Antiulcerogenic Effects of Aqueous Stem-Bark Extracts of Pterocarpus erinaceus on Indomethacin-Induced Ulcer in Albino Rats
Received: 25-Apr-2018 / Accepted Date: 21-Jun-2018 / Published Date: 30-Jun-2018
Keywords: Anti-ulcerogenic; Albino rats; Indomethacin; Ulcer; Phytochemical; Stem-bark extract
Introduction
Ulcers are deep lesions penetrating through the entire thickness of the gastrointestinal tract (G.I.T) mucosa and surrounding musculature. Ulcers create discontinuities or breaks in membranes and so impede tissue or organ functions. This disease and its complications have shown striking geographical variations in incidence and prevalence across the globe typifying the different types that include peptic and gastric ulcers, which are consistent with the linings of the stomach, and duodenum respectively [1]. Ulcers are associated with excessive secretion of acid by the stomach [2].
Medicinal plants remain a rich source of bioactive phytochemicals that have proved to be richest resource of drugs used in traditional and modern medicine as pharmaceutical intermediate and chemical entities for synthetic drugs [3]. These plants contain a wide variety of chemical compounds of therapeutic value which can be sorted out by their chemical classes and functional groups. Some of them have been documented for their antioxidant potentials [4].
Herbal and traditional medicines are widely used in Nigeria. There is the need to promote research in drug development, especially herbal medicine. Previous studies by [5] showed that the ethanolic stem bark extracts of P. erinaceus possess significant and dose-dependent analgesic and anti-inflammatory activities in laboratory rats and mice. Other reports [6] revealed that the phytochemical constituents of P. erinaceus include steroids, flavonoids, tannins, carbohydrate, proteins and amino acids. We report have other effects of the stem bark extracts of P. erinaceus
Materials and Method
Plant materials
The stem-bark specimens of P. erinaceus were collected in June 2015 from Vunoklang Village in Girei L.G.A of Adamawa State, Nigeria which lies between latitudes 9°11’ to 9°19’ and longitude 12°20’ to 12°30’ [7]. The plant specimen identified and authenticated in the Department of Plant Science of Modibbo Adama University of Technology, Yola, Nigeria. A voucher specimen was also kept in the department herbarium.
Stem bark preparation
The stem-bark was air-dried for 30 days and then reduced to powder form using mortar and pestle [8]. One hundred and eighty grams (180 g) of the powdered stem bark were cold macerated in 100 ml of distilled water for a minimum of 24 hours with constant shaking and filtered using Whatman’s filter paper No. 1. The extract was then concentrated to dryness on a water bath at 40°C. The extract was further dried in vacuum oven. The dried aqueous extract was dissolved in water to get clear solution, which was used for the analysis.
Animals
Forty two (42) adult male albino rats weighing (80 ± 20 g) were procured from Animal House Unit; National Veterinary Research Institute, Vom, Jos, Nigeria. The animals were housed in polypropylene cages and were given Broiler finisher feed and water ad libitum and maintained under laboratory conditions of temperature of about 25°C and 12 hours light and dark cycles before any work was carried out using them.
Determination of phytochemicals presents in P. erinaceus stem bark extracts
Qualitative analysis was determined using the method of Trease and Evans, 1989, Sofowora 1984 and Harborne 1973.
Determination of Concentration of Some Phytochemicals in the Aqueous Stem-Bark Extract of P. erinaceus
Determination of tannins using Folin-Denis reagent
Procedure: To 0.20 gram of the extract was added 20 ml of 50% methanol. This was shaken thoroughly and placed in a water bath at 80°C for 1 hour to ensure uniform mixing. The extract was filtered into a 100 ml volumetric flask, followed by the addition of 20 ml of distilled water, 2.5 ml of Folin-Denis reagent and 10 ml of 17% aq. Na2CO3 and was thoroughly mixed. The mixture was made up to 100 ml with distilled water, mixed and allowed to stand for 20 min. The bluishgreen color developed at the end of the reaction mixture of different concentrations. The absorbance of the tannic acid standard solutions as well as sample was measured after color development at 760 nm (Association of Official Analytical Chemistry (AOAC), 1980).
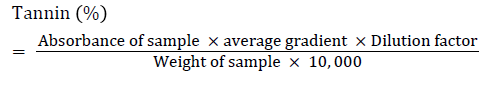
Estimation of total alkaloid
5 gram of the sample was weighed into 250 ml beaker and 200 ml of 10% acetic acid in ethanol was added and covered and allowed to stand for 4 hour. It was then be filtered and the extract was concentrated on a water bath to one quarter of the original volume. Concentrated ammonia hydroxide was then added drop wise to the extract until the precipitation was complete. The whole solution was allowed to settle and the precipitated was collected and was washed with 0.1% dilute ammonium hydroxide and was then filtered. The residue was the alkaloid, which was then dried and weighed (Harborne).

Flavonoids determination
10 gram of the plants sample was extracted repeatedly with 100 ml of 80% aqueous methanol at room temperature. The whole solution was filtered through Whatman’s No 42 filter paper (125 mm). The filtrate was then transferred into crucible and evaporated into dryness over a water bath and weighed to a constant weight (Bohm and Koapai-Abyazan).
