-9652.1000220
Copyright: © 2017 Kapoor R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
The pandemic of viral diseases during recent years has forced the scientific community to investigate less toxic antiviral phytomolecules instead of using nucleic acid analogues, protease inhibitors or other toxic synthetic molecules as antiviral therapeutics. Plants and many of their secondary metabolites because of the healing properties have been in traditional use throughout the world since ancient times. They provide us diverse bioactive phytochemicals which play synergetic role in maintaining human health. The development of clinical products from phyto-pharmaceuticals is a trending approach to look for ecofriendly therapeutic molecules. More than 50% of drugs used in Western nations are derived from plants or their constituents. Many plants have significant antiviral properties too. Very little information is available about plants of antiviral significance. This article briefly reviews various phytochemicals/bioactive molecules which have been isolated from plants and possess antiviral constituents, their mode of action and potential applications in treating/preventing viral diseases.
Keywords
Viral diseases; Antiviral phytochemicals; Isolation; Mode of action
Introduction
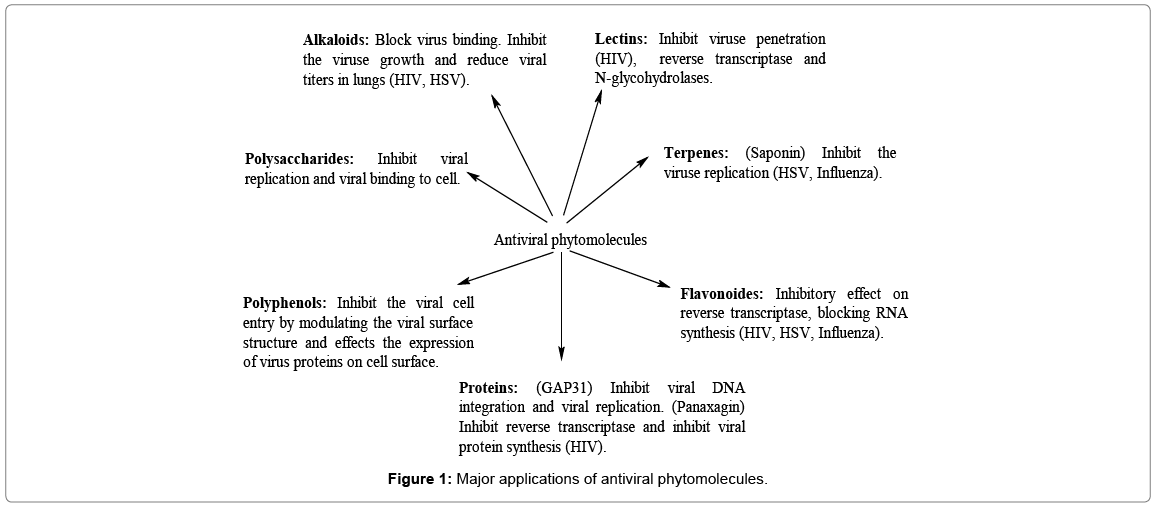
Several infectious viral diseases have been reported till date and newer ones are occurring frequently. Among emerging diseases, most of the diseases involve viruses such as HIV, Influenza, Herpes simplex virus (HSV), Dengue, Chikungunya, Zika, Hepatitis A (HSV), Hepatitis B (HSB), Hepatitis C (HCV), etc. [1-3]. Viral diseases pose great risk to human health as viral infections are tough to control due to mutative nature of the viral genomes [4]. There is constant emergence of new resistant viral strains which demands novel antiviral agents with fewer side effects and cell toxicity [5]. In the past, deadly viruses caused pandemics in the world there by increasing the risk of spreading viral diseases between continents. Very few drugs have been developed till date to effectively treat viral diseases [6]. Majority of the approved antiviral drugs possess adverse drug reactions and have also developed viral resistance in long-term therapy [7]. Plants offer us a variety of therapeutic metabolites which have potential to inhibit viral replication by regulating viral adsorption, binding to cell receptors, inhibition of virus penetration into the host cell and by competing for pathways of activation of intracellular signals [8-10] (Figure 1).
Antiviral molecules of plant origin
Natural medicine is a valuable field of research to explore, extract and establish curative properties. However, a very little percentage of phytochemicals has been systematically investigated for their therapeutic potential [11,12]. Natural products provide an unusual approach for the discovery of antiviral agents with remarkable pharmacological effects [13,14]. At present, approximately 25% of the drugs prescribed are of plants origin [15]. Many anticancer and anti-infective drugs are derived from plant products [16]. Herbal practitioners use traditional plants since ancient times to heal several human and animal diseases especially in Asia [17]. People still rely on traditional plants and their products for their health, living and primary health care in many parts of the world [18]. Approximately 2500 medicinal plant species have been recorded globally [19,20] to treat a myriad of inflictions and diseases. Polyphenols, alkaloids, flavonoids, saponins, quinones, terpenes, proanthocyanidins, lignins, tannins, polysaccharides, steroids, thiosulfonates and coumarins are prominent bioactive phytochemicals, which have been observed to combat viral infections [21-40] (Table 1).
