Research Article Open Access
Antioxidative and Anti-Inflammatory Potentials of Ambroxol in Ameliorating Vincristine Induced Peripheral Neuropathic Pain in Rats
| Bhardwaj HC*, Muthuraman A, Hari KSL and Navis S | |
| Rayat Bahra University, India | |
| *Corresponding Author : | Hukkam Chand Bhardwaj Rayat Bahra University, India Tel: 1605009665 E-mail: bhardwaj21003@gmail.com |
| Received: December 13, 2015; Accepted: January 12, 2016;Published: January 16, 2016 | |
| Citation: Bhardwaj HC, Muthuraman A, Hari KSL, Navis S (2016) Antioxidative and Anti-Inflammatory Potentials of Ambroxol in Ameliorating Vincristine Induced Peripheral Neuropathic Pain in Rats. J Neuroinfect Dis 7:202. doi:10.4172/2314-7326.1000202 | |
| Copyright: © 2016 Bhardwaj HC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
The present study was designed to investigate the ameliorative potential of Ambroxol, a voltage gated sodium channel 1.8 (VGSC 1.8) inhibitor in Vincristine induced neuropathic pain in rats. Administration with Vincristine (75 μg/mg, intraperitoneal) for 10 consecutive days induces neuropathic pain. Hyperalgesia and allodynic symptoms were assessed with various behavioral models i.e., paw thermal-heat hyperalgesia, tail cold hyperalgesia and paw cold allodynia via hot-plate test, cold-water tail immersion test and acetone drop test at different time intervals of 0,1,7,14, and 21 days. Oxidative stress markers i.e., thio-barbituric acid reactive substances, superoxide anion content and inflammatory mediators like tumor necrosis factor-alpha and myeloperoxidase were biochemically assessed from sciatic nerve tissue and surrounding muscular tissue homogenates respectively. Pharmacological cotreatments with Ambroxol (1000 mg/kg, per oral), Carbamazepine (100 mg/kg, per oral) and combination of Ambroxol with Pregabalin (10 mg/kg, per oral) for 14 days, significantly ameliorate the Vincristine induced neuropathic pain in terms of attenuating paw thermal hyperalgesia, tail-cold hyperalgesia and paw cold allodynia along with decrease in oxidative stress markers and inflammatory mediators. Therefore, on the basis of data in hand from present study, it has been concluded that Ambroxol have neuroprotective potential in ameliorating Vincristine induced neuropathic pain in rats.
| Keywords |
| Vincristine induced neuropathic pain; Hyperalgesia; Allodynia; Sciatic nerve; Oxidative stress; TNF-alpha; Myeloperoxidase |
| Introduction |
| Neuropathic pain is a persistent chronic pain resulting from damage to the central or peripheral pain signaling pathways [1]. Neuropathic pain is typically characterized by sensory abnormalities such as Hyperalgesia (Increased pain perception/response to painful stimuli), allodynia (pain in response to stimuli that doesn’t normally provoked pain) and dysesthesia (Unpleasant abnormal sensation) [2,3]. Neuropathic pain is a symptomatic condition and not a disease which is caused by various disorders or dysfunctioning of somatosensory components of nervous system [4]. Recent definition of neuropathic pain proposed by International consensus and adopted by International Association for Standardization of Pain (IASP) is ‘Pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” [5,6]. |
| Neuropathic pain can be caused by several factors such as infections, trauma, metabolic abnormalities, chemotherapy, surgery, radiation, neurotoxins, nerve compression, inflammation and tumor invasion [2,7]. It is difficult to diagnose and distinguish neuropathic pain from other pain types [8]. Chemotherapy induced peripheral neuropathic pain is most common and toxic neurological complication and is observed in about 30-40% patients treated with antineoplastic agents [9,10]. Vincristine, a vinca alkaloid is a widely used antineoplastic drug that is used alone or in a combination with other drugs for the treatment of many tumor types [10-13]. Vincristine induces dose dependent peripheral neuropathic pain [11,13,14] that occur in two major stages. In early stage, Vincristine damages the peripheral axons and produces the symptoms like paresthesias and dysesthesias. At higher