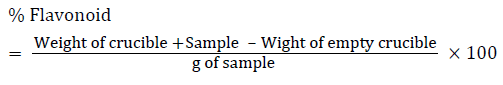
Determination of total saponins
The plant sample (20 gram) was added to 100 ml of 20% aqueous ethanol and kept in a shaker for 30 min. The samples were heated over a water bath for 4 hour at 55°C. The mixture was then filtered and the residue re-extracted with another 200 ml of 20% aqueous ethanol. The combined extracts were reduced to approximately 40 ml over the water bath at 50°C. The concentrate was transferred into a 250 ml separatory funnel and extracted twice with 20 ml diethyl ether. The ether layer was discarded while the aqueous layer was retained and to which 60 ml n-butanol was added. The n-butanol extracts were washed twice with 10 ml of 5% aqueous sodium chloride. The remaining solution was heated on a water bath. After evaporation, the samples were dried in the oven at 40°C to a constant weight (Obadoni and Ochuko). The Saponins content was calculated using the formula:
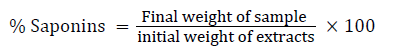
Determination of total phenols
The fat free sample was boiled with 50 milliliter of ether for the extraction of the phenolic component for 15 min. 5 ml of the extract was taken into a 50 ml flask, and then 10 ml of distilled water was added. 2 ml of ammonium hydroxide solution and 5 ml of concentrated amyl alcohol was also added. The samples were made up to mark and left to react for 30 minutes for colour development. It was then measured at 505 nm. These data was used to estimate the total phenolic content using a standard calibration curve that was obtained from various diluted concentrations of gallic acid [9].
Experimental design
Forty two (42) male albino’s rats weighing 80 ± 20 g used in the study were randomized into seven groups of six rats each. Ulceration was induced in the animals with a single oral dose of indomethacin (30 mg/kg/body weight) on the first day of the experiment. The animals were deprived of food but had access to water 24 hours prior to ulcer induction [10]. The groupings of the animals were as follows:
Group A. (normal control) animals received only broiler finisher feed with water. Group B. animals were administered respectively with 900 mg/kg/b.w of aqueous extracts (AQE) only. Rats in Group C. were given cimetidine 100 mg/kg/b.w orally once daily as a reference drug. Animals in Group D were given indomethacin 30 mg/kg/b.w single oral doses as a negative control (ulcer control). Groups E, F, and G, comprised ulcerated rats treated with AQE (300, 600 and 900 mg/kg/ b.w), respectively. Treatments with the cimetidine and plant extract lasted for fourteen (14) days on indomethacin- ulcer induced animals. Cimetidine and extract administered orally once daily using oral intubator. Food and water were given ad libitum throughout the experimental period. Determinations were done weekly by sacrificing three (3) animals each week.
Microscopic examination
On the twenty-first day, the animals were humanely sacrificed under anaesthesia (with an overdose of chloroform) and the stomachs were opened along the greater curvature, rinsed with normal saline to remove gastric contents and blood clots and examined with the aid of magnifier lens (10X) to assess the formation of ulcer. The numbers of ulcers were counted. Mean Ulcer Score for each animal were expressed as ulcer index. Ulcer index was calculated by the method [11].
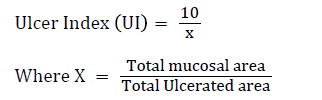
The Ulcer area (UA) was calculated using the sum of the areas of all lesions for each stomach according to previously published protocol [12].
The percentage healing was calculated as:

Biochemical Estimation
Homogenization process
The stomach tissue from the glandular portion of stomach was excised and washed in ice cold 1.15% potassium chloride solution, blotted with filter paper. They were then chopped into bits (0.5 g each) and homogenized in four volumes of phosphate saline buffer (1: 4 (w/v), pH 7.4) using mortar and pestle. The resulting homogenate was centrifuged at 3500 rpm for 10 min at temperature of 4°C. The resulting supernatants were frozen in a freezer to ensure maximum release of enzymes located in the tissue before being used for the enzymes assay and other parameters [12].
Volume of gastric juice
Principle: This method is based on the principle that gastric volume reflects the processes of secretions and emptying and allows the simultaneous measurement of gastric volume, ions and fluid secretion and emptying. This point is important as intragastric volume influences gastric acid secretion and intragastric H+ concentration regulates in part, gastric emptying.
Procedure: Contents of the dissected stomachs of the rats were taken in graduated test tubes and allowed to centrifuge at 2000 rpm for 15 min. The supernatant fluid was measured for volume of gastric juice and expressed as ml/4 hours [12,13].
pH of gastric juice
Principle: A pH meter provides a value as to how acidic or alkaline a liquid is. The basic principle of the pH meter is to measure the concentration of hydrogen ions; Acids dissolve in water forming positively charged hydrogen ions (H+). The greater this concentration of hydrogen ions, the stronger the acid is.
Procedure: The gastric juices of the stomach previously kept in the blank serum bottle were centrifuge at 2000 rpm for 15 min to determine the pH using a pH meter.
Free and total acidity
Principle: The determination of free and total acidity is based upon a simple acid-base titration with the difference that two indicators are used for the two end points, instead of the usual one indicator used in such titrations.
Color change during titration:
Red → Orange → Yellow → Pink
Free acidity is completely neutralized at pH 3.5 and total acidity at pH 8.5
Procedure: 1 ml of the supernatant liquid was pipetted out and diluted to10 ml with distilled water. To the end point when the solution turned to orange colour, the volume of NaOH needed was taken as corresponding to be free acidity [14].
Titration was further continued till the solution regained pink colour; the volume of NaOH required was noted and was taken as corresponding to the total acidity.