| Phytochemicals | Class | Active against virus | Plant (part) | References |
|---|---|---|---|---|
| Baicalin | Flavonoid | DENV | Scutellaria baicalensis (roots) | [23] |
| Chalcones | Ketone | Influenza A (H1N1) | Glycyrrhiza inflate (roots) | [24] |
| Dammarenolic acid | Triterpenoid | Retroviruses | Aglaia sp. (bark) | [25] |
| Decanoylphorbol-13 acetate | Diterpene | CHIKV | Croton mauritianus (leaves) | [26] |
| Excoecarianin, Loliolide |
Tannins | HSV- 2, HCV | Phyllanthus urinaria (whole plant) | [27,28] |
| Honokiol | Lignin | DENV-2 | Magnolia tree (roots, bark) | [29 |
| Jubanines | Alkaloids | PEDV | Ziziphus jujuba (roots) | [30 |
| Limonoids | Lignin | HCV | Swietenia macrophylla (stem) | [31,32] |
| Oleanane | Triterpenes | PDEV | Camellia japonica (flowers) | [33] |
| Quercetin | Flavonoid | HCV | Embelia ribes (seeds) | [34] |
| Saikosaponins | Terpenoid | HCV | Bupleurum kaoi (roots) | [35] |
| Sennoside A | Glycoside | HIV-1 | Rheum palmatum (roots) | [36] |
| Silvestrol | Benzofuran | Ebola virus | Aglaia foveolata (leaves, bark) | [37] |
| SJP-L-5 | Ligningomisin | HIV-1 | Schisandra micrantha (roots) | [38] |
| Spiroketalenol | Ether | HSV-1 HSV-2 |
Tanacetum vulgare (rhizome) | [39] |
| Swerilactones | Lactones | HBV | Swertia mileensis (whole plant) | [40] |
| Xanthohumol | Chalcone | BVDV | Humulus lupulus (whole plant) | [41] |
Table 1: Some antiviral phytochemicals from plants.
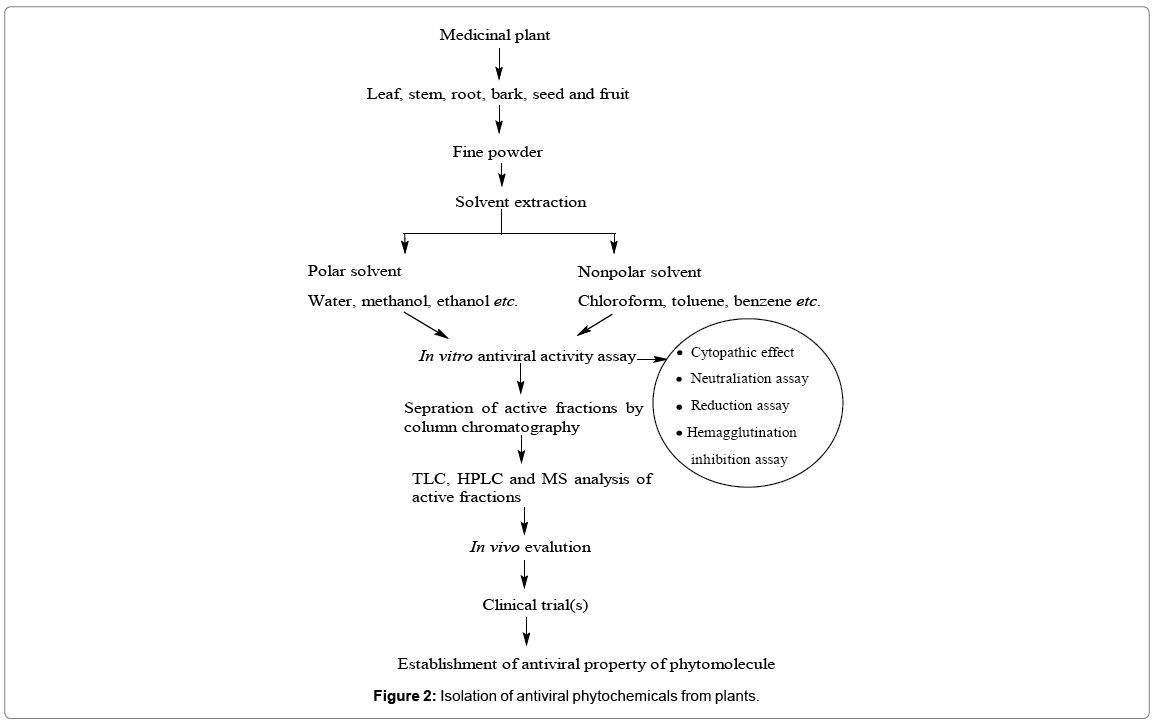
Plants have naturally evolved over the years in diverse climate conditions on earth and have been endowed with rich complex of secondary metabolites/phytochemicals with wide pharmacokinetic spectrum. Very few of the phytochemicals have been purified and studies for their structure and therapeutic properties. Most of the crude plant products have been marketed as pharmaceutical products without certified quality and efficacy. Many traditional medicinal plants and herbs have been reported to have strong antiviral activity against HCV, HBV and H1N1, etc. These phytochemicals must be subjected to animal and human studies to determine their effectiveness in whole-organism systems including reactogenecity and toxicity studies (Figure 2).
Gathering traditional information from local or indigenous people or using ethnomedicinally important plant(s) to extract bioactive molecules/phytochemicals for curing various diseases are quite challenging approaches. Many factors such as different solvents (polar, nonpolar) employed for the extraction of bioactive constituent(s), choice of plant part/tissue for extraction bioactive constituent(s) often play important roles in extracting the biologically active phytochemicals/natural constituents from plants efficiently. To appraise the antiviral activity of plants systematic approach to isolate and characterize the bioactive molecules/phytochemicals and virus replication inhibition assays in animals or mammalian cell system are indeed needed before such phytomolecules could actually be employed to treat viral infection [41]. Different methods for isolation, purification of bioactive molecules/phytochemicals from the extracts of plant to carry out biological activity such as their antibacterial, antifungal and antiviral properties are requires to be established. A variety of biological assay such as antiviral properties such as cytopathic effect screen, neutralization assays, yield reduction assays and haemagglutination inhibition test have been successfully used to study the. Extraction of bioactive principle is an important first step in the analysis of plants to extract the desired bioactive molecules/phytochemicals. The traditional practices in isolation of these bioactive molecules/phytochemicals using different separation techniques such as TLC, column chromatography, flash chromatography, HPSCCC, HPLC, FTIR, NMR and MS have been extensively exploited to obtain and facilitate the identification of the bioactive molecules/phytochemicals. The ability to identify bioactive molecules/phytochemicals from large chemical libraries accurately and rapidly has been the ultimate goal in developing high throughput screening assays (Figure 2). To firmly establish the antiviral activity and adverse reactions like reactogenecity or toxicity of the purified phytomolecules, appropriate in vivo studies (animal models) and subsequent clinical trials are necessary. A bioactive flavonoid ‘Baicalein’ isolated from Chinese medicinal plant Scutellaria baicalensis Georgi showed antiviral properties using high-speed counter-current chromatography (HSCCC) technique [42-58].
