Research Article Open Access
Anti-Obesity Effects and Metabolism in DIO Mice of a Novel Hybrid Lipid with Lauric and Lipoic Acid
| Samanthi RP Madawala1, Manjunath Manubolu2, Michaela Asp3, Camilla Gokturk4, Kjell Malmlöf3 and Paresh C Dutta1* | |
| 1Department of Food Science, Uppsala BioCenter, Swedish University of Agricultural Sciences, SLU, P.O. Box 7051, 750 07 Uppsala, Sweden | |
| 2Department of Anatomy, Physiology and Biochemistry, Swedish University of Agricultural Sciences, SLU, P.O. Box 7011, 750 07 Uppsala, Sweden | |
| 3Uppsala Genome Center, Science for Life Laboratory, Department of Immunology, Genetics and Pathology, Uppsala University, Rudbeck Laboratory, Dag Hammarskjölds v 20, SE-751 85 Uppsala, Sweden | |
| 4Sweden Contract In Vivo Design AB, Krusbärsg. 20, 754 49 Uppsala, Sweden | |
| Corresponding Author : | Paresh C. Dutta Department of Food Science, Uppsala BioCenter Swedish University of Agricultural Sciences SLU, P.O. Box 7051, 750 07 Uppsala Sweden Tel: +4618303749 Fax: 1-718-997-4163 E-mail: Paresh.Dutta@slu.se |
| Received February 02, 2015; Accepted April 16, 2015; Published April 25, 2015 | |
| Citation: Madawala SRP, Manubolu M, Asp M, Gokturk C, Malmlöf K, et al. (2015) Anti-Obesity Effects and Metabolism in DIO Mice of a Novel Hybrid Lipid with Lauric and Lipoic Acid. J Obes Weight Loss Ther 5:256. doi:10.4172/2165-7904.1000256 | |
| Copyright: © 2015 Madawala SRP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Obesity is a world-wide epidemic and available anti-obesity drugs are associated with side-effects. The effects of oral administration of a novel hybrid lipid obtained by conjugating fatty acid and α-lipoic acid esterified on a glycerol molecule, 1,3-dilauroyl-2-lipoyl-sn-glycerol (diLaLA), on the body weight and food intake of DIO male mice, as well as some other selected metabolic parameters in such mice, was investigated. DIO mice groups on an HFD were fed diLaLA in doses of 10, 50 or 250 mg/kg/day (DIO-10, -50 or -250) for 6 weeks. A fourth group of mice fed with the vehicle (rapeseed oil) alone and served as the control (DIO-V). In comparison with the DIO-V, the highest dose of diLaLA (DIO-250) significantly reduced the body weight, plasma and hepatic Free Fatty Acids (FFAs), as well as the SCD-16 activity index (C16:1n-7/C16:0) in the white adipose tissue of the DIO mice, whereas the liver weight was similar among all the groups. No significant differences were found in the cumulative food intake over the period of 6 weeks among all the groups. Exploratory RT-qPCR analysis indicated that, in the liver, diLaLA induced up-regulation of PPARα, CD 36, CPT1α, Acox, Acca, and AMPK isoforms α1 and γ1, while Acot isoforms 2, 3 and 12 and AMPK isoforms α2 and β1 were down-regulated. The diLaLA reduced the HFD-induced body weight of the mice, possibly through enhancement of the energy expenditure via β-oxidation and suppression of the in vivo lipogenesis, suggesting the potential of diLaLA as a promising candidate against obesity
| Keywords |
| Anti-obesity agent; Body weight; Hybrid lipid; 1,3- Dilauroyl-2-lipoyl-sn-glycerol; Lipid metabolism; Lypogenesis; Obesity; β-Oxidation gene expression |
| Introduction |
| Obesity is a world-wide epidemic caused by a combination of genetic and environmental factors, resulting in an imbalance between energy intake and energy expenditure [1,2]. Weight reduction through lifestyle changes is the first-line therapy for obesity and related metabolic syndromes [3]. The few anti-obesity drugs currently available are associated with side-effects and alternative supplements and drugs are urgently required. With a view to reducing these sideeffects, intensive research is underway to develop new anti-obesity drugs acting on multiple targets such as peripheral tissues, instead of only achieving CNS-regulated appetite control [4,5]. Obesity is associated with dyslipidemia, which is characterized by increased levels of serum total cholesterol, low-density lipoprotein and triacylglycerol, and decreased levels of high-density lipoprotein cholesterol. Abnormalities in the serum lipid profile are typically associated with elevated serum Free Fatty Acid (FFA) levels, insulin resistance and inflammation in the major insulin target tissues, such as skeletal muscle, the liver and endothelial cells [6-8]. Reduced activity of a key enzyme in fatty acid de novo synthesis, namely Stearoyl- Coenzyme A Desaturase-1 (SCD-1), has been found to enhance energy expenditure through increased fatty acid oxidation in tissues, and has been suggested as a marker for the evaluation of different anti-obesity measures [9-13]. |
| Studies in animals and humans have shown that long-term exposure to 1,3-Diacylglycerol (DAG) reduces postprandial Triacylglycerol (TAG) levels, body weight and visceral fat accumulation compared with long-term exposure to conventional TAG with a similar fatty acid composition [14,15]. The mechanism of weight loss induced by DAG is not fully understood. It has been suggested that 1(3)-Monoacylglycerol (MAG) derived from the digestion of DAG by pancreatic lipase is poorly utilized in the resynthesis of TAG, and the resulting Free Fatty Acids (FFAs) are used for energy production through enhanced β-oxidation in the liver [14-16]. Another mechanism involving the increased expression of β- oxidation genes due to DAG mediated by serotonin has also been suggested [17]. |