Acidity was expressed as:
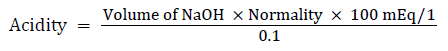
Determination of pepsin activity
Principle: Pepsin is the principal proteolytic enzymes of vertebrate gastric juice. Its inactive precursor form, pepsinogen, is released by the chief cells in the stomach wall, and upon mixing with hydrochloric acid of the gastric juice, pepsinogen activate to become pepsin.
Procedure: Aliquots of 20 μl of the gastric content were incubated with 500 μl of albumin solution (5 mg/ml) in 0.06 N HCl acid at 37°C for 10 min. The reaction was stopped with 200 μl of 10% TCA and the samples were centrifuge at 1500 rpm for 20 min. The supernatant was alkalinized with 2.5 ml of 0.55 M sodium carbonate and 400 μl of 1.0 N Folin’s reagent was added to the tubes, which were incubated for 30 min at room temperature. The absorbance of the samples was determined by spectrophotometry at 660 nm [15].

Determination of protein content of the stomach tissue
Principle: The assay was based on polypeptide chelating of cupric ion (colored chelate) in strong alkali. The colour intensity was measured using spectrophotometer, and it is directly proportional to the amount of total protein.
Procedure: Stomach homogenate sample of 0.4 ml was added to 6.0 ml of sulphate-sulphide solution. The Sulphate-sulphide (2.0 ml) was added to 5.0 ml Biuret reagent and Tartrate-iodide solutions. The mixtures were mixed well and were incubated at room temperature for 15 mins. The spectrophotometer was set to zero using the blank at 540 nm the absorbance of the standard test and QC was measured [16,17].
Calculation:

Determination of the lipid peroxidation (MDA level)
Principle: The principle of this method being that malondialdehyde (MDA), an end product of lipid peroxidation, reacts with Thiobarbituric acid (TBA) to form a pink chromogen. The level of peroxidation (peroxides) can be expressed as nmoles of MDA formed/mg protein.
Procedure: The supernatant (300 μL) of various groups were pipetted into test tubes already containing 300 μL of 8.1% SDS (sodium duodecyl sulphate), 600 μL of 1.34 M acetate buffer (pH 3.4) and 600 μL of 0.8% thiobarbituric acid. The reaction mixtures were incubated at 100°C for one hour and allowed to cool. The absorbance was taken against the blank at 532 nm [12].
Calculation:
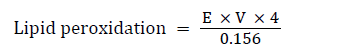
Where E=absorbance; V=Total volume in test tube; 0.156=Molar Extinction coefficient at 532 nm; 4=Conversion factor to hour
Results/Discussion
The relatively high percentage yield of aqueous stem bark extract of P. erinaceus (Table 1) could suggest some abundance of phytochemicals in the stem bark extract. Alkaloids, flavonoids, saponins, tannins, steroids, terpenoids, phenols (Table 2) were detected in the extract with saponins and tannins occurring in substantial amounts: Flavonoids and phenols occurred in reasonable amounts (Table 3). The occurrence of alkaloids was negligible. It would therefore be reasonable to suggest that saponins and tannins could be the principal bioactive substances in the extract.
| Solvent | Initial weight | Yield | Percentage Yield (%) |
|---|---|---|---|
| Aqueous Extract | 400 | 144 | 36 |
Table 1: Percentage yield of aqueous stem-bark extract of P. erinaceus
| Phytochemical | Aqueous Extract |
|---|---|
| Alkaloids | + |
| Flavonoids | + |
| Glycosides | - |
| Tannins | + |
| Terpenoids | - |
| Saponins | + |
| Steroids | + |
| Phenols | + |
| Key: += present, -= absent | |
Table 2: Some phytochemicals detected in the aqueous stem-bark extract of P. erinaceus
| Phytochemical | Aqueous Extract (% w/w) |
|---|---|
| Alkaloids | 0.67 ± 0.29 |
| Flavonoids | 8.99 ± 2.03a |
| Saponins | 35.53 ± 0.64a |
| Tannins | 26.29 ± 0.04a |
| Phenols | 19.86 ± 0.41 |
a significantly higher (p˂0.05) compared to values horizontally
Table 3: Concentration of some phytochemicals in the aqueous stem-bark extract of P. erinaceus
Flavonoids are believed to exert their anti-ulcerogenic effects by promoting mucus formation, directly activating gastric membrane protective factors, or stimulating inherent protective mechanism via irritation of the gastric mucosa [18]. Saponins as complex compounds with a saccharide attached to a steroid or triterpene have haemolytic, expectorative, anti-inflammatory and immune-stimulating activities.
Tannins are used in medicine primary because of their astringent properties, since they react with proteins in tissues. Tannins precipitate micro proteins at the site of peptic ulcer, forming a protective layer that prevents absorption of toxic substances, and promote resistance to the action of proteolytic enzymes, an associated activity against H. pylori [19].