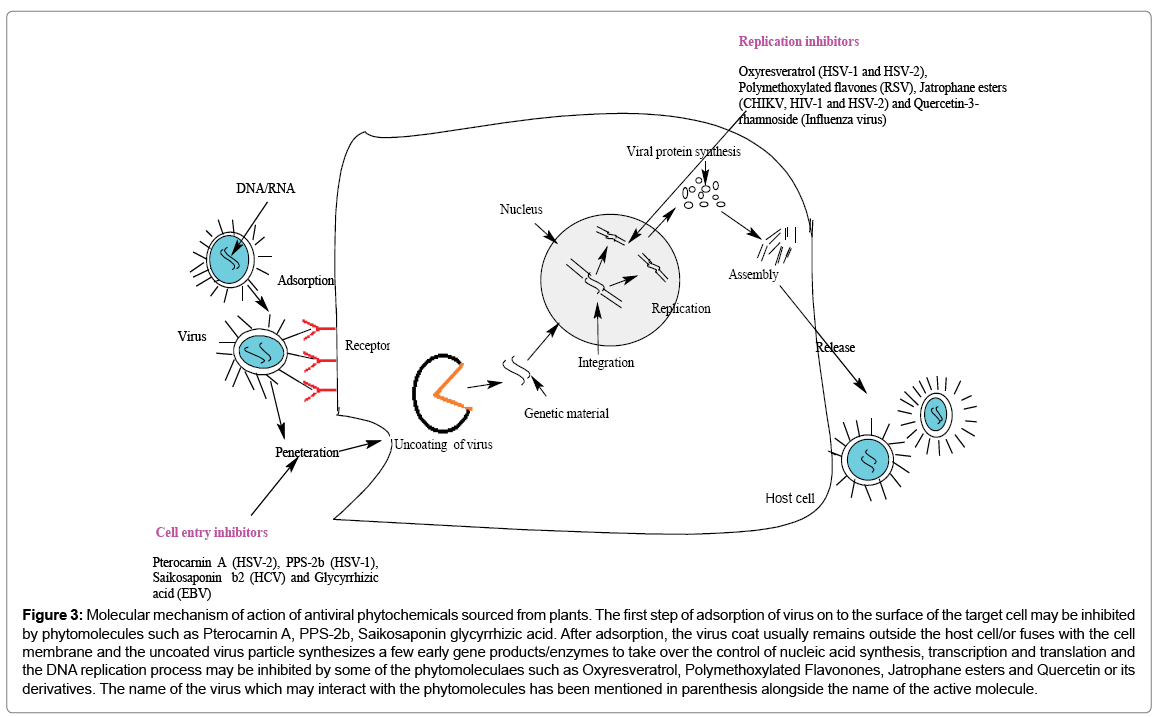
Virus attacks the host cells specifically through adsorption to receptors, penetrating through cell wall where there is uncoating of virus, the genetic material liberates out and integrates or episomally exists inside the nucleus with genetic material of host cell, interfere with their transcription, replication and translation process, respectively, assembles there and releases out during host cell lysis. Various steps of virus interaction with the host cell till the release of the virions from the host cells have been depicted schematically (Figure 3). Plant produces a diverse array of more than 100,000 secondary metabolites and can be classified, on the basis of composition and the pathway through which they are synthesized [59]. Enormously improved methods of genetically engineering the structural complexity of natural products from plants have been successfully mastered now [60-62]. Herpes simplex virus 1 and 2 (HSV-1 and HSV-2) which have double-stranded linear DNA genome and icosahedral protein [63] show symptoms of infection including blisters on the skin, lips, nose or genitals [64]. HSV enter into the host cell through interaction with glycoproteins present on the surface of the virus envelope and trans-membrane receptors of the cell surface of host cell [64]. Plants preferred as impending sources of novel bioactive molecules for development of new antiviral drugs [65] often on the basis of their ethnomedicinal use. Compounds such as Spiroketalenol ether derivatives isolated from rhizome extract of Tanacetum vulgare, which function as cell entry inhibitors had been reported to block virus entry and also arrest the synthesis of HSV-1 gC and HSV-2 gG glycoproteins. Samarangenin B cut-off from roots of Limonium sinense exhibited inhibition of HSV-1 α gene expression. Artocarpus lakoocha plant with Oxyresveratrol compound was found to inhibit early and late phase of viral replication of HSV-1 and HSV- 2, respectively (Figure 3). Pterocarnin A compound inaccessible from Pterocarya stenoptera inhibited HSV-2 from binding and penetration to the host cells. Hepatitis B (HBV), C (HCV) causes hepatic infectious diseases that affects the liver, out of which around 143 million people worldwide are estimated to be infected by Hepatitis C virus [64,66]. The primary route of their transmission through blood transfusions and unsafe medical procedures [67]. Saikosaponin b2 compound from Bupleurum koi roots has been reported to inhibit early HCV entry. Chalepin and Pseudane IX reported from Ruta angustifolia (Leaves) inhibited HCV at the post-entry step, RNA replication and viral protein synthesis. LPRP-97543 compound isolated from root part of Liriope platyphylla demonstrated to inhibit viral gene expression, replication and viral promoter activity by affecting the binding activity of NF-κB to HBV surface gene CS1 element. The HIV virion enters macrophages and CD4+ T cells by the adsorption of glycoproteins on their surface to receptors on the target cell(s) followed by fusion of the viral envelope with the cell membrane leading to the release of the HIV capsid into the cell.. Entry to the cell begins through interaction of the trimeric envelope complex (gp160 spike) to both CD4 and a chemokine receptor (generally either CCR5 or CXCR4) present on the host cell surface. The gp160 spike contains binding domains for both CD4 and chemokine receptors [68,69]. Considerable advancement has been made on the use of natural products of plant origin as anti-HIV agents which could assist in the prevention of the disease. Jatrophane esters interfered with viral entry by inducing internalization and down regulation of HIV receptors (CD4, CXCR4 and CCR5). RSV (Respiratory syncytial virus) has a linear negative-sense RNA genome with F and G lipoproteins which are the only two that target the cell membrane, and are highly conserved among RSV isolates [70]. Tangeretin and Nobiletin (Polymethoxylated flavones) isolated from pericarps of Citrus reticulate (Pericarps) affected the intracellular replication of RSV. Tangeretin down regulated the expression of RSV phosphoprotein (P protein).The reports through inhibition of virus-cell fusion in the early stage, and the inhibition of cell-cell fusion at the end of replication cycle. Dicaffeoylquinic acids from Schefflera heptaphylla inhibited the replication of RSV. An inhibitory effect of Manassantin B isolated from the root part of Saururus chinensis towards Epstein-Barr virus (EBV) lytic replication has been reported. The first step of entry of EBV into the host cell is inhibited by the Glycirrhizic acid. EBV infects B cells of the immune system and epithelial cells. Once EBV’s initial lytic infection is brought under control, EBV latency persists in the individual’s B cells for the rest of the individual’s life [71]. Some of the other examples of antiviral compounds with their mode of action have been summarized in Table 2.