doses in the later stage, axons are lost that result into loss of motor functions. Vincristine also increases oxidative stress and calcium levels [15]. Systemic administration of Vincristine (100 μg/kg) by intra venous route for 2 week period, produced hyperalgesia that persists for more than one week after the last injection of Vincristine. Vincristine (100 μg/kg/day) administration resulted into central sensitization of spinal wide dynamic range (WDR) neurons which contributes to hyperalgesia in patients and hyperresponsiveness to sensory stimuli in animals [13]. Also, Vincristine (75 μg/kg) by intra peritoneal route for 10 consecutive days (5 days/week) significantly produced neuropathic pain assessed by different behavioral models for hyperalgesia and allodynia. Moreover, Vincristine (75 μg/kg) significantly increase oxidative stress and inflammatory markers [16]. Vincristine binds to microtubules and causes morphological changes in myelinated and unmyelinated fibers and these morphological changes contribute to hyperalgesia in rats and paresthesias and dysesthesias in patients. Vincristine is highly sensitive to peripheral neurons and does not cross the blood-brain barrier [17]. |
| Materials and Methods |
| Experimental animals |
| The experimental animals Sprague Dawley rats weighing 180-250 grams, employed in the present research study were purchased from National Institute of Pharmaceutical Education and Research (NIPER) S.A.S. Nagar Mohali (India), were maintained on standard laboratory diet (Ashirwaad Feeds Ltd., Kharar, Chandigarh, India) and having free access to tap water. They were housed in the Rayat and Bahra Institute of Pharmacy departmental animal house and were exposed to normal cycle of light and dark. The experimental protocol (RBIP/ IAEC/CPCSEA PROTOCOL NO. 11) was approved by the Institutional Animal Ethics Committee (IAEC) and the care of the animals was carried out as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India (Reg. No-1380/a/10/CPCSEA). |
| Drugs and chemicals |
| Vincristine sulfate injections (1 mg/ml) were purchased from post graduate institute of medical education and research (PGIMER) Chandigarh, India. The drugs Ambroxol and Pregabalin were taken as a gift samples from Zee Laboratories Ltd. Poanta Sahib (H.P.), India. All the other chemicals used in present study were of analytical grade. |
| Treatment schedule |
| The present study consists of 5 experimental groups and each group comprises of five sprague dawley rats. All the animals were acclimatized to laboratory environment for at least one week before starting experiments. After one week animals were divided into the following groups and respective treatments were given to these groups. All the behavioral testing were carried out two hours prior to any drug treatment. |
| Induction of neuropathic pain |
| Peripheral neuropathic pain was induced in rats by injecting Vincristine sulfate 0.075 mg/kg intraperitoneal 5 days per week (for two weeks) as described previously [18] Vincristine sulfate injections were given daily (Monday through Friday) followed by 2 days gap per week. |
| Behavioral examinations |
| The experimental animals were subjected for various behavioral studies for assessment of hyperalgesia and allodynia carried out on the different time intervals of 0,1,7,14 and 21 days. |
| Paw thermal hyperalgesia (Hot Plate Test) |
| Thermal hyperalgesia (noxious thermal stimuli) of the hind paw was assessed using Eddy’s hot plate as described by method of Eddy [19]. The temperature of the plate was maintained 52.5 ± 0.5°C. The rats were placed on the top of a controlled preheated plate to assess the withdrawal response of hind paw to the nociceptive threshold. The cutoff times of 20 seconds was maintained for thermal hyperalgesia. |
| Paw cold allodynia (Acetone Drop Method) |
| Cold allodynia were assessed using the acetone drop method as described by Choi et al. and Muthuraman et al., [20]. Rats were placed on wire mesh grid and 0.1 ml (100 μl) of acetone was sprayed with the help of plastic syringe on plantar surface of hind paw. Normal rats either ignored the stimulus or occasionally responded with a small and brief withdrawal of hind paw. Responses with respect to either paw licking, shaking or rubbing the hind paw was observed and recorded as paw lifting duration. The cut off time was 20 seconds. |