| Lipoic Acid (LA) is a bioactive compound which has multiple therapeutic effects against obesity and related metabolic complications when administered in doses from 600-1800 mg/kg of body weight [18-20]. It has been suggested that LA-reduced body weight and food intake in rodents are caused by the suppression of AMPK in the hypothalamus and its activation in peripheral tissues [21,22]. Supplementation of LA has been shown to up-regulate the genes for fatty acid β-oxidation and free radical scavenger enzymes, downregulate the cholesterol synthesis enzymes, and inhibit liver lipogenic genes. In addition, LA lowers hepatic TAG secretion, stimulates the clearance of TAG-rich lipoproteins, and enhances the uncoupling of ATP generation, resulting in an increased thermogenesis [23-25]. |
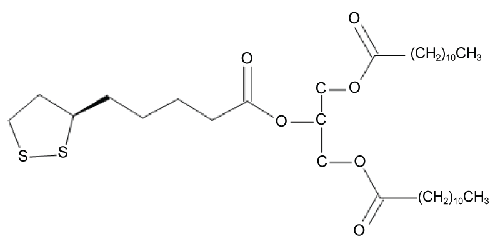
| We have previously reported on the synthesis of novel TAG, obtained by conjugating oleic acid and Lipoic Acid (LA) or dihydrolipoic acid [26]. This study was undertaken involving a novel analogue 1,3-dilauroyl-2-lipoyl-sn-glycerol (diLaLA) (Figure 1) in DIO mice. We hypothesized that this hybrid molecule possesses improved physicochemical properties such as solubility and bioavailability, compared with its constituent components, and thereby increases its physiological effects by targeting multiple regulatory mechanisms, leading to a significant body weight reduction in an animal model of obesity. In this paper we present the effects, in DIO mice, of oral administration of diLaLA on the body weight, food intake, some selected metabolic parameters, and the expression of a few hepatic lipid metabolism genes. |
| Methods |
| Materials |
| The test compound (Figure 1), 1,3-dilauroyl-2-lipoyl-sn-glycerol (diLaLA), was purchased from Onco Targeting AB, Uppsala, Sweden, having been synthesized following the published method [26]. |
| Animal study |
| This study was approved by the Animal Experiment Ethics Committee, Uppsala, Sweden (Ref. no: C189/11). The animal experiment was conducted by Sweden Contract in vivo Design, at its animal experiment facility in Uppsala, Sweden. Diet-Induced Obese (DIO) male C57BL/6J mice were obtained from Taconic, USA. After the animals were transported to Uppsala, Sweden, they were fed with a new HFD (Product no. D12451, Research Diet Inc., New Brunswick, NJ, USA) containing 45 kcal% fat and 35 kcal% carbohydrates for an acclimatization period of 11 days. The purpose of this change was to mimic the Western diet, with its high fat content. Four groups (n=7) of DIO mice on the HFD were orally administered (through gavage) either vehicle (DIO-V) (edible rapeseed oil; Zeta Rapsolja, Di Luca & Di Luca AB, Stockholm, Sweden) or diLaLA in doses of 10, 50 or 250 mg/kg (DIO-10, -50 or -250) once daily for 6 weeks. The doses of the conjugate diLaLA selected in this study were based on an exploratory 4 hour acute study in Wistar rats (unpublished results). Two or three animals were housed per cage for ethical and practical reasons. |
| Termination of experiment |
| The animals were anesthetized using isoflurane (IsoFlo® Vet) and blood was drawn from the heart and transferred to Li-heparinized collection tubes. The plasma was immediately separated by centrifugation and kept on dry ice. Tissues were dissected out, frozen immediately on dry ice, and stored at -80°C until future analysis. The liver tissue was inspected for steatosis and the liver weight was recorded for all the animals. |
| Analysis of plasma glucose |
| The blood glucose levels of the non-fasting animals were determined using a measurement kit (Accu-Chek® Aviva from Roche, Mannheim, Germany). |
| Analysis of vitamin E (a-tocopherol) in the plasma and liver |
| Lipids from 25 μL of plasma and 50 mg of liver samples were extracted with 1 mL of hexane: 2-propanol, and 20 μL of lipid extract in hexane was directly analyzed using HPLC [27,28]. |
| Analysis of the total cholesterol in the plasma and liver with GC |
| In brief, 15 g of 5-α-cholestane was added, as an Internal Standard (IS), to 25 μL of plasma and saponified, and the unsaponifiable fraction was silylated to trimethylsilyl ether derivatives and analyzed with GC. The liver cholesterol was analyzed similarly, except that saponification was carried out on 50-100 mg liver samples [29]. |
| Analysis of the triacylglycerols (TAGs), free fatty acids (FFAs) and fatty acids (FAs) in the plasma, liver and white adipose tissue (WAT) |
| Plasma (100 μL) and liver (50-100 mg) were extracted with chloroform/methanol [30] along with triheptadecanoin as an internal standard (IS). The TAG and FFA fractions were separated using prep- TLC, and methylated to Fatty Acid Methylesters (FAMEs) in situ with H2SO4 in methanol. In order to quantify the TAGs and FFAs using the IS, the FAMEs were analyzed with GC as described below. To estimate the SCD-1 activity, the FAs were analyzed by means of direct saponification [31], using 25 μL plasma samples, 25 mg liver samples, and 10-15 mg WAT samples, with the IS added. The FAMEs were prepared using BF3 reagent and analyzed with a GC (GC 6890 N, Agilent Technologies, Wilmington DE, USA) equipped with a flame ionization detector. A CP-Wax 58 (FFAP) CB capillary column (50 m × 0.32 mm × 0.45 mm, with a 0.2 μm film thickness) was used. Hexane (1 μL) containing FAMEs were injected in the splitless mode using a GC PAL autosampler (CTC Analytics AG, Swingen, Switzerland). The injector and detector temperatures were 240°C and 270°C, respectively. Helium was used as the carrier gas (0.5 mL/min) and nitrogen as the make-up gas (30 mL/min). The initial oven temperature of 100°C was held for 1 min, after which the temperature was increased to 160°C at a rate of 15°C/min, where it was maintained for 1 min, after which it was ramped to 250°C at 10°C/min, where it was maintained for 10 min, after which it was ramped to 260°C at the same rate and kept at 260°C for 10 min. The SCD-1 activity was estimated following a published method [10]. |