Figure 3: Molecular mechanism of action of antiviral phytochemicals sourced from plants. The first step of adsorption of virus on to the surface of the target cell may be inhibited by phytomolecules such as Pterocarnin A, PPS-2b, Saikosaponin glycyrrhizic acid. After adsorption, the virus coat usually remains outside the host cell/or fuses with the cell membrane and the uncoated virus particle synthesizes a few early gene products/enzymes to take over the control of nucleic acid synthesis, transcription and translation and the DNA replication process may be inhibited by some of the phytomoleculaes such as Oxyresveratrol, Polymethoxylated Flavonones, Jatrophane esters and Quercetin or its derivatives. The name of the virus which may interact with the phytomolecules has been mentioned in parenthesis alongside the name of the active molecule.
| Plant (part) | Phytochemicals | Target | Mode of action | References |
|---|---|---|---|---|
| Artocarpus lakoocha (Heartwood) |
Oxyresveratrol | HSV-1 HSV-2 | Inhibitory activity at the early and late phase of viral replication of HSV-1 and HSV-2. Inhibition of late protein synthesis | [44] |
| Bupleurum kaoi (Root) |
Saikosaponin b2 (Terpenoid) |
HCV | Inhibiting early HCV entry, including neutralization of virus particles, preventing viral attachment | [45] |
| Citrus reticulate (Pericarps) |
Tangeretin and nobiletin (Polymethoxylated flavones) | RSV | Affected the intracellular replication of RSV. Tangeretin down regulated the expression of RSV phosphoprotein (P protein) | [46] |
| Euphorbia amygdaloides spp.and semiperfoliata (Whole plant) |
*Compound 3 (Jatrophane esters) | CHIKV HIV-1 HIV -2 |
Selective inhibitor of the replication and inducing down regulation of HIV receptors (CD4, CXCR4 and CCR5 | [47] |
| Glycyrrhiza radix (Roots) |
Glycyrrhizic acid (GL) | EBV | GL interferes with an early step of EBV replication cycle. | [48] |
| Houttuynia cordata (Aerial parts) |
Quercetin 3rhamnoside (Q3R) |
Anti-influenza A/WS/33 virus | Inhibit replication in the initial stage of virus infection by indirect interaction with virus particles | [49] |
| Limonium sinense (Root) |
Samarangenin B | HSV-1 | Inhibit HSV-1 α gene expression, including expression of the ICP0 and ICP4 genes, by blocking β transcripts such as DNA polymerase mRNA, and by arresting HSV-1 DNA synthesis and structural protein expression in Vero cells. | [50] |
| Liriope platyphylla (Root) |
LPRP-Et-97543 | HBV | Inhibit viral gene expression and replication. Inhibit viral promoter activity. | [51] |
| Melia azedarach L. (Leaves) |
Tetranortriterpenoid 1-cinnamoyl-3, 11-dihydroxymeliacarpin (CDM) | VSV HSV-1 |
CDM blocks VSV entry and the intracellular transport of VSV-G protein and confined it only to the Golgi apparatus and also modulates the NF-κB signaling pathway by slowing down its activation in HSV-1-infected conjunctival cells which leads to the accumulation of p65 NF-κB subunit in the cytoplasm of uninfected treated Vero cells | [52] |
| Prunella vulgaris (Fruit spikes) |
Lignin–carbohydrate complex (PPS-2b) |
HSV-1 HSV-2 | Block HSV-1 binding and inhibiting penetration into Vero cells | [53] |
| Pterocarya stenoptera (Bark) |
Pterocarnin A | HSV-2 | Inhibit HSV-2 from attaching and penetrating into cells. It also actively suppressed HSV-2 multiplication in Vero cells even when added 12 h after infection | [54] |
| Ruta angustifolia (Leaves) |
Chalepin and pseudane IX | HCV | Inhibited HCV at the post-entry step and decreased the levels of HCV RNA replication and viral protein synthesis | [55] |
| Saururus chinensis (Root) |
Manassantin B (Dineolignans) |
EBV | Inhibitory effects toward EBV lytic replication | [56] |
| Schefflera heptaphylla (Leaf stalks) |
Dicaffeoylquinic acids | RSV | Inhibition of virus–cell fusion in the early stage and the inhibition of cell–cell fusion at the end of the RSV replication cycle. | [57] |
| Scoparia dulcis L. (Whole plant) | Scopadulcic acid B | HSV-1 | Inhibit the viral replication. | [58] |
| Scutellaria baicalensis (Root) |
5,7,4' trihydroxy-8-methoxyflavone (F36) | A/PR8 (mouse-adapted influenza virus) |
Reduces single-cycle replication of A/PR8from 4 h to 12 h after incubation by dose-dependent manner but did not inhibit the adsorption of A/PR8 to MDCK cells. | [59] |
| Tanacetum vulgare (Rhizome) |
Spiroketalenol ether derivative | HSV-1 HSV-2 |
Block virus entry and arrested the synthesis of HSV-1 gC and HSV-2 gG glycoproteins. The viral glycoprotein reduction was due to the inhibition of Gc and gG coding mRNA synthesis. | [60] |
*(2R,3R,4S,5R,7S,8R,13R,15R)-3,5,7,15-Tetraacetoxy-2-hydroxy-8-tigloyloxy-9,14-dioxojatropha-6(17),11E-diene
Table 2: Mode of action of specific phytochemicals with antiviral activity.