Indomethacin caused significant increase in the ulcer index (Table 4) suggesting that indomethacin induced gastric ulcer model was useful in inducing severe ulceration in experimental animals [20]. It has been suggested that indomethacin induces gastric damage via inhibiting the release of protective factors like cyclooxygenase-1, prostaglandins E2, bicarbonate and mucus; increasing the aggressive factors like gastric acid; and increasing oxidant parameters while decreasing antioxidant parameters [21]. The significant decreases observed in the ulcer index on treatment with aqueous stem bark extract of P. erinaceus at different doses, suggest reduced severity of indomethacin induced ulcer following recovery through increased protective factors; alongside other natural recovery mechanisms and the percentage healing was calculated as percentage curative which signifies the level of healing as a result of administration of aqueous extracts (Table 5).
| Groups | Treatment | Wk 1 | Wk 2 |
|---|---|---|---|
| 1 | Normal Control | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 2 | Aqueous Extract treated 900 mg/kg/b.w | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 3 | Ulcerated rats treated with Cimetidine 100 mg/kg/b.w | 3.29 ± 0.09 | 1.85 ± 0.14ac |
| 4 | Indomethacin 30 mg/kg/b.w | 8.28 ± 1.11b | 9.12 ± 0.98b |
| 5 | Ulcerated rat treated with 300 mg/kg/b.w AQE | 6.00 ± 1.82 | 3.00 ± 1.05ac |
| 6 | Ulcerated rat treated with 600 mg/kg/b.w AQE | 5.10 ± 1.43 | 2.50 ± 0.29a |
a significantly lower (p˂0.05) compared to values of indomethacin group in each column
b significantly higher (p˂0.05) compared to values in each column
c significantly lower (p˂0.05) compared to values in week 1
Key: AQE=Aqueous Extract; b.w=body weight
Table 4: Effects of aqueous stem-bark extract of P. erinaceus on ulcer index (mm2)
| Groups | Treatment | Wk 1 | Wk 2 |
|---|---|---|---|
| 1 | Normal Control | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 2 | Aqueous Extract Treated 900 mg/kg/b.w | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 3 | Ulcerated rats treated with Cimetidine 100 mg/kg/b.w | 49.75 ± 3.39 | 80.59 ± 2.24a |
| 4 | Indomethacin 30mg/kg/b.w | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 5 | Ulcerated rat treated with 300 mg/kg/b.w AQE | 21.23 ± 0.56 | 57.08 ± 2.83a |
| 6 | Ulcerated rat treated with 600 mg/kg/b.w AQE | 36.20 ± 1.72 | 69.85 ± 3.87a |
| 7 | Ulcerated rat treated with 900 mg/kg/b.w AQE | 42.52 ± 1.33 | 78.71 ± 3.87a |
Table 5: Percentage (%) curative index
Some phytochemicals may have been responsible for the decrease as earlier suggested by Goel RK et al. [22] and Agrawal AK et al. [23] who reported decreases in ulcer index on treatments with medicinal plants. The aqueous extract of P. erinaceus remarkably dose and duration dependable ulcer index at 900 mg/kg/b.w extract by the second week of treatment, signifying the effects of treatments and duration of treatment on ulcer index. The aqueous extract at 900 mg/kg body weight and cimetidine at 100 mg/kg seemed to confer similar activity or the same work in this study. Significantly decreased free and total acidity, pepsin activity (Tables 6, 7, 8, 9 and 10) and volume of gastric juice, on administration of 300, 600 and 900 mg/kg body weight of aqueous stem bark extract of P. erinaceus and cimetidine at 100 mg/kg could have resulted from phenols and saponins contained in the plant extract [5,6,24]. Our results clearly suggest that ulcer induced by oral exposure of rats to indomethacin as demonstrated by significant increases in volume of gastric juice, pepsin activity, free and total acidity as previously reported by [25,26] who noted the relationship between acid production, pH, volume of gastric juice and pepsin activity, could be reversed by the plant extract when administered to rats.
| Groups | Treatment | Week 1 | Week 2 |
|---|---|---|---|
| 1 | Normal Control | 1.13 ± 0.06 | 1.23 ± 0.10 |
| 2 | Aqueous Extract Treated 900 mg/kg/b.w | 1.27 ± 0.12 | 1.32 ± 0.22 |
| 3 | Ulcerated rats treated with Cimetidine 100 mg/kg/b.w | 2.57 ± 0.12 | 2.15 ± 0.51b |
| 4 | Indomethacin 30 mg/kg/b.w | 3.00 ± 0.98a | 4.00 ± 0.77a |
| 5 | Ulcerated rats treated with AQE 300 mg/kg/b.w | 2.97 ± 0.77 | 2.71 ± 0.43b |
| 6 | Ulcerated rats treated with AQE 600 mg/kg/b.w | 2.80 ± 0.73 | 2.15 ± 0.39b |
| 7 | Ulcerated rats treated with AQE 900 mg/kg/b.w | 2.70 ± 0.51 | 2.01 ± 0.42b |
a Significantly higher (p˂0.05) compared to values of aqueous extract