| Tail cold hyperalgesia (Tail Immersion Test) |
| The cold hyperalgesia was assessed by using tail immersion in cold water as described by Necker [21]. The terminal tip (1 cm) of tail was immersed in cold water (temperature maintained 0-4°C). The withdrawal latency of tail from cold water was recorded. The cut off time for cold water tail immersion test was taken as 20 seconds. |
| Biochemical estimations |
| All experimental animals were sacrificed at the end of experiment by cervical dislocation. The sciatic nerve and the muscular tissue beneath the sciatic nerve were located and isolated immediately. Isolated sciatic nerve was excised into small pieces and the uniform sciatic nerve homogenates were prepared with 0.1 M Tris-HCl buffer (pH 7.4) and phosphate buffer (pH 7.4). The test tubes with homogenate were kept in ice water for 30 minutes and centrifuged at 4°C (2500 rpm, 10 min). The supernatants of homogenates were separated, and employed to estimate superoxide anion generation [22], total protein [23], thio-barbituric acid reactive substances [24], and tumor necrosis factor-alpha (TNF-α) respectively. Also, homogenate of surrounding muscular tissue was prepared with phosphate buffer (pH 7.4) for myeloperoxidase (MPO) activity [25]. |
| Estimation of tissue total protein |
| Total protein content in sciatic nerve was determined by Lowry’s method [23] using bovine serum albumin (BSA) as a standard. The protein content was determined spectrophotometrically at 750 nm and expressed as mg per ml of 10% sciatic nerve homogenate. |
| Estimation of superoxide anion generation |
| The superoxide anion generation was measured by Wang [22] method. A weighed amount of tissue was taken in 5 ml phosphate buffered saline containing 100 μM of nitro-blue tetrazolium (NBT) and incubated at 37°C for 1.5 hours. The reduction of NBT was stopped by adding 5 ml of 0.5 M HCl. Then, the tissue was taken out and was minced and homogenized in a mixture of 0.1 M sodium hydroxide and 0.1% sodium dodecyl sulphate in water containing 40 mg/L diethylene triamine penta acetic acid. Then mixture was centrifuged at 20,000 rpm for 20 min and the resultant pellet was suspended in 1.5 ml of pyridine and kept at 80°C for 1.5 hours to extract formazan (an adduct formed after reaction of NBT with superoxide anions). The mixture was again centrifuged at 10,000 rpm for 10 min and absorbance of formazan was determined spectrophotometrically at 540 nm. The amount of reduced NBT was calculated using the formula |
| Amount of reduced NBT = A×V/[T]Wt×Æ�×1 |
| Where A is absorbance, V is volume of pyridine (1.5 ml), T is time for which thew tissue was incubated with NBT (1.5 hours), Wt is blotted wet weight of tissue (mg), ε is extinction coefficient (0.72 L/ mmol/mm) and l is length of light path (1 cm). Results were expressed as reduced NBT picomole per min per mg of wet tissue. |
| Estimation of thio-barbituric acid reactive substances |
| The lipid peroxidation in the sciatic nerve was estimated by measuring the thio-barbituric acid reactive substances method of Okhawa et al., [24] The reaction mixture was prepared by mixing 0.2 ml of sciatic nerve homogenate, 0.2 ml of 8.1% sodium dodecyl sulphate (SDS), 1.5 ml of 20% acetic acid solution adjusted to pH 3.5 with NaOH, and 1.5 ml of 0.8% aqueous solution of thio-barbituric acid (TBA). The reaction mixture was made up to 4.0 ml with distilled water, and then heated in water bath at 95°C for 60 min. After cooling with tap water, 1.0 ml of distilled water and 5.0 ml of mixture of nbutanol and pyridine (15:1 v/v) were added to reaction mixture and shaken vigorously. After centrifugation at 4000 rpm for 10 min, the organic layer was separated out and its absorbance at 532 nm was measured. TBARS level (MDA concentrations) were calculated from the standard curve of 1,1,3,3-tetramethoxypropane (TMP). |