Challenges and future avenues
Plants possessing minute amount of invaluable natural compounds are great source for pharmaceuticals invention. Modern drug discovery which has its roots in traditional medicine provides avenues to newer phytomolecules-based therapies [72]. Now a days major pharmaceutical industry are reducing their research focus and are indulging towards profit-making venture by extracting and selling phyto-constituents [73]. Various challenges appear during the drug discovery or identification of natural compound from plants or their extracts. The isolation of active biological compound(s) starts primarily with their isolation, purification and characterization involving multistep procedures with low yields. In addition, reduction of biological activity of compound in each step during fractionation of extracts leads to the loss of synergistic effects between analogue constituents [74]. In modern time, many efforts have been focused on the production of bioactive natural compounds/products from plants through metabolically engineered process using versatile E. coli to sustain the availability of potential drug candidates; however, it has one possible drawback that is nonfunctionality of many key plant enzymes [75]. Metabolical engineering of plant derived natural products/compounds no doubt has high potential but with a little success rate. A gene encoded Hyoscyanin 6β-hydroxylase isolated from Hyoscyamus niger and incorporated into Atropa belladonna resulted in the accumulation of high value of endproduct Scopolamine but this compound was however, overexpressed in Hyoscyamus muticus hairy roots. So, along with large amount of Scopolamine, high level of Hyoscyamine also accumulated in the hairy roots [76,77]. Alternative biotechnology tools to enhance the production of natural products/compounds using plant cell cultures are indeed needed. Such examples include skinonin production from Lithospermum erythrorhizon and berberine production by Copis japonica cell culture [78]. Another big challenge in natural compounds/ drug development is that it hardly focuses on single site(s) of drug action and ignores the multiple pharmacological actions of many drugs and molecules. So, with the development of science of network pharmacology, which involves interpretation of mechanism of drug action, shifts one target or one drug to target a disease, considers multicomponent plant mixtures (which contains 10-20 plants in the formula) has been expected as a significant alternative model for the drug discovery [79,80]. A number of electrophilic [81], nucleophilic [82] and scavengers [83] are commercially available now to avoid repeated purifications of the crude compound prior to final HPLC purification. Today, nanomedicine gained tremendous attention in pharmaceutical science [84]. Phytonanotechnology provides ecofriendly, simple, rapid, stable and cost effective new avenues for synthesis of nanoparticles. It has advantages including biocompatibility and has medical applicability [85]. As plant constituents, they act as capping and stabilizing agents [86]. Many natural compounds such as Quercetin (phytochemical) with low aqueous solubility and bioavailability is quickly metabolized in the body, which unfortunately reduces its efficacy in treating diseases [87]. The encapsulation of Quercetin into biodegradable and biocompatible nanoparticles might help in delaying its metabolism and maintaining adequate free Quercetin level in blood. But major hurdle is potential toxicity of nanoparticles. So, incorporation of targeted ligand on the surface of nanoparticles can increase delivery of encapsulated phytochemicals [88]. Data exist about and bioavailability of nanocariers, and tissue specific pharmokinetics are limited [89]. The precise mechanism and the components responsible for plantmediated synthetic nanoparticles remain to be elucidated. The use of in silico information systems further provide avenues that might involve databases to relate constituents to their network profile in order to integrate networks which would decrease the exploitation of plants. Such strategies may also permit the development of new drug discovery from plants which have ubiquitous existence on all corners of earth.
Conclusion
Viruses are obligate intracellular parasites that have evolved genetic variation, transmission, and replication, and have the ability to persist within the host for short or long period of time. By understanding the molecular mechanisms of viral invasion and replication in the host cell(s), researchers and scientists will get help to design effective and inexpensive antiviral drugs and target them to their specific site. Most of the known clinical essential antiviral drugs can specifically target a single viral enzyme (proteolytic viral enzymes, viral polymerase, integrase and reverse transcriptase) during different stages of viral replication. For new drug development, the replication inhibitors are midst of the top priorities as many virus diseases are yet non-curable. Acyclo-guanosine, popularly known as acyclovir is a nucleoside analogue that drastically slows down the Herpes virus infection [90,91]. Carbohydrate-binding agents might also act as inhibitors binds by masking the glycans present on the viral envelope. This step subsequently blocks virus entry and cause some deletions in the envelope glycan shield triggering the immune system to act against epitopes of the viral envelope. Glycans have been reported to be present on the HIV and HCV and often play important role in enabling an efficient entry of the virus into permissive target cells [92]. For creative revelation of modern drug development, introduction of high-throughput technologies and traditional medicines together could play a crucial role to identify the potential plant derived compounds. They act against viral diseases but still have long way to cover for discovery, isolation and mechanistic studies before final utilization in the clinic. Considering vast range and complexity of bioactive molecules/phytochemicals present in plants, a strong unrelenting and persistent approach is needed to explore unknown phyto-constituents with potent antiviral activities. This obviously requires more investments, systematic exploration to prove the desired activity of the phyto-constituent in an in vivo and/or in vitro model in a reasonable period of time.
Conflict of Interest
The authors have no conflict regarding publication of this paper among themselves or with the parent institute.