b Significantly lower compared (p˂0.05) to values of indomethacin group in each column b.w=body weight
Table 6: Effects of aqueous stem-bark extract of P. erinaceus on volume of gastric juice (ml/4 hour)
| Groups | Treatment | pH of gastric juice |
|---|---|---|
| 1 | Normal Control | 6.70 ± 0.15d |
| 2 | Aqueous Extract Treated 900 mg/kg/b.w | 6.66 ± 0.15d |
| 3 | Ulcerated rats treated with Cimetidine 100 mg/kg/b.w | 6.47± 0.29d |
| 4 | Indomethacin 30mg/kg/b.w | 3.50 ± 0.50 |
| 5 | Ulcerated rat treated with 300 mg/kg/b.w AQE | 5.47 ± 0.06 |
| 6 | Ulcerated rat treated with 600 mg/kg/b.w AQE | 6.00 ± 0.50 |
| 7 | Ulcerated rat treated with 900 mg/kg/b.w AQE | 6.33 ± 0.29d |
d significantly higher (p˂0.05) compared to values of indomethacin group
b.w=body weight
Table 7: Effects of aqueous stem-bark extract of P. erinaceus on pH of gastric juice
| Groups | Treatment | Week 1 | Week 2 |
|---|---|---|---|
| 1 | Normal Control | 2.10 ± 0.13b | 6.32 ± 0.39b |
| 2 | Aqueous Extract Treated 900 mg/kg/b.w | 2.40 ± 0.15b | 7.20 ± 0.45b |
| 3 | Ulcerated rats treated with Cimetidine 100 mg/kg/b.w | 3.84 ± 0.08b | 11.53 ± 0.24b |
| 4 | Indomethacin 30 mg/kg/b.w | 11.29 ± 0.42a | 33.86 ± 1.26a |
| 5 | Ulcerated rats treated with AQE 300 mg/kg/b.w | 8.62 ± 0.42 | 25.45 ± 1.26b |
| 6 | Ulcerated rats treated with AQE 600 mg/kg/b.w | 6.06 ± 0.05 | 18.17 ± 0.15b |
| 7 | Ulcerated rats treated with AQE 900 mg/kg/b.w | 4.91 ± 0.03b | 14.72 ± 0.08bc |
a Significantly higher (p˂0.05) compared to values of aqueous extract
b Significantly lower compared (p˂0.05) to values of indomethacin group in each column
c significantly lower compared (p˂0.05) to values in week 1
b.w=body weight<
Table 8: Effects of aqueous stem-bark extract of P. erinaceus on free acidity (FA) mEq/l
| Groups | Treatment | Week 1 | Week 2 |
|---|---|---|---|
| 1 | Normal Control | 11.13 ± 1.00b | 33.40 ± 1.85b |
| 2 | Aqueous Extract Treated 900 mg/kg/b.w | 11.30 ± 0.92b | 33.90 ± 1.85b |
| 3 | Ulcerated rats treated with Cimetidine 100 mg/kg/b.w | 16.98 ± 0.82b | 50.95 ± 1.65b |
| 4 | Indomethacin 30 mg/kg/b.w | 38.31 ± 0.90a | 114.94 ± 1.80a |
| 5 | Ulcerated rats treated with AQE 300 mg/kg/b.w | 27.24 ± 0.92 | 81.73± 1.85 |
| 6 | Ulcerated rats treated with AQE 600 mg/kg/b.w | 22.76 ± 0.05 | 68.27 ± 1.10 |
| 7 | Ulcerated rats treated with AQE 900 mg/kg/b.w | 17.53 ± 1.00bc | 52.60 ± 2.00bc |
a Significantly higher (p˂0.05) compared to values of aqueous extract
b Significantly lower compared (p˂0.05) to values of indomethacin group in each column
c significantly lower compared (p˂0.05) to values in week 1
b.w=body weight
Table 9: Effects of aqueous stem-bark extract of P. erinaceus on total acidity (TA) mEq/l
| Groups | Treatment | Week 1 | Week 2 |
|---|---|---|---|
| 1 | Normal Control | 0.67 ± 1.75b | 2.00 ±1.15b |
| 2 | Aqueous Extract Treated 900 mg/kg/b.w | 0.68 ± 1.09b | 2.05 ± 0.19b |
| 3 | Ulcerated rats treated with Cimetidine 100 mg/kg/b.w | 0.83 ± 1.57b | 2.51 ± 1.14b |
| 4 | Indomethacin 30 mg/kg/b.w | 2.90 ± 1.33a | 8.7 ± 0.65a |
| 5 | Ulcerated rats treated with AQE 300 mg/kg/b.w | 2.11 ± 1.44 | 6.35 ± 0.87 |
| 6 | Ulcerated rats treated with AQE 600 mg/kg/b.w | 1.65 ± 1.81 | 4.96 ± 1.62 |
| 7 | Ulcerated rats treated with AQE 900 mg/kg/b.w | 0.99 ± 1.86bc | 2.98 ± 1.73bc |
a Significantly higher (p˂0.05) compared to values of aqueous extract
b Significantly lower compared (p˂0.05) to values of indomethacin group in each column
c significantly lower compared (p˂0.05) to values in week 1
b.w=body weight
Table 10: Effects of aqueous stem-bark extracts of P. erinaceus on pepsin activity units/mg of protein
Significant increases in pH of gastric juice possibly followed anti-secretory and acidity neutralizing effects exerted by the extract. This increase in pH in the aqueous extract treated groups may also be associated with the re-creation of mucin content and the modulations of other indices that contribute to conferring gastrointestinal protection on the animals. Similar findings by [27] reported a decrease in free and total acidity, and increase in pH on treatment with fixed oil of Ocimum basilicum linn in ulcer induced rats. The decreased total protein (Table 11) suggests further damage to the stomach following indomethacin treatment. However, significant improvement in protein concentrations in the groups treated with extract would be an indication of protein synthesis that includes those of antibodies and enzymes. At high dose over a long period of treatments better effects were observed.