| Gene expression analysis of the liver samples using RT-qPCR |
| The total RNAs from the liver tissues were isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The input RNA was quality-controlled using an RNA 6000 Pico chip in a Bioanalyzer (Agilent Technologies). The samples which had an RNA Integrity Number (RIN) from 4.6 to 9.8 were used for further analysis. RNA samples were then removed from the genomic DNA and synthesized to cDNA with the RT2 First Strand Kit according to the RT2 Profiler PCR Array Handbook (February 2012, Qiagen). To measure the reproducibility of the results, technical replicates of cDNA were synthesized in two different sets. The cDNA was then analyzed using modified RT2 Profiler PCR Arrays in 384-well (4 × 96) plates (Custom Catalogue Number: CAPM11082-PAMM-007E-4, Qiagen), in combination with an RT2 SYBR Green ROXTM qPCR Mastermix (Qiagen). PCR was performed in a 7900 HT Fast Real-Time PCR System (Applied Biosystems) with the SDS software, version 2.3, rev. A (Applied Biosystems), and the gene expression analyses were carried out using the ΔΔCt method. As mentioned above, the expression analyses were performed only on a limited number of liver samples from the DIO-250 group (n=3) and the DIO-V group (n=2). For this reason no statistical t-tests comparing the two groups were performed, but for each sample the duplicate analyses were checked for consistency. |
| Statistical analyses |
| All the data are based on observations of individual animals, except for the data recorded for the cumulative food intake, which were calculated on a cage basis. The differences between the treatments were analyzed using one-way analysis of variance (one-way ANOVA), and were compared using Bonferroni’s or Dunnett’s multiple comparison test. The data from the cholesterol, TAG, and vitamin E analysis of the plasma and liver samples from the DIO-250 and DIO-V groups were compared using a two-sample t-test. The group mean values were considered to be significantly different at p<0.05. Statistical analyses were performed using the software GraphPad Prism 5 (GraphPad Software Inc., California, USA) and Minitab Statistical Software, Release 16 for Windows (Minitab Inc. (2009), PA, USA). The average expression levels of the genes for all the replicates were analyzed twice online using RT2 ProfilerTM PCR Array Data Analysis, version 3.5 (Qiagen Gmbh, Hilden, Germany). |
| Results |
| Body weight change and cumulative food intake (CFI) |
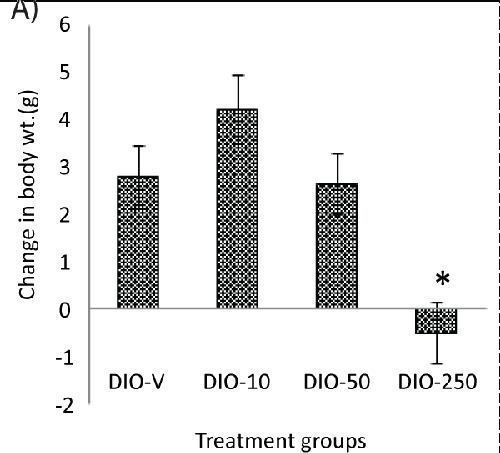
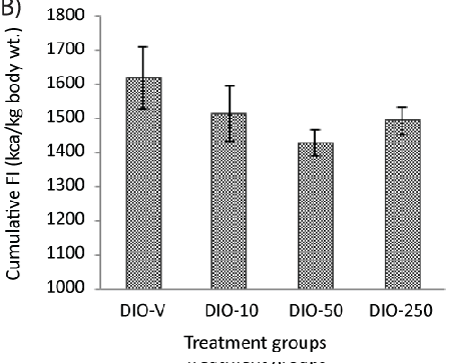
| A continuous increase in the body weight could be seen after approximately 10 days of treatment in all the animals, but this occurred to a lesser extent in the animals receiving 250 mg/kg of diLaLA. No difference was seen between the other treatment groups (receiving 10 or 50 mg/kg of diLaLA) compared with the DIO-V group (Table 1). The body weight of the DIO animals receiving 250 mg/kg of diLaLA was significantly lower (Table 1) compared to that of the DIO animals receiving vehicle at the end of treatment period (one-way ANOVA, Dunnett’s post hoc test gave p<0.05).The global body weight loss in the DIO-250 group at the end of the experimental period was significantly different compared with that in the DIO-V group (p<0.05, Figure 2A). The CFI was calculated for each group based on FI data on a cage basis (Figure 2B). Concerning the CFI over the period of 6 weeks, no significant difference was found among the groups (according to GLM, one-way ANOVA and Dunnett’s post hoc test). Individual FI could not be measured without compromising the animals’ welfare. However, the trend shows a reduction in the FI of 10-15%, suggesting that individual measurements might be able to show a significant difference. |
| Clinical chemistry and pathological evaluations |
| No clinical signs of adverse effects could be detected in any of the animals with regard to the treatment. In addition, at macroscopy no changes or adverse effects were found in the animals that could be attributed to the diLaLA treatment. |
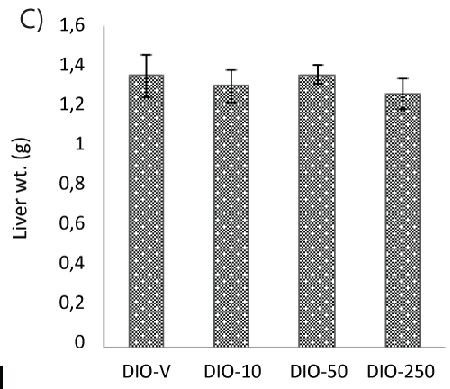
| However, steatosis was found in four animals, one in each group on the HFD, and no difference in the liver weight could be detected (Figure 2C). |
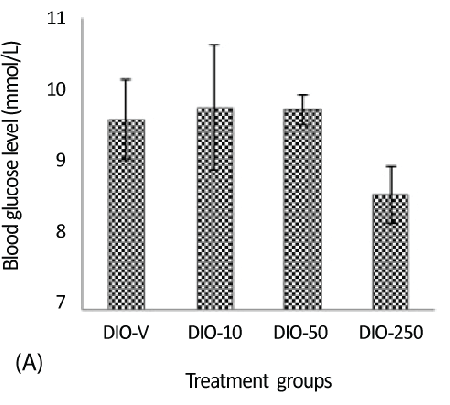
| A considerable decrease was detected in the glucose levels in the DIO-250 group (8.6 mmol/L) compared with those in the DIO-10 and DIO-50 groups, 9.6 and 9.8 mmol/L, respectively (Figure 3A). It should be mentioned that the animals had not been fasting overnight before the measurement of blood glucose. |