| Estimation of myeloperoxidase activity |
| Myeloperoxidase (MPO) activity was measured as described by Grisham et al., [25]. The presence of MPO was measured at 460 nm for 3 minutes. MPO activity is expressed as U per g tissue. One unit of MPO activity is defined as that degrading 1 μmol peroxide per min at 25°C. The results were expressed as myeloperoxidase activity units per milligram of protein at one minute. |
| Estimation of tumor necrosis factor-alpha (TNF-α) level |
| Sciatic nerve samples were utilized for determination of TNF-α level. TNF-α levels (sensitivity: 25 pg/ml) were determined by using rat TNF-alpha ELISA kit and procedure was followed according to the manufacturer instructions. Sciatic nerve homogenate was prepared with phosphate buffer (pH 7.4). Recombinant anti-Rat TNF-alpha was used as a standard to generate a standard curve. The absorbance was measured spectrophotometrically at 450 nm. The results were expressed as pictograms of TNF-α per mg of protein. |
| Experimental protocol |
| Group 1: Normal group-Animals of this group were not subjected to any treatment. They were kept for 21 days. Behavioral tests were assessed on different time intervals, i.e., 0,1,7,14 and 21st day. All the animals were sacrificed and subjected to biochemical estimations of total protein, tumor necrosis factor-alpha (TNF-α), thio-barbituric acid reactive substances (TBARS), superoxide anion generation from sciatic nerve tissue and myeloperoxidase (MPO) activity in the surrounding muscular tissue sample. |
| Group 2: Vincristine control group-Animals of this group were treated with Vincristine sulfate injections 75 μg/kg intraperitoneal 5 days per week for 10 days. All the behavioral tests and biochemical parameters were assessed as mentioned in group I. |
| Group 3: Vincristine+Ambroxol treated group-Animals of this group were treated with Ambroxol 1000 mg/kg, per oral for consecutive 14 days before 60 minutes prior to Vincristine injection. All the behavioral tests and biochemical parameters were assessed as mentioned in group I. |
| Group 4: Vincristine + Carbamazepine treated group- Animals of this group were treated with Carbamazepine 100 mg/kg, per oral for consecutive 14 days 60 minutes prior to Vincristine injection. All the behavioral tests and biochemical parameters were assessed as mentioned in group I. |
| Group 5: Vincristine+Ambroxol+Pregabalin treated group-Animals of this group were treated with Ambroxol 1000 mg/kg, peroral and Pregabalin 10 mg/kg peroral for consecutive 14 days before each Vincristine injection. All the behavioral tests and biochemical parameters were assessed as mentioned in group I. |
| Statistical analysis |
| All the result values were expressed as mean ± S.E.M. the data of behavioral results and biochemical parameters were statistically analyzed by two way analysis of variance (ANOVA) followed by |
| Bonferroni’s post-hoc test and Tukey’s multiple comparison test respectively using Graph Pad Prism version-6.0 and Graph Pad-Instat softwares. Results having probability value P ≤ 0.05 were considered as statistically significant. |
| Results |
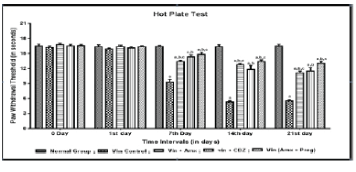
| Effect of Ambroxol, Carbamazepine and combination of Ambroxol with Pregabalin on paw thermal hyperalgesia |
| Vincristine administration significantly resulted into development of thermal heat hyperalgesia assessed by hot plate test. Vincristine produces a significant decrease in the nociceptive threshold for thermal conduction heat hyperalgesia as reflected by decrease in paw withdrawal threshold, when compared to normal group. Treatment with Ambroxol (1000 mg/kg, peroral) and Carbamazepine (100 mg/kg, peroral) significantly attenuated Vincristine induced decrease in the noxious nociceptive threshold for thermal conduction heat hyperalgesia and the ameliorative effect of Ambroxol in attenuating Vincristine induced decrease in paw withdrawal threshold was significantly comparable to the Carbamazepine. Similar effects were observed in Ambroxol with Pregabalin (10 mg/kg, p.o.) treated rats (Figure 1). |
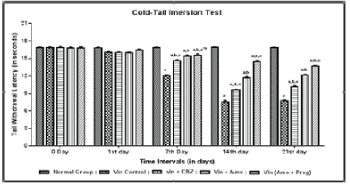