| Groups | Treatment | Week 1 | Week 2 |
|---|---|---|---|
| 1 | Normal Control | 71.77 ± 1.76b | 181.20±3.80b |
| 2 | Aqueous Extract Treated 900 mg/kg/b.w | 69.80 ± 2.01b | 176.67 ±1.03b |
| 3 | Ulcerated rats treated with Cimetidine 100 mg/kg/b.w | 36.73 ± 0.42b | 179.70±1.81b |
| 4 | Indomethacin 30 mg/kg/b.w | 19.57 ± 0.65a | 22.70 ± 0.38a |
| 5 | Ulcerated rats treated with AQE 300 mg/kg/b.w | 26.13 ± 1.00 | 78.63±2.01bc |
| 6 | Ulcerated rats treated with AQE 600 mg/kg/b.w | 39.30 ± 1.15b | 110.20±0.45bc |
| 7 | Ulcerated rats treated with AQE 900 mg/kg/b.w | 42.90 ± 1.81b | 150.50±1.00bc |
a Significantly lower (p˂0.05) compared to values of aqueous extract
b Significantly higher (p˂0.05) compared to values of indomethacin group in each column
c significantly higher (p˂0.05) compared to values in week 1
b.w=body weight
Table 11: Effects of aqueous stem-bark extract of P. erinaceus on total protein of stomach tissue homogenate (g/l)
Indomethacin also increased malonaldehyde (MDA) levels in animals (Table 12) indicating the generation of reactive oxygen species (ROS), hydroxyl radicals that caused lipid peroxidation especially in membranes. The reasons for lipid peroxidation after exposure to indomethacin are not completely known, but it is believed that disturbance in glutathione and metallothionine levels may allow free radicals such as HO and O2 radicals to attack double bonds in membrane lipids [28]. Significantly reduced effects of indomethacin with regards to lipid peroxidation, especially in animals treated with high dose. It is likely that the anti-ulceration mechanism of P. erinaceus stem bark extract involved the ability of the stem bark extract to reduce lipid peroxidation due to the presence of iso flavonoids in the extract [29,30].
| Groups | Treatment | Week 1 | Week 2 |
|---|---|---|---|
| 1 | Normal Control | 9.80 ± 1.62d | 10.25 ± 1.17d |
| 2 | Aqueous Extract Treated 900 mg/kg/b.w | 8.80 ± 2.44d | 9.20 ± 0.87d |
| 3 | Cimetidine 100 mg/kg/b.w | 11.78 ± 1.02b | 16.76 ± 0.75b |
| 4 | Indomethacin 30 mg/kg/b.w | 26.71 ± 1.56a | 67.39 ± 1.99a |
| 5 | Ulcerated rats treated with AQE 300 mg/kg/b.w | 20.07 ± 1.36 | 40.85 ± 1.33bc |
| 6 | Ulcerated rats treated with AQE 600 mg/kg/b.w | 16.11 ± 1.12b | 30.34 ±1.05bc |
| 7 | Ulcerated rats treated with AQE 900 mg/kg/b.w | 13.05 ± 2.34b | 20.51 ± 1.35bc |
a Significantly higher (p˂0.05) compared to values of aqueous extract
b Significantly lower (p˂0.05) compared to values of indomethacin group in each column
c significantly lower (p˂0.05) compared to values in week 1
d significantly lower (p˂0.05) compared to values of treated groups
b.w=body weight
Table 12: Effects of aqueous stem-bark extract of P. erinaceus on lipid peroxidation (MDA level) of stomach tissue homogenate in n moles of MDA formed/mg protein
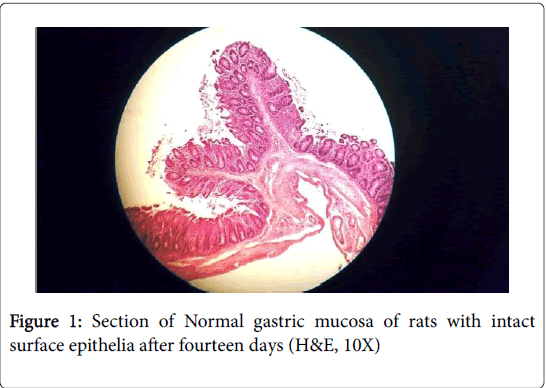
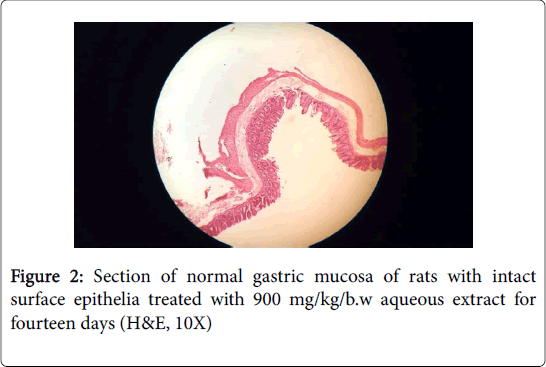
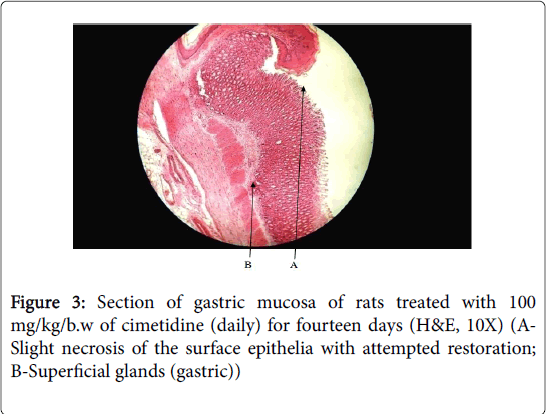
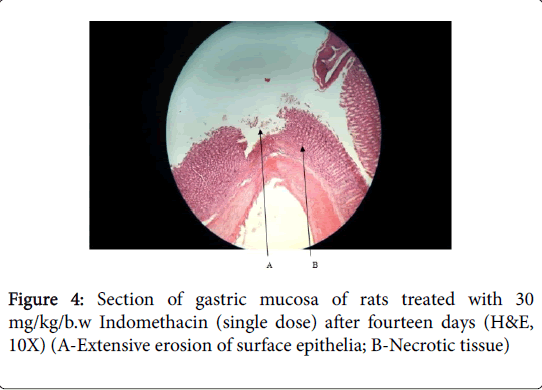
Histopathology of stomach of normal control groups showed normal cyto-architecture of cells and normal gastric mucosa (Figures 1 and 2). Indomethacin induction in disease control group caused heavy mucosal damage, oedema and with necrotic lesion penetrating deeply into the mucosa accompanied by extensive edema and leukocyte infiltration of the sub-mucosal layer cell necrosis (Figures 3 and 4).