| Total cholesterol levels in the plasma and liver |
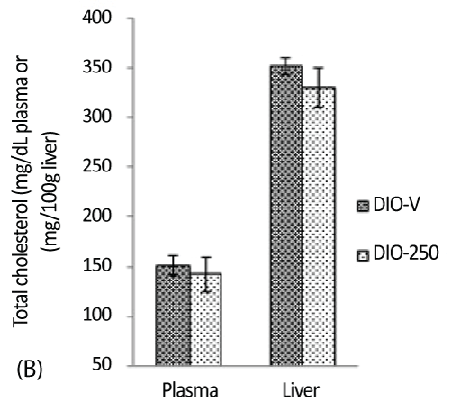
| The cholesterol analysis and the other analyses presented below were limited to the DIO-250 and DIO-V groups. The plasma total cholesterol levels in the DIO-250 and DIO-V mice were 141.9 ± 7.8 mg/dL and 151.1 ± 9.8 mg/dL, respectively, giving a marginal reduction of 6% in the DIO-250 mice. A similar cholesterol-reducing trend was observed in the liver samples (Figure 3B). |
| TAG in the plasma and liver |
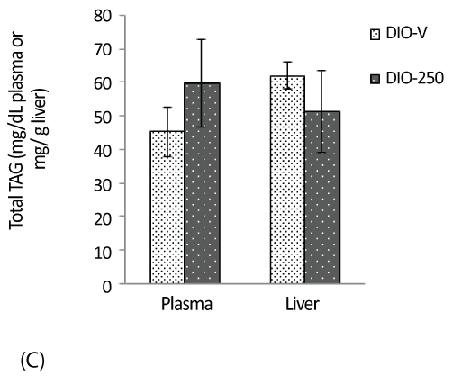
| The content of TAG in the liver samples of the DIO-250 mice was 51 ± 12 mg/g, which is considerably lower compared with 62 ± 13 mg/g in the DIO-V group; an opposite trend was observed for the plasma TAG levels (Figure 3C). |
| FFAs in the plasma and liver |
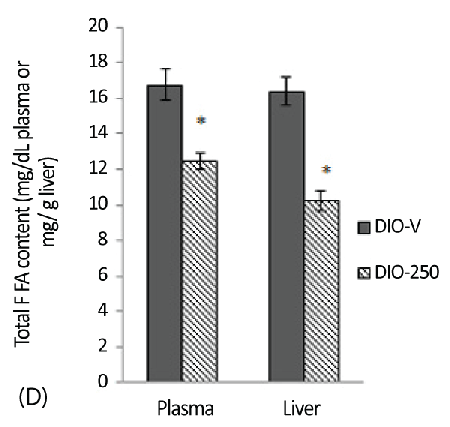
| The plasma and hepatic FFA levels in the DIO-250 mice were significantly reduced (p<0.05) compared with those in the DIO-V mice (Figure 3D). The total FFAs in the plasma and liver of the DIO-250 mice were 13 mg/dl and 1023 mg/100 g, respectively, compared with 17 mg/dL and 1638 mg/100 g, respectively, in the DIOV group. |
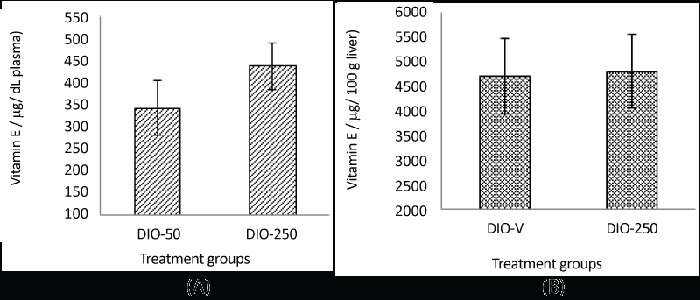
| Vitamin E in the plasma and liver |
| Due to the insufficient amount of plasma samples from the DIO-V group, the vitamin E content was compared only between the DIO-250 and the DIO-50 groups. |
| In the DIO-250 mice the plasma level was considerably higher, 438 μg/dL, compared with 344 μg/dL in the DIO-50 mice. However, no clear difference in the vitamin E content was observed in the liver samples (Figure 4). |
| Stearoyl-CoA desaturase I (SCD-1) activity |
| The estimated SCD-16 index (C16:1n-7/C16:0) showed significantly reduced (p<0.05) SCD-1 enzyme activity in the WAT of the DIO-250 mice compared with that in the WAT of the DIO-V mice. A similar trend was observed in the liver, although the difference in this case was not significant, and no difference was observed in the plasma (Figure 5A). |
| In the liver, the SCD-18 index (C18:1n-9/C18:0) was lower in the DIO-250 group than in the DIO-V group. In contrast, the SCD-18 index in the plasma and the WAT appeared to be slightly higher in the DIO-250 mice than in the DIO-V mice (Figure 5B). |
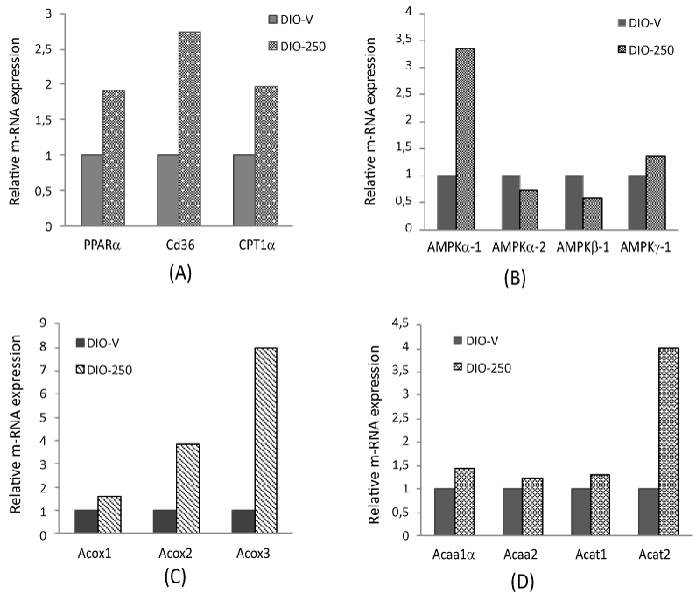
| Gene expression analysis with RT-qPCR in the liver |
| The peroxisome proliferator-activated receptor-alpha (PPAR-α) expression was increased 1.9-fold, the Fatty Acid Translocase (FAT/CD 36) expression 2.7-fold, and the Carnitine Palmitoyl Transferase 1a (CPT1α) expression 1.9-fold in the DIO-250 group (Figure 6A). The isoforms AMPK-α1 and -γ1 were up-regulated, whereas AMPK-α2 and -β1 were down-regulated (Figure 6B). An increased expression was observed for the Acyl-Coa Oxidases (Acox-1, -2, and -3), Acetyl-Coa Acyltransferase (Acaa-1 and -2), and Acetyl- Coa Acetyltransferase (Acat-1 and -2) in the DIO-250 group compared with the corresponding expressions in the DIO-V group (Figure 6C and 6D). |
| Discussion |
| Our data demonstrate that daily administration of diLaLA at 250 mg/kg of body weight for 6 weeks significantly reduced the body weight of the DIO-250 male mice compared with that of the mice given vehicle alone. All the animals exhibited a dip in weight gain in the first period of treatment. This dip was not substance-related, since it also occurred in the vehicle-treated animals. The animals were handled daily during the acclimatisation period of 11 days when the food shift was being made; suggesting that the initial body weight drop was possibly due to the first two weeks of gavage treatment. In order to avoid effects on the eating behaviour and thereby the body weight due to an aversive taste of the test item, the administration of the test item was performed once daily in the mornings, a sufficiently long time before dawn, when the wakening and eating period starts for rodents. |
| There are no published data to compare with; however, the two components of the conjugate have been tested for their weight loss and safety aspects in their own right. The significant reduction in body weight observed in DIO-250 mice in this study is in agreement with previously published findings for pure LA in rodents and humans [20-22]. Pharmacological effects have been observed for LA administered in doses from 600-1800 mg/kg body weight in humans [18-20]. Therefore, our finding of an effect at 250 mg/kg diLaLA is interesting and may suggest that a combination of DAG and LA offers an advantage in terms of the minimum dose needed to obtain an effect. Verification of this would require a different dose response setup, but merits further investigation. |
| LA causes weight loss in rodents partly due to the effect of reduced Food Intake (FI) and partly to enhanced β-oxidation by suppressed hypothalamic AMPK, whereas in skeletal muscle LA enhances fatty acid oxidation by activating AMPK [21,22]. It is to be mentioned that AMPK is the principal regulator in peripheral tissues other than hypothalamus and skeletal muscle, which are integrated in maintaining energy balance and food intake within the complex physiological system [19,32]. Data from showed that dietary DAG suppresses weight gain and the accumulation of body fat along with intestinal expression of genes involved in lipid metabolism and thermogenesis, an effect which may be related to the characteristic metabolism of DAG in the small intestine [1,2,33,34]. |