| Effect of Ambroxol, Carbamazepine and combination of Ambroxol with Pregabalin on tail cold hyperalgesia |
| Tail cold hyperalgesia was assessed by cold water tail-immersion test (0-4°C). Vincristine administration was associated with the development of tail cold hyperalgesia as reflected by decrease in tail withdrawal threshold to cold water (0-4°C), when compared to normal group. Vincristine administration for two weeks decreased tail withdrawal duration. Pharmacological treatments with Ambroxol (1000 mg/kg, peroral) and Carbamazepine (100 mg/kg, peroral) significantly attenuated the tail cold allodynia to cold stimuli. Effect of Ambroxol in attenuating tail withdrawal duration was significantly comparable to the Carbamazepine. Similar effects were observed in Ambroxol with Pregabalin (10 mg/kg, peroral) treated rats (Figure 2). |
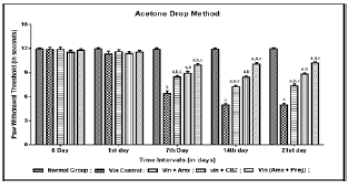
| Effect of Ambroxol, Carbamazepine and combination of Ambroxol with Pregabalin on paw cold allodynia |
| Vincristine administration was associated with the development of paw cold allodynia. Vincristine resulted into increased non-noxious nociceptive threshold as reflected by decrease in hind paw withdrawal duration on application of acetone to plantar surface of paw, when compared to normal group. Pharmacological co-treatments with Ambroxol (1000 mg/kg peroral) and Carbamazepine (100 mg/kg peroral) significantly attenuated Vincristine induced increased in the non-noxious nociceptive threshold and the effect of Ambroxol was significantly comparable to Carbamazepine. Similar results were observed in Ambroxol with Pregabalin (10 mg/kg, peroral) treated rats, showed significant combinational effect in attenuating Vincristine induced non-noxious nociceptive threshold (Figure 3). |
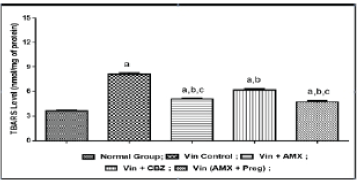
| Effect of Ambroxol, Carbamazepine and combination of Ambroxol with Pregabalin on biochemical parameters |
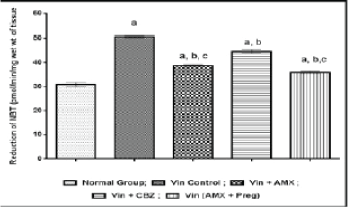
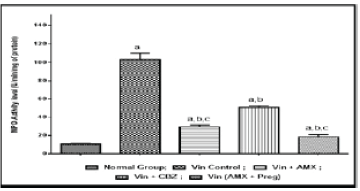
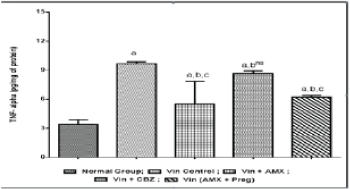
| Vincristine administration was associated with an increase in the oxidative stress markers i.e, thio-barbituric acid reactive substances, superoxide anion generation and inflammatory mediators like myeloperoxidase and tumor necrosis factor-alpha in the sciatic nerve tissue, when compared to the normal control group (Figures 4-7). However, pharmacological co-treatments with Ambroxol (1000 mg/kg peroral) and Carbamazepine (100 mg/kg peroral) significantly (p ≤ 0.05) prevent Vincristine induced increase in oxidative stress markers and inflammatory mediators when compared to Vincristine control group. Similar effects were observed in Ambroxol with Pregabalin treated rats (Table 1). |
| Discussion |
| Vincristine is a well-known antineoplastic drug clinically used from last 3 or 4 decades for the treatment of cancer but associated with neurotoxicities and development of peripheral neuropathic pain. Though, there is no specific pharmacological class of drugs for treating neuropathic pain, but clinically available other classes of drugs are being potentially effective in providing symptomatic relief from acute as well as chronic painful neuropathies. Some antidepressants, antiepileptics and opioid analgesics are found to be clinically effective as single or in combinational therapy with other drugs in the management of neuropathic pain, but their full clinical exploitation is limited due to their life threatening adverse effects associated with their clinical use [15,26,27]. Moreover, none of the medication has been found clinically effective in chemotherapy induced neuropathic pain. Therefore, there have been an urgent need of alternative medicines for the management of chemotherapy induced neuropathic pain. The present study has been designed to explore the possible therapeutic and preventive management of chemotherapy induced neuropathic pain. |