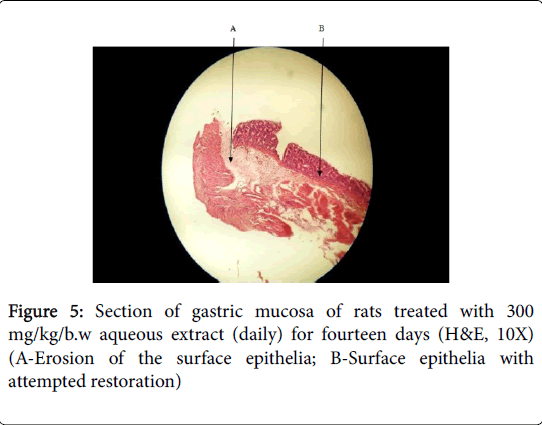
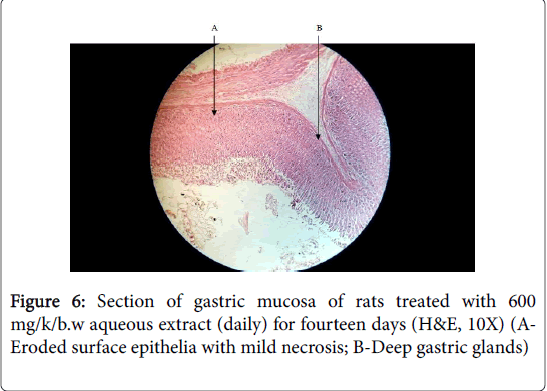
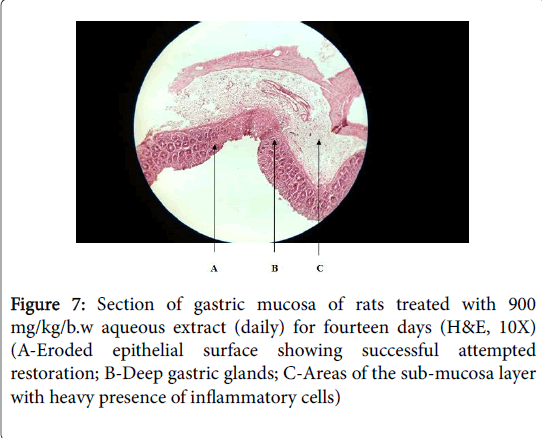
Cimetidine treated group showed mild oedema and mild to moderate mucosal damage which was comparatively quite lower than disease control group. Treatment with plant extract has shown significant cytoprotective activity or healing effect and reduction in ulcer severity. Histology of aqueous stem bark extract treated groups showed reduction in depth and extent of ulceration in comparison to the indomethacin control group Figures 5, 6 and 7).
These indicate its antiulcer potential. These results demonstrated that the plants extract exerted cytoprotective effects in a dose dependent manner which at 900 mg/kg/body weight proved to have exerted more effects.
Conclusion
Our results suggest that treatment with the stem bark extract of P. erinaceus caused a beneficial effect on indomethacin-induced ulcers in rats as evidenced by the reduction in the ulcer index. The effects of the aqueous stem bark extract was dose and duration dependent and this may justify its trial as an anti-ulcerogenic agent. The result of this study demonstrated that the treatment with aqueous stem bark extract of P. erinaceus has anti-ulcer activity.
Acknowledgement
Authors are sincerely thankful to Mr. Alex of the Histopathology department and Mr. Ferdinand of Chemical Pathology department of Federal Medical Centre Yola for their time and contribution during the laboratory analysis.
Conflict of Interest
There is no conflict of Interest regarding the study or accompanying report
References
- Rang HP, Dale MM, Ritter M, Moore PK (2003) Pharmacology (5th edition) Churchill Livingstones, Edinburgh, New York, pp: 797.
- Kumar AVS, Jonnalagadda VG, Jyothi MJ, Prathyusha S, Lakshmi M, et al. (2013) Pharmacognostic, phytochemical and pharmacology evaluation for the antiulcer activity of Poly herbal suspension. J Pharmacogn Phytochem 2: 128-135.
- Rastogi RP, Mehrotra BN (2002) Glossary of Indian Medicinal Plants, National Institute of Science Communication and Information Resources, New Delhi, India.
- Gorinstein S, Yamamota K, Katrich E, Leontowkz H, Lojek A, et al. (2003) Antioxidative properties of Jaffa sweeties and grapefruit and their influence on lipid metabolism and plasma antioxidative potentials in rats. Biosci Biotechnol Biochem 67: 907-910.