| This study showed that the plasma and hepatic total cholesterol levels in the DIO-250 mice tended to be about 6% lower than those in the mice given vehicle alone. It has previously been shown that mice supplemented with 0.1% LA in an HFD decreased their plasma total cholesterol and LDL-cholesterol, and increased their HDL-cholesterol [23]. These changes have been reported to be mediated by the downregulation of genes involved in cholesterol synthesis. The cholesterolreducing effect of LA has been reported as a parallel and long-term benefit of weight reduction [35]. |
| Reports on the effects of DAG on plasma TAG levels have been variable. In one study on diabetic apoE-deficient mice, the plasma total cholesterol and TAG levels were significantly lower in the DAG-fed animals than in the TAG-fed animals [36]. In another study [37], no effects on fasting and postprandial plasma TAG levels, the hepatic TAG content, or the rate of secretion of TAG from the liver were observed in brown adipose tissue-deficient mice fed with either DAG oil or TAG oil. It was suggested that the effects of DAG were associated with the maintenance of normal insulin sensitivity. Furthermore, gene expression data showed that the consumption of DAG stimulated fatty acid oxidation in skeletal muscle (and possibly in the liver and intestines) and caused suppression of hepatic gluconeogenesis, which may explain the molecular changes underlying these favourable effects of dietary DAG. A later study indicated that DAG in test oil was effective in altering the postprandial serum TAG and chylomicron-TAG concentrations [38]. |
| The total hepatic TAG in the DIO-250 mice appeared to be 17% lower than that in the vehicle-treated group. This might indicate that diLaLA reduces the de novo synthesis of TAG in the liver, since it has been shown that endogenous FA synthesis is reflected more by hepatic TAG than by plasma TAG [39,40]. In male Sprague-Dawley rats fed with DAG and TAG, the DAG diet resulted in a significant reduction in the liver and serum TAG levels compared with the levels in the TAG group [41]. It was suggested in [41] that these effects were associated with the up-regulation of acyl-CoA carnitine acyltransferase and the down-regulation of acyl-CoA DAG acyltransferase. |
| The plasma vitamin E level has recently been suggested as one of the factors associated with obesity in adolescents. Alone, or in combination with other dietary supplements, LA can favourably modulate multiple metabolic targets, such as TAG, oxidative stress and cholesterol homeostasis, to reduce the CVD risk [42]. The DIO-250 mice had approximately 22% higher plasma vitamin E levels than the DIO-50 mice, which indicate a better antioxidant status in the former group. |
| The DIO-250 significantly reduced both the plasma and the liver FFA levels in obese mice. This interesting finding is in accordance with the findings of Kim et al. [21] and Park et al. [39]. Our result is very encouraging, since elevated concentrations of plasma FFAs associated with obesity have been identified as an important factor behind insulin resistance [6] and indicate defects in regulation of the storage and mobilization of FAs in the WAT [43]. Therefore, a reduction in both the plasma and the liver FFA concentrations can be expected to reduce the risk of developing insulin resistance and Type 2 diabetes. The portion of LA in the diLaLA molecule is only 32%, and therefore 250 mg of diLaLA/kg of body weight represents only 80 mg of LA, which means that a surprisingly low dose of LA alone can still produce a significant effect. The reduced concentration of FFAs induced by diLaLA suggests that FA oxidation in the peripheral tissues is increased, at the same time as the synthesis of hepatic TAGs is reduced. Such a conclusion is consistent with our data on liver transcripts discussed below. Future studies will have to show whether this is dependent on a synergistic effect of LA and DAG on lipid metabolism. Additional investigation of the effects of diLaLA on fasting plasma glucose and post prandial glucose and related biochemical parameters is highly warranted. |
| The critical question arises as to how diLaLA brings about these positive reductions in FFA levels. A possible mediation through a different intake of nutrients can be excluded, as in this study both the treatment and vehicle groups were fed with same HFD with a similar fatty acid composition. Moreover, as discussed above the cumulative food intake measured between the two groups showed no significant differences. Enhanced concentrations of plasma FFAs associated with obesity are due to high loads of FFAs mainly from WAT, while the clearance via oxidation in peripheral tissues remains relatively low [8,44]. Thus the reduced concentration of FFAs induced by diLaLA suggests an increase in FA oxidation in peripheral tissues, while reducing synthesis of hepatic TAGs. Such a conclusion is consistent with our exploratory data on liver transcripts discussed below. |