Acknowledgement
The financial supports in the form of a Senior Research Fellowship [F. No. 17-4- /08(SA-1)] to Ms. Rita Kapoor from University Grants Commission, New Delhi, and DST Inspire-Senior Research Fellowship to Mr. Bhupender Sharma (IF 130378), Department of Science and Technology, Ministry of Science and Technology, Govt. of India are gratefully acknowledged. The authors are thankful to the Department of Biotechnology, Himachal Pradesh University, Shimla for providing the basic infrastructure and other facilities to the authors.
References
- Orhan I, Deliorman-Orhan D, Ozçelik B (2009) Lipophilic extracts of various edible plants and their fatty acids. Food Chem 115: 701-705.
- Monath TP, Woodall JP, Gubler DJ, Yuill TM, Mackenzie JS, et al. (2016) Yellow fever vaccine supply: A possible solution. Lancet 387: 1599-1600.
- Dyer O (2015) Zika virus spreads across Americas as concerns mount over birth defects. BMJ 2015: 351: h6983.
- Yasuhara-Bell J, Yang Y, Barlow R, Trapido RH, Lu Y (2010) In vitro evaluation of marine-microorganism extracts for antiviral activity. Virology 7: 182.
- Farrar J, Focks D, Gubler D, Barrera R, Guzman MG, et al. (2007) Towards a global dengue research agenda. Trop Med Int Health 12: 695-699.
- Muller V, Chávez JH, Reginatto FH, Zucolotto SM, Niero R, et al. (2007) Evaluation of antiviral activity of South American plant extracts against herpes simplex virus type 1 and rabies virus. Phytother Res 21: 970-974.
- Moscana AN (2005) Neuraminidase inhibitors for influenza. N Eng J Med 353: 1363-1373.
- Khan MTH, Ather A, Thompson KD, Gambari R (2005) Extracts and molecules from medicinal plants against Herpes simplex viruses. Antiviral Res 67: 107-111.
- Ghosh T, Chattopadhyay K, Marschall M, Karmakar P, Mandal P, et al. ( 2009) Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiol 19: 2-15.
- Dikid T, Jain SK, Sharma A, Kumar A, Narain JP (2013) Emerging and remerging infections in India. Indian J Med Res 138: 19-31.
- Dikid T, Jain SK, Sharma A, Kumar A, Narain JP (2013) Emerging and remerging infections in India. Indian J Med Res 138: 19-31.
- De Clercq E (2005) Recent highlights in the development of new antiviral drugs. Curr Opin Microbiol 8: 552-560
- Hostettmann KM, Marston A, Ndjoko K, Wolfender JL (2000) The potential of African plants as a source of drugs. Curr Org Chem 4: 973-1010.
- Cos P, Maes L, Vanden Berghe D., Hermans N, Pieters L, et al. (2004) Plant substances as anti-HIV agents selected according to their putative mechanism of action. J Nat Prod 67: 284-293
- Hostettmann, K, Marston A (2002) Twenty years of research into medicinal plants: Results and perspectives. Phytochem Rev 1: 275-285.
- Sala E, Guasch L, Iwaszkiewicz J, Mulero M, Salvado MJ, et al. (2011) Identification of human IKK-2 inhibitors of natural origin (Part II): In silico prediction of IKK-2 inhibitors in natural extracts with known anti-inflammatory activity. Eur J Med Chem 46: 6098-6103.
- Cragg GA, Newman DJ (2005) Biodiversity: A continuing source of novel drug leads. Pure Appl Chem 77: 7-24.
- Farnsworth NR (1985) Medicinal plants in therapy. Bull World Health Organ 63: 965-981.
- Sala E, Guasch L, Iwaszkiewicz J, Mulero M, Salvado MJ, et al. (2011) Identification of human IKK-2 inhibitors of natural origin (Part II): In silico prediction of IKK-2 inhibitors in natural extracts with known anti-inflammatory activity. Eur J Med Chem 46: 6098-6103.
- Slikkerveer L (2006) The challenge of non-experimental validation of mac plants, towards a multivariate model of transcultural utilization of medicinal, aromatic and cosmetic plants. In: medicinal and aromatic plants: agricultural, commercial, ecological, legal, pharmacological and social aspects RJ Bogers, LE Craker, D Lange (Eds) Springer 17: 1-28.
- Shinwari ZK (2010) Medicinal plants research in Pakistan. J Med Plants Res 4: 161-176.
- De Smet PA (2002) Herbal remedies. N Engl J Med 347: 2046-2056.
- Chattopadhyay D, Chawla SM, Chatterjee T, Dey R, Bag P, et al. (2009) Recent advancements for the evaluation of antiviral activities of natural products. N Biotechnol 25: 347-368.
- Hupfeld J, Efferth T (2009) Drug resistance of human immunodeficiency virus and overcoming it by natural products. In Vivo 23: 1-6.
- Moghaddam E, Teoh BT, Sam SS, Lani R, Hassandarvish P, et al. (2014) Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci Rep 4: 5452.
- Dao TT, Nguyen PH, Lee HS, Kim E, Park J, et al. (2011) Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflate. Bioorg Med Chem Lett 21: 294-298.
- Esimone CO, Gero Eck, Nworu, CS, Hoffmann D, Uberla K, et al. (2010) Dammarenolic acid, as ecodammaranetriterpenoid from Aglaia sp. shows potent anti-retroviral activity in vitro. Phytomed 17: 540-547.
- Corlay N, Delang L, Valenciennes EG, Neyts J, Clerc P, et al. (2014) Tigliane diterpenes from Croton mauritianus as inhibitors of Chikungunya virus replication. Fitoterapia 97: 87-91.
- Fang CY, Chen SJ, Wu HN, Ping YH , Lin CY, et al. (2015) Honokiol, a lignanbiphenol derived from the magnolia tree, inhibits dengue virus type 2 infection. Viruses 7: 4894-4910.