- Aliyu M, Salawu OA, Wannang NN, Yaro AH, Bichi LA, et al. (2005) Analgesic and anti-inflammatory activities of the ethanolic extract of the stem bark of Pterocarpus erinaceus in mice and rats. Niger J Pharmacol Res 4: 12-17.
- Aliyu M, Chedi B (2010) Effects of the ethanolic stem bark extract of Pterocarpus erinaceus poir (Fabaceae) on some isolated smooth muscles. Bayero J Pure Appl Sci 3: 34-38.
- Upper Benue River Basin Development Authority (2005), Annual Book Report, Nigeria.
- Harborne JB (1984) Phytochemical methods-A guide to modern techniques of plant Analysis (2nd Edition) New York, Chapman and Hall, London.
- Edeogal HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 4: 685-688.
- Al-Harb MM, Qureshi S, Raza M, Ahmed MM, Afzal M, et al. (1997) Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. J Ethnopharmacol 55: 141-150.
- Goyal RK, Sairam K (2002) Antiulcer drugs from indigenous sources with emphasis on Musa sapientum, tamrabhasma, Asparagus racemosus and Zingiber officinale. Indian J Pharmacol 34: 100-110.
- Olaleye MT, Ojo CT, Adetuyi AO (2013) Evaluation of the anti-ulcerogenic properties of Ethanolic extract of Pterocarpus erinaceus and homopterocarpin against aspirin-induced ulcer in albino rats. FUTA J Res Sci 1: 135-146.
- Deshpande SS, Shah GB, Parmar NS (2003) Anti-ulcer activity of Tephrosia purpurea in rats. Indian J Pharmacol 35: 168-172.
- Kulkarni SK (2010) Handbook of experimental pharmacology (3rd edition) Vallabh Prakashan, Delhi.
- Anson ML (1938) The estimation of pepsin, trypsin, papain and catepsin with haemoglobin. J Gen Physiol 22: 78-89.
- Rang HP, Ritter JM, Flower RJ, Henderson G (2007) Rang & Dale’s Pharmacology (6th edition) Churchill Livingstone/​Elsevier Publication, UK, pp: 829.
- Rang HP, Dale MM, Ritter JM, Flower RJ (2007) The heamopoeitic system. In: Rang and Dale’s Pharmacology (6th Edition) Churchill Livingstone, Philadelphia, pp: 350-353.
- Borrelli F1, Izzo AA (2000) The plant kingdom as a source of anti-ulcer remedies. Phytother Res 14: 581-591.
- de Jesus NZ, de Souza Falcão H, Gomes IF, de Almeida Leite TJ, de Morais Lima GR, et al. (2012) Tannins, peptic ulcers and related mechanism. Int J Mol Sci 13: 3203-3228.
- Nakamura C, Otaka M, Odashima M, Jin M, Konishi N, et al. (2003) Rolipram, a specific type IV phosphodiesterase inhibitor, ameliorates indomethacin-induced gastric mucosal injury in rats. Pathophysiology 9: 195-200.
- Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z, et al. (2010) Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 33: 224-234.
- Goel RK, Chakrabarti A, Sanyal AK (2000) The effect of biological variables on the anti-ulcerogenic effect of vegetable plantain banana. Planta Med 51: 85-88.
- Agrawal AK, Rao CV, Sairam K, Josh VK, Goel RK, et al. (2000) Effect of piper longum Linn, Zingiber officinale Linn and Ferula species on gastric ulceration and secretion in rats. Indian J Exp Biol 38: 994-998.
- Aliyu AB, Musa AM, Oshanimi JA, Ibrahim HA, Oyewale AO, et al. (2008) Phytochemical analysis and mineral elements composition of some medicinal plants of Northern. Niger J Pharm Sci 7: 119-125.
- Brodie DA, Marshall RW, Moreno OM (2006) Effect of restraint on gastric acidity in the rat. Am J Physiol 202: 812-814.
- Dai S, Ogle CW (2005) Histamine H2 receptor blockade: Effect on methacholine induced gastric secretion in rats. IRCS J Med Sci 32: 61-65.
- Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, et al. (2007) Natural antibiotic function of a human gastric mucin against helicobacter pylori infection. Science 305: 1003-1006.
- Nuhu AM, Mshellio MS, Yakubu Y (2000) Antimicrobial screening of the bark extract of Pterocarpus erinaceus tree. J Cheml Soc Niger 25: 85-87.
- Mota KS, Dias GE, Pinto ME, Luiz-Ferreira A, Souza-Brito AR, et al. (2009) Flavonoids with gastroprotective activity. Molecules 14: 979-1012.
- Niero R, de Andrade SF, Cechinel Filho V (2011) A review of the Ethnopharmacology, Phytochemistry and pharmacology of plants of the Maytenus genus. Curr Pharm Des 17: 1851-1871.
Citation: Patrick AT, Samson FP, Madusolumuo MA (2018) Antiulcerogenic Effects of Aqueous Stem-Bark Extracts of Pterocarpus erinaceus on Indomethacin-Induced Ulcer in Albino Rats. J Biochem Cell Biol 1: 107.
Copyright: © 2018 Patrick AT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 8288
- [From(publication date): 0-2018 - Nov 02, 2025]
- Breakdown by view type
- HTML page views: 7146
- PDF downloads: 1142