| In order to get a measure of de novo synthesis of FAs, we have selected SCD1 activity as a relevant biomarker. The product of this enzyme, C16:1n-7 is the major monounsaturated FA generated by de novo synthesis [45]. Thus, SCD16 index reflects the rate of de novo lipogenesis, while SCD18 reflects the subsequent elongation of FAs. The significant decrease in SCD16 index in WAT along with a similar trend observed in liver samples of diLaLA-treated mice suggest a reduction in de novo lipogenesis and can thus help to explain the reduced body weight and also the fall in circulating levels of FAs. It is of note that in obesity prone Sprague-Dawley the SCD-16 index is elevated in adipose tissue early in the development of obesity may precede elevations in the liver or plasma Cedernaes et al. [11]. |
| The relatively low SCD18 index observed in liver samples might indicate reduced FA elongation and desaturation activity in diLaLA treated mice compared with the vehicle group, though the difference was not statistically significant. Somewhat in contrast to this, and to the effects on SCD16 index, a slightly higher SCD18 index was registered in WAT and plasma samples from mice given diLaLA. Such seemingly opposing responses in SCD16 index and SCD18 index in WAT might be due to the high MUFA content in the diet and to the use of rapeseed oil as vehicle. Despite alterations of the endogenous FA pool through several physiological processes, the overall FA composition of WAT in rats resembles that of the diet they consume [46]. In spite of the surprising results observed for elongation index in WAT, the overall suppression induced by diLaLA on de novo lipogenesis in the liver was further supported by the low levels of hepatic FFAs clearly observed in DlaLA-treated mice. |
| Data from our exploratory RT-qPCR analysis on target gene expression in liver samples provided further indications as to how these metabolic effects were mediated. It must be mentioned, in this connection, that the number of observations per group were limited, because only the samples which had an RIN from 4.6 to 9.8 were analysed, as described below in the methods section. However, the majority of these data are well in line with the other results obtained in this study. The activation of PPARα is known to induce key genes encoding for mitochondrial proteins involved in the β-oxidation pathway, such as CD 36 and CPT1 [47]. Accordingly, in our study FA translocase, CD 36, and CPT1 were up-regulated in the diLaLAtreated mice compared with the corresponding levels in the vehicle group. The expression of the peroxisomal enzymes Acox-1, -2, and -3 was increased by diLaLA, indicating a possible increase in the initial oxidation of long-chain acetyl-CoA in peroxisomes, which is a crucial step before mitochondrial β-oxidation. This was followed by the upregulation of Acetyl-Coa Acyltransferase (Acaa) and Acetyl-Coa Acetyltransferase (Acat), catalyzing the subsequent steps of β- oxidation. Valdecantos et al. [25] suggested that the ability of LA to increase the transcriptional activity of PPARα is an important mechanism mediating positive effects of LA, since PPARα is a key transcriptional factor involved in lipid metabolism. |
| In the diLaLA-treated mice, AMPK-α1 and -γ1 were up-regulated, whereas AMPK-α2 and AMPK-β1 were down-regulated. The reason for this differential regulation is currently not completely elucidated, but it has been shown that hepatic AMPK can limit anabolic pathways and facilitate catabolic pathways to increase ATP production. A number of previous studies have suggested that LA mediates the expression of AMPK in the liver and skeletal muscles [20-22]. According to Park et al. [39], pure LA could reduce FFA and TAG through both AMPK-dependent and -independent ways. |
| The few studies available on the effects of DAG on lipid metabolic gene expressions show results in line with our results. For example, in a study of male Sprague-Dawley rats fed with DAG and TAG, the high-DAG diet resulted in a significant reduction in the body weight gain and in the liver TAG and serum TAG levels compared with the high-TAG diet. These effects were associated with the up-regulation of acyl-CoA carnitine acyltransferase gene expression in the liver, the down-regulation of acyl-CoA DAG Acyltransferase (DGAT) and phosphoenolpyruvate carboxykinase gene expression in the liver, and increased expression of the genes for peroxisome proliferator-activated receptor α, lipoprotein lipase, and uncoupling proteins 2 and 3 in skeletal muscle [38,41]. |
| The metabolic fate of diLaLA has not been studied before, and we propose that it is hydrolyzed by pancreatic lipase in the small intestine, producing mixtures of 1(3)-lauroyl-2-lipoyl-sn-glycerol, 2-lipoyl-snglycerol, free lauric acid, and some free LA and other possible metabolites. Since 1(3)-2-lipoyl-sn-glycerol and 2-lipoyl-sn-glycerol are not natural lipid molecules, they may not be easily absorbed by the enterocytes or used as precursors for re-esterification to TAG. Instead, the free lauric acid will preferentially be channelled to the liver for β- oxidation and result in a prolonged absorption time and the bioavailability of free LA. However, how these anticipated events are integrated in the metabolic processes with other signal pathways remains be studied. |
| Conclusion |