| Chronic administration of Vincristine for two weeks resulted into development of painful behaviors such as paw thermal hyperalgesia, tail cold hyperalgesia and paw cold allodynia in experimental animals. An increased level or overproduction of oxidative stress and inflammatory biomarkers in peripheral nerve tissues has also been reported that resulted into oxidative damage to neuronal cells and neuro-inflammation to peripheral nerves. An increased level of thiobarbituric acid reactive substances (TBARS), superoxide anion generation, tumor necrosis factor-alpha (TNF-α) and myeloperoxidase were biochemically estimated in the present study, confirming the pathophysiological role of oxidative stress and neuro-inflammation in Vincristine induced peripheral neuropathic pain. Present results revealed that ameliorative potential of current pharmacological treatments is due to attenuating chemotherapy induced painful behaviors, reducing oxidative stress and neuro-inflammation in peripheral neuronal tissues. |
| Previous some studies revealed that oxidative stress play a key pathophysiological role in the pathogenesis of Vincristine induced peripheral painful neuropathy. Various studies has been focused on oxidative stress as a major target in Vincristine induced neuropathic pain [1,15,28]. Also, many antioxidants therapies ameliorate chemotherapy induced oxidative stress in the development of neuropathic pain. Beside, oxidative stress, neuro-inflammation is also an important pathophysiological marker in the pathogenesis of Vincristine induced neuropathic pain. Development of painful behaviors and biochemical alterations, in present study are in consistent with the earlier researches [1,15,28]. Chronic administration of Vincristine resulted into increase in tumor necrosis factor-alpha that play a crucial pathophysiological role in the pathogenesis of inflammatory and neuropathic pain. An increased TNF- α level in sciatic nerve tissue were reported in Vincristine induced neuropathic models [15,18,28]. |
| In present study, pharmacological co-treatments with Ambroxol, Carbamazepine and combination of Ambroxol with Pregabalin significantly ameliorate Vincristine induced peripheral neuropathic pain. The ameliorative effect of Ambroxol is due its anti-nociceptive and neuro-protective potentials. Antinociceptive potential may supposed due to alleviating hyperalgesic and allodynic painful behaviors while neuroprotective potential is due to antioxidative and anti-inflammatory actions in neuronal tissues. This contention is supported by the evidences that Ambroxol possesses antioxidative and anti-inflammatory actions [29-32]. However, pain alleviating mechanisms of Ambroxol is not so clear but it may be hypothesized that Ambroxol may have antinociceptive potential due to inhibition of neuronal voltage gated sodium channels, nociceptors and signal transduction mechanisms in A- δ and C fibers. |
| Carbamazepine and Pregabalin are well known agents being clinically used in management of neuropathic pain. Both of drugs show beneficial effects in management of neuropathic pain. Carbamazepine clinically being used in treatment of trigeminal neuralgia and epilepsy via inhibiting neuronal voltage gated sodium channels [33-36], and additionally have antioxidantive [37] and antiinflammatory potentials therefore, was selected as a positive standard drug in present investigational work. Pregabalin has been shown its potential effect via inhibition of voltage gated calcium channel and additionally possesses good antioxidant and anti-inflammatory actions [18]. |
| Conclusion |
| It has been concluded from present study that Ambroxol may have a good ameliorative effect in Vincristine induced neuropathic pain due to its antinociceptive and neuroprotective potential via alleviating hyperalgesic and allodynic painful behaviors, decreasing neuronal oxidative stress and neuro-inflammation. Also, combination of Ambroxol with Pregabalin shows a safe and additive ameliorative effect in terms of reducing oxidative stress markers and inflammatory mediators in Vincristine induced neuropathic pain. |
References
- Abad ANA, Mir HadiKhayate MH, Nouri, Tavakkoli F (2011) Effect of Salvia officinalishydroalcoholic extract on vincristine-induced neuropathy in mice. Chinese Journal of Natural Medicines 9: 354-358.
- Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, et al. (2010) European Federation of Neurological Societies: EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 17: 1113-1188.
- Bennett MI, Attal N, Backonja MM, Baron R, Bouhassira D, et al. (2007) Using screening tools to identify neuropathic pain. Pain 127: 199-203.
- Chand H, Kumar-Hari SL, Navis S (2013) Neuropathic pain review: Current potentials interventions and recent advances in management of neuropathic pain. International Journal of Pharmacotherapy and Therapeutics 3: 34-52.
- De la Calle JL, Mena MA, Gonzalez-Escalada JR, Paino CL (2002) Intrathecal transplantation of neuroblastoma cells decreases heat hyperalgesia and cold allodynia in a rat model of neuropathic pain. Brain Res Bull 59: 205-211.
- Eddy NB, Touchberry CF, Lieberman JE (1950) Synthetic analgesics; methadone isomers and derivatives. J Pharmacol Exp Ther 98: 121-137.
- Felix K, Pairet M, Zimmermann R (1996) The antioxidative activity of the mucoregulatory agents: ambroxol, bromhexine and N-acetyl-L-cysteine. A pulse radiolysis study. Life Sci 59: 1141-1147.
- Ficarra S, Misiti F, Russo A, Carelli-Alinovi C, Bellocco E, et al. (2013) Antiepileptic carbamazepine drug treatment induces alteration of membrane in red blood cells: possible positive effects on metabolism and oxidative stress. Biochimie 95: 833-841.
- Gaida W, Klinder K, Arndt K, Weiser T (2005) Ambroxol, a Nav1.8-preferring Na(+) channel blocker, effectively suppresses pain symptoms in animal models of chronic, neuropathic and inflammatory pain. Neuropharmacology 49: 1220-1227.
- Geis C, Beyreuther BK, Stohr T, Sommer C (2011) Lacosamide has protective disease modifying properties in experimental vincristine neuropathy. Neuropharmacology 61: 600-607.
- Grisham MB, Specian RD, Zimmerman TE (1994) Effects of nitric oxide synthase inhibition on the pathophysiology observed in a model of chronic granulomatous colitis. J Pharmacol Exp Ther 271: 1114-1121.
- Horowitz SH (2007) The diagnostic workup of patients with neuropathic pain. Anesthesiology Clinic 25: 699–708.
- Kamei J, Tamura N, Saitoh A (2005) Possible involvement of the spinal nitric oxide/cGMP pathway in vincristine-induced painful neuropathy in mice. Pain 117: 112-120.
- Kaur G, Jaggi AS, Singh N (2010) Exploring the potential effect of Ocimum sanctum in vincristine-induced neuropathic pain in rats. J Brachial PlexPeripher Nerve Inj 5: 3.
- Kiguchi N, Maeda T, Kobayashi Y, Kondo T, Ozaki M, et al. (2008) The critical role of invading peripheral macrophage-derived interleukin-6 in vincristine-induced mechanical allodynia in mice. Eur J Pharmacol 592: 87-92.