- Kang KB, Ming G, Kim GJ, Ha TKQ, Cho Hi, et al. (2015) Cyclopeptide alkaloids from the roots of Ziziphus jujuba. Phytochem 43: 264-267.
- Cheng HY, Yang CM, Lin TC, Lin LT, Chiang LC, et al. (2011) Excoecarianin, isolated from Phyllanthus urinaria Linnea, inhibits herpes simplex virus type 2 infection through inactivation of viral particles. Evid Based Compl Alt Med 8: 1.
- Chung CY, Liu CH, Burnouf T, Wang GH, Chang SP, et al. (2016) Activity-based and fraction-guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of Hepatitis C virus entry. Antiviral Res 130: 58-68.
- Fang CY, Chen SJ, Wu HN, Ping YH , Lin CY, et al. (2015) Honokiol, a lignanbiphenol derived from the magnolia tree, inhibits dengue virus type 2 infection. Viruses 7: 4894-4910.
- Kang KB, Ming G, Kim GJ, Ha TKQ, Cho Hi, et al. (2015) Cyclopeptide alkaloids from the roots of Ziziphus jujuba. Phytochem 43: 264-267.
- Lin LT, Chung CY, Hsu WC, Chang SP, Hung TC, et al. (2015) Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J Hepatol 62: 541-548.
- Wu SF, Lin CK, Chuang YS, Chang FR, Tseng CK, et al. (2012) Anti-hepatitis C virus activity of 3-hydroxy caruilignan C from Swietenia macrophylla stems. J Viral Hepat 19: 364-370.
- Cheng YB, Chien YT, Lee JC, Tseng CK, Wang HC, et al. (2014) Limonoids from the seeds of Swietenia macrophylla with inhibitory activity against Dengue virus 2. J Nat Prod 26: 2367-2374.
- Yang JL, Ha TKQ, Dhodary B, Pyo E, Nguyen NH, et al. (2015) Oleanane triterpenes from the flowers of Camellia japonica inhibit porcine epidemic diarrhea virus (PEDV) replication. J Med Chem 58: 1268-1280.
- Bachmetov L, Tanamy MG, Shapira A, Vorobeychik M, Giterman-Galam T, et al. (2012) Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J Viral Hepat 19: e81-e88.
- Lin LT, Chung CY, Hsu WC, Chang SP, Hung TC, et al. (2015) Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J Hepatol 62: 541-548.
- Esposito F, Carli I, Vecchio CD, Xu L, Corona A, et al. (2016) Sennoside A, derived from the traditional Chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomed 23: 1383-1391.
- Biedenkopf N, Lange-Grünweller K, Schulte FW, Weiber A, Muller C, et al. (2016) The natural compound Silvestrolis a potent inhibitor of Ebola virus replication. Antiviral Res 137: 76-81.
- Bai R, Zhang XJ, Li YL, Liu JP, Zhang HB, et al. (2015) SJP-L-5 a novel small-molecule compound, inhibits HIV-1 infection by blocking viral DNA nuclear entry. BMC Microbiol 15: 274.
- Alvarez AL, Habtemariam S, Moneim AEA, Melón S, Dalton KP, et al. (2015) A spiroketal-enol ether derivative from Tanacetum vulgare selectively inhibits HSV-1 and HSV-2 glycoprotein accumulation in Vero cells. Antiviral Res 119: 8-18.
- Geng CA, Jiang ZY, Ma YB, Luo J, Zhang XM, et al. (2009) Swerilactones A and B, anti-HBV new lactones from a traditional Chinese herb: Swertia mileensis as a treatment for viral hepatitis. Org Lett 11: 4120-4123.
- Zhang N, Liu Z, Han Q, Chen J , Lv Y (2010) Xanthohumol enhances antiviral effect of interferon a-2b against bovine viral diarrhea virus, a surrogate of hepatitis C virus. Phytomed 17: 310-316.
- Serkedjieva J, Ivancheva S (1999) Antiherpes virus activity of extracts from the medicinal plant Geranium sanguineum L. J Ethnopharmacol 64: 59-68.
- Li HB, Chen F (2005) Isolation and purification of baicalein, wogonin and oroxylin A from the medicinal plant Scutellaria baicalensis by high-speed counter-current chromatography. J Chromatogr A 1074: 107-110.
- Chuanasa T, Phromjai J, Lipipunc V, Likhitwitayawuid K, Suzuki M, et al. (2008) Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: Mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antiviral Res 80: 62-70
- Xu JJ, Wu X, Li MM, Li GQ, Yang YT, et al. (2014) Antiviral activity of polymethoxylated flavones from Guangchenpi, the edible and medicinal pericarps of Citrus reticulata ‘Chachi’. J Agric Food Chem 62: 2182-2189.
- Scaglia LFN, Retailleau P, Paolini J, Pannecouque C, Neyts J, et al. (2014) Jatrophane diterpenes as inhibitors of chikungunya virus replication: Structure-activity relationship and discovery of a potent lead. J Nat Prod 77: 1505-1512.
- Lin JC (2003) Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antiviral Res 59: 41-47.
- Choi HJ, Song JH, Park KS, Kwon DH (2009) Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur J Pharm Sci 37: 329-333.
- Kuo YC, Lin LC, Tsai WJ, Chou CH, Kung SH, et al. (2002) Samarangenin B from Limonium sinense suppress herpes simplex virus type 1 replication in vero cells by regulation of viral macromolecular synthesis. Antimicrob Agents Chemother 46: 2854-2864.
- Huanga TJ, Tsai YC, Chiang SY, Wang GJ, Kuoe YC, et al. (2014) Anti-viral effect of a compound isolated from Liriope platyphylla against hepatitis B virus in vitro. Virus Res 192: 16-24.
- Barquero AA, Michelini FM, Alche LE (2006) 1-Cinnamoyl-3, 11-dihydroxymeliacarpin is a natural bioactive compound with antiviral and nuclear factor-kappa B modulating properties. Biochem Biophys Res Commun 344: 955-962.