| This study demonstrated that the oral administration of novel diLaLA at 250 mg/kg of body weight for 6 weeks gave promising effects in reducing the body weight of DIO mice on an HFD, and that this effect possibly not primarily due to a decrease in the food intake. In addition to the weight reduction property, improvements were observed here in the plasma glucose and vitamin E status and the lipid profile, and a reduction in the plasma and hepatic FFA levels was observed. These interesting results are most probably caused by a decreased de novo lipogenesis and increased β-oxidation of fatty acids in the liver in the obese mice following the diLaLA treatment. Therefore, this approach may open a new research area for the treatment and prevention of obesity and related metabolic disorders. |
| Authors’ Contributions |
| The animal experiment was designed and conducted by CG with support from PCD. Lipid analyses were performed by SRPM. RNA sample preparation was conducted by MM and Microarray analysis was done by MA. Cross-checking and data analyses and interpretation were carried out by CG, SRPM and MM, with support from MA, KM, PCD. All the authors contributed in writing and revising the manuscript. |
| Acknowledgements |
| Samanthi R. P. Madawala (SRPM) gratefully acknowledges the Department of Food Science of the Swedish University of Agricultural Sciences (SLU) for providing the opportunity to pursue PhD studies. Ms Inger Jonasson and Dr Olga Vinnere Pettersson at UGC, Uppsala, Sweden are thanked for their support in the RT-qPCR analysis. |
| Financial Support |
| The authors gratefully acknowledge SLU in Uppsala for their financial support for this study, and Uppsala Genome Center (UGC) and UPPMAX for providing assistance in massive parallel sequencing and with the computational infrastructure. The work performed at UGC was partly funded by RFI/VR “SNISS” (the Swedish National Infrastructure for Large-Scale Sequencing) and the Science for Life Laboratory, Uppsala, Sweden. PCD thankfully acknowledges a stipend awarded by FOKUS Verifiering II, Innovationsbron, Uppsala Science Park, Uppsala, Sweden. |
| Conflict of Interest |
| Authors PCD and KM declare conflict of interest. |
References
- Murase T, Mizuno T, Omachi T, Onizawa K, Komine Y, et al. (2001) Dietary diacylglycerol suppresses high fat and high sucrose diet-induced body fat accumulation in C57BL/6J mice.J Lipid Res 42: 372-378.
- Osaki N, Meguro S, Onizawa K, Mizuno T, Shimotoyodome A, et al. (2008) Effects of a single and short-term ingestion of diacylglycerol on fat oxidation in rats.Lipids 43: 409-417.
- Robinson JR, Niswender KD (2009) What are the risks and the benefits of current and emerging weight-loss medications?CurrDiab Rep 9: 368-375.
- Trigueros L, Peña S, Ugidos AV, Sayas-Barberá E, Pérez-Álvarez JA, et al. (2013) Food ingredients as anti-obesity agents: a review.Crit Rev Food SciNutr 53: 929-942.
- Alvarado M, Decara J, Luque MJ, Hernandez-Folgado L, Gómez-Cañas M, et al. (2013) Novel antiobesity agents: Synthesis and pharmacological evaluation of analogues of Rimonabant and of LH21. Bioorg Medici Chem 21: 1708-1716.
- Boden G (2008) Obesity and free fatty acids.EndocrinolMetabClin North Am 37: 635-646, viii-ix.
- Klop B, Elte JW, Cabezas MC (2013) Dyslipidemia in obesity: mechanisms and potential targets.Nutrients 5: 1218-1240.
- Karpe F, Dickmann JR, Frayn KN (2011) Fatty acids, obesity, and insulin resistance: time for a reevaluation.Diabetes 60: 2441-2449.
- Flowers MT, Ntambi JM (2008) Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism.CurrOpinLipidol 19: 248-256.
- Warensjö E, Rosell M, Hellenius M-L, Vessby B, Faire UD, et al. (2009) Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis 8:37.
- Cedernaes J, Alsiö J, Västermark Å, Risérus U, Schiöth HB (2013) Adipose tissue stearoyl-CoA desaturase 1 index is increased and linoleic acid is decreased in obesity-prone rats fed a high-fat diet. Lipids Health Dis 12:2.
- Vinknes KJ, Elshorbagy AK, Nurk E, Drevon CA, Gjesdal CG, et al. (2013) Plasma stearoyl-CoA desaturase indices: association with lifestyle, diet, and body composition.Obesity (Silver Spring) 21: E294-302.
- Jeyakumar SM, Lopamudra P, Padmini S, Balakrishna N, Giridharan NV, et al. (2009) Fatty acid desaturation index correlates with body mass and adiposity indices of obesity in Wistar NIN obese mutant rat strains WNIN/Ob and WNIN/GR-Ob.NutrMetab (Lond) 6: 27.
- Yasunaga K, Saito S, Zhang YL, Hernandez-Ono A, Ginsberg HN (2007) Effects of triacylglycerol and diacylglycerol oils on blood clearance, tissue uptake, and hepatic apolipoprotein B secretion in mice.J Lipid Res 48: 1108-1121.
- Yanai H, Tomono Y, Ito K, Furutani N, Yoshida H, et al. (2007) Diacylglycerol oil for the metabolic syndrome.Nutr J 6: 43.
- Rudkowska I, Roynette CE, Demonty I, Vanstone CA, Jew S, et al. (2005) Diacylglycerol: efficacy and mechanism of action of an anti-obesity agent.Obes Res 13: 1864-1876.
- Yanai H, Tomono Y, Ito K, Hirowatari Y, Yoshida H, et al. (2009) A molecular mechanism for diacylglycerol-mediated promotion of negative caloric balance.Diabetes MetabSyndrObes 3: 1-6.
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM (2009) Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential.BiochimBiophysActa 1790: 1149-1160.
- Carbonelli MG, Di Renzo L, Bigioni M, Di Daniele N, De Lorenzo A, et al. (2010) Alpha-lipoic acid supplementation: a tool for obesity therapy?Curr Pharm Des 16: 840-846.