- Lau W, Dykstra C, Thevarkunnel S, Silenieks LB, de Lannoy IA, et al. (2013) A back translation of pregabalin and carbamazepine against evoked and non-evoked endpoints in the rat spared nerve injury model of neuropathic pain. Neuropharmacology 73: 204-215.
- Li ZH, Zlabek V, Velisek J, Grabic R, Machova J, et al. (2010) Modulation of antioxidant defence system in brain of rainbow trout (Oncorhynchusmykiss) after chronic carbamazepine treatment. Comp Biochem Physiol C ToxicolPharmacol 151: 137-141.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- Meyer HP (2008) Neuropathic pain - Current concepts. Family Practice 50: 40-49.
- Muthuraman A, Jaggi AS, Singh N, Singh D (2008) Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. Eur J Pharmacol 587: 104-111.
- Muthuraman A, Ramesh M, Sood S (2012) Ameliorative potential of montelukast on ischemia-reperfusion injury induced vasculitic neuropathic pain in rat. Life Sci 90: 755-762.
- Muthuraman A, Singh N, Jaggi AS (2011) Protective effect of Acorus calamus L. in rat model of vincristine induced painful neuropathy: an evidence of anti-inflammatory and anti-oxidative activity. Food ChemToxicol 49: 2557-2563.
- Necker R, Hellon RF (1978) Noxious thermal input from the rat tail: modulation by descending inhibitory influences. Pain 4: 231-242.
- Nirogi R, Jabaris SL, Jayarajan P, Abraham R, Shanmuganathan D, et al. (2011) Antinociceptive activity of a4ß2* neuronal nicotinic receptor agonist A-366833 in experimental models of neuropathic and inflammatory pain. Eur J Pharmacol 668: 155-162.
- Nowak D, Antczak A, Krol M, Bialasiewicz P, Pietras T (1994) Antioxidant properties of Ambroxol. Free Radic Biol Med 16: 517-522.
- Okhawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Park BY, Park SH, Kim WM, Yoon MH, Lee HG (2010) Antinociceptive Effect of Memantine and Morphine on Vincristine-induced Peripheral Neuropathy in Rats. Korean J Pain 23: 179-185.
- Rizzo MA (1997) Successful treatment of painful traumatic mononeuropathy with carbamazepine: insights into a possible molecular pain mechanism. J Neurol Sci 152: 103-106.
- Rush AM, Elliott JR (1997) Phenytoin and carbamazepine: differential inhibition of sodium currents in small cells from adult rat dorsal root ganglia. Neurosci Lett 226: 95-98.
- Shaikh S, Yaacob HB, AbdRahman RB (2011) Lamotrigine for trigeminal neuralgia: efficacy and safety in comparison with carbamazepine. J Chin Med Assoc 74: 243-249.
- Spruce MC, Potter J, Coppini DV (2003) The pathogenesis and management of painful diabetic neuropathy: a review. Diabet Med 20: 88-98.
- Tamba M, Torreggiani A (2001) Physico-chemical and biological properties of ambroxol under irradiation. Radiation Physics and Chemistry. 60: 43-52.
- Tanner KD, Reichling DB, Levine JD (1998) Nociceptor hyper-responsiveness during vincristine-induced painful peripheral neuropathy in the rat. J Neurosci 18: 6480-6491.
- Theile JW, Cummins TR (2011) Recent developments regarding voltage-gated sodium channel blockers for the treatment of inherited and acquired neuropathic pain syndromes. Front Pharmacol 2: 54.
- Velasco R, Bruna J (2010) Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia 25: 116-131.
- Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, et al. (1998) Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide, Circulation Research 82: 810-818.
- Weng HR, Cordella JV, Dougherty PM (2003) Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain 103: 131-138.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 |
Relevant Topics
- Bacteria Induced Neuropathies
- Brain Infection
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neurosyphilis
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 12558
- [From(publication date):
March-2016 - Jul 03, 2024] - Breakdown by view type
- HTML page views : 11669
- PDF downloads : 889