- Zhang Y, But PPH, Ooi VEC, Xu HX, Delaney GD, et al. (2007) Chemical properties, mode of action, and in vivo anti-herpes activities of a lignin-carbohydrate complex from Prunella vulgaris. Antiviral Res 75: 242-249
- Cheng HY, Lin TC, Yang CM, Wang KC, Lin CC (2004) Mechanism of action of the suppression of herpes simplex virus type 2 replication by pterocarnin A. Microbes Infect 6: 738-744.
- Wahyuni TS, Widyawaruyanti A, Lusida MI, Fuad A, Soetjipto, et al. (2014) Inhibition of hepatitis C virus replication by chalepin and pseudane IX isolated from Ruta angustifolia leaves. Fitoterapia 99: 276-283.
- Cui H, Xu B, Wu T, Xu J, Yuan Y, et al. (2014) Potential antiviral lignans from the roots of Saururus chinensis with activity against Epstein-barr virus lytic replication. J Nat Prod 77: 100-110.
- Li FY, But PPH, Ooi VEC (2005) Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L) Antiviral Res 68: 1-9.
- Hayashi K, Niwayama S, Hayashi T, Nago R, Ochiai H, et al. (1988) In vitro and in vivo antiviral activity of scopadulcic acid B from Scoparia dulcis, Scrophulariaceae, against herpes simplex virus type 1. Antiviral Res 9: 345-354
- Nagai T, Moriguchi R, Suzuki Y, Tomimori T, Yamada H (1995) Mode of action of the anti-influenza virus activity of plant flavonoid, 5,7,4'-trihydroxy-8-methoxyflavone, from the roots of Scutellaria baicalensis. Antiviral Res 26: 11-25
- Qin N, Li CB, Jin MN, Shi LH, Duan HQ, Niu WY (2011) Synthesis and biological activity of novel tiliroside derivants Eur J Med Chem 46: 5189-5195.
- Bauer A, Brönstrup M (2014) Industrial natural product chemistry for drug discovery and development. Nat Prod Rep 31: 35-60
- Fernandes MJB, Barros AV, Melo MS, Simoni IC (2012) Screening of Brazilian plants for antiviral activity against animal herpes viruses. J Med Plant Res 6: 2261-2265.
- Mettenleiter TC, Klupp BG, Granzow H (2006) Herpes virus assembly: A tale of two membranes. Curr Opin Microbiol 9: 423-429.
- Ryan KJ, Ray CG (2004) Sherris Medical Microbiology (4th ed) McGraw Hill 551-552.
- Clarke RW (2015) Forces and structures of the herpes simplex virus (HSV) entry mechanism. ACS Infect Dis 1: 403-415.
- Cos P, Berghe VD, Bruyne TD, Vlietinck AJ (2003) Plant substances as antiviral agents: An update (1997-2001). Curr Org Chem 7: 1163-1180.
- GBD 2015 disease and injury incidence and prevalence, collaborators (2016) Global, regional, and national incidence, prevalence and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet 388: 1545–1602.
- Maheshwari A, Thuluvath, PJ (2010). Management of acute hepatitis C. Clin Liver Dis 14: 169-176.
- Chan DC, Kim PS (1998) HIV entry and its inhibition. Cell 93: 681-684.
- Wyatt R, Sodroski J (1998) The HIV-1 envelope glycoproteins: fusogens, antigens and immunogens. Science 280: 1884-1888.
- Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, et al. (2015) A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun 6: 8143.
- Wolfgang A, Farrell PJ (2004) Reactivation of Epstein-Barr virus from latency. Rev Med Virol 15: 149-156.
- Paterson I, Anderson EA (2005) The renaissance of natural products as drug candidates. Science 310: 451-453.
- Jarvis MF (2010) The neural–glial purinergic receptor ensemble in chronic pain states. Trends Neurosci 33: 48-57.
- Zak O, Sande MA (1999) Handbook of animal models of infection. Academic Press, London.
- Leonard E, Yan Y, Fowler ZL, Li Z, Lim CG, et al. (2008) Strain improvement of recombinant Escherichia coli for efficient production of plant flavonoids. Mol Pharm 5: 257-265.
- Jouhikainen K, Lindgren L, Jokelainen T, Hiltunen R, Teeri TH, et al. (1999) Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta 208: 545-551.
- Yun DJ, Hashimoto T, Yamada Y (1992) Metabolic engineering of medicinal plants: transgenic Atropa belladonna with an improved alkaloid composition. Proc Natl Acad Sci USA 89: 11799-11803.
- Fujita Y, Tabata M (1987) Secondary metabolites from plant cells: pharmaceutical applications and progress in commercial production. In plant tissue and cell cultures. Green CE, Somers DA, Hackett WP, Biesboer DD (Ed), Alan R Liss, New York 169-185.
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, et al. (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2: 751-760.
- Noruzi M (2015) Biosynthesis of gold nanoparticles using plant extracts. Bioprocess Biosyst Eng 38: 1-14.
- Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, et al. (2014) Green nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat 6: 35-44.
- Hopkins AL (2007) Network pharmacology. Nat Biotechnol 25: 1110-1111.
- Hopkins AL (2008) Network pharmacology: The next paradigm in drug discovery. Nat Chem Biol 4: 682-690.
- Shanmuganathan S, Greiner L, Domínguez de María P (2010) Silica immobilized piperazine: A sustainable organocatalyst for aldol and knoevenagel reactions. Tetrahedron Lett 51: 6670-6672.
- Kennedy AL, Fryer AM, Josey JA (2002) A new resin-bound universal isonitrile for the ugi 4CC reaction: Preparation and applications to the synthesis of 2,5-diketopiperazines and 1,4-benzodiazepine-2,5-diones. Org Lett 4: 1167-1170.
- Eames J, Watkinson M (2001) Polymeric scavenger reagents in organic synthesis. Eur J Org Chem 7: 1213-1224.
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, et al. (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2: 751-760.
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 12175
- [From(publication date):
June-2017 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 10218
- PDF downloads : 1957