- Koh EH, Cho EH, Kim M-S, Park J-Y, Lee K-U (2008) Effects of alpha-lipoic acid on AMP-activated protein kinase in different tissues: Therapeutic implications for the metabolic syndrome. Lipoic Acid: Energy Production, Antioxidant Activity and Health Effects, eds Patel S, Packer L (Boca Raton, CRC Press) 495-519.
- Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, et al. (2004) Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase.Nat Med 10: 727-733.
- Wang Y, Li X, Guoa Y, Chan L, Guan X (2010) Lipoic acid increases energy expenditure by enhancing adenosine monophosphate–activated protein kinase-peroxisome proliferator-activated receptor- coactivator-1 signaling in the skeletal muscle of aged mice. MetabolClinExper 59: 967-976.
- Yang RL, Li W, Shi YH, Le GW (2008) Lipoic acid prevents high-fat diet-induced dyslipidemia and oxidative stress: a microarray analysis.Nutrition 24: 582-588.
- Butler JA, Hagen TM, Moreau R (2009) Lipoic acid improves hypertriglyceridemia by stimulating triacylglycerol clearance and downregulating liver triacylglycerol secretion. ArchivBiochemBiophys 485: 63-71.
- Valdecantos MP, Perez-Matute P, Gonzalez-Muniesa P, Prieto-Hontoria PL, Moreno-Aliaga MJ, et al. (2012) Lipoic acid administration prevents nonalcoholic steatosis linked to long-term high-fat feeding by modulating mitochondrial function. J NutrBiochem 23: 1676-1684.
- Madawala SR, Andersson RE, Jastrebova JA, Almeida M, Dutta PC (2011) Novel Conjugates of 1,3-Diacylglycerol and Lipoic Acid: Synthesis, DPPH Assay, and RP-LC-MS-APCI Analysis.J Lipids 2011: 419809.
- Hara A, Radin NS (1978) Lipid extraction of tissues with a low-toxicity solvent.Anal Biochem 90: 420-426.
- Azadmard-Damirchi S, Dutta PC (2008) Stability of minor lipid components with emphasis on phytosterols during chemical interesterification of a blend of refined olive oil and palm stearin. J Am Oil ChemSoc 85: 13-21.
- Ubhayasekera SJKA, Tres A, Codony R, Dutta PC (2010) Effect of feed fat by-products with trans fatty acids and heated oil on cholesterol and oxycholesterols in chicken. J Am Oil ChemSoc 87: 173-184.
- Folch J, Lees M, Sloame Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J BiolChem 226: 497-509.
- Rule DC, Broughton KS, Shellito SM, Maiorano G (2002) Comparison of muscle fatty acid profiles and cholesterol concentrations of bison, beef cattle, elk, and chicken.J AnimSci 80: 1202-1211.
- Stark R, Ashley SE, Andrews ZB (2013) AMPK and the neuroendocrine regulation of appetite and energy expenditure.Mol Cell Endocrinol 366: 215-223.
- Murase T, Aoki M, Wakisaka T, Hase T, Tokimitsu I (2002) Anti-obesity effect of dietary diacylglycerol in C57BL/6J mice: dietary diacylglycerol stimulates intestinal lipid metabolism.J Lipid Res 43: 1312-1319.
- Murase T, Nagasawa A, Suzuki J, Wakisaka T, Hase T, et al. (2002) Dietary alpha-linolenic acid-rich diacylglycerols reduce body weight gain accompanying the stimulation of intestinal beta-oxidation and related gene expressions in C57BL/KsJ-db/db mice.J Nutr 132: 3018-3022.
- Poobalan A, Aucott L, Smith WCS, Avenell A, Jung R, et al. (2004) Effects of weight loss in overweight/obese individuals and long-term lipid outcomes - a systematic review. Obes Rev 5: 43-50.
- Fujii A, Allen TJ, Nestel PJ (2007) A 1,3-diacylglycerol-rich oil induces less atherosclerosis and lowers plasma cholesterol in diabetic apoE-deficient mice.Atherosclerosis 193: 55-61.
- Saito S, Hernandez-Ono A, Ginsberg HN (2007) Dietary 1,3-diacylglycerol protects against diet-induced obesity and insulin resistance.Metabolism 56: 1566-1575.
- Saito S, Yamaguchi T, Shoji K, Hibi M, Sugita T, et al. (2010) Effect of low concentration of diacylglycerol on mildly postprandial hypertriglyceridemia.Atherosclerosis 213: 539-544.
- Park KG, Min AK, Koh EH, Kim HS, Kim MO, et al. (2008) Alpha-lipoic acid decreases hepatic lipogenesis, through adenosine monophosphate-activated protein kinase (AMPK)-Dependent and AMPK-independent pathways. Hepatol 48: 1477-1486.
- Lambert JE, Parks EJ (2012) Postprandial metabolism of meal triglyceride in humans.BiochimBiophysActa 1821: 721-726.
- Meng X, Zou D, Shi Z, Duan Z, Mao Z (2004) Dietary diacylglycerol prevents high-fat diet-induced lipid accumulation in rat liver and abdominal adipose tissue.Lipids 39: 37-41.
- Harding SV, Rideout TC, Jones PJ (2012) Evidence for using alpha-lipoic acid in reducing lipoprotein and inflammatory related atherosclerotic risk.J Diet Suppl 9: 116-127.
- Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB (2010) Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK.Am J Physiol Cell Physiol 298: C961-971.
- Fried SK, Horenstein RB (2011) Obesity. Lippincott´s Illustrated Reviews: Biochemistry, ed Harvey RA (Lippincott Williams & Wilkins. Baltimore) 349-356.
- Ntambi JM (1999) Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol.J Lipid Res 40: 1549-1558.
- Oosterveer MH, van Dijk TH, Tietge UJ, Boer T, Havinga R, et al. (2009) High fat feeding induces hepatic fatty acid elongation in mice.PLoS One 4: e6066.
- Burri L, Thoresen GH, Berge RK (2010) The Role of PPARα Activation in Liver and Muscle. PPAR Res 2010.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
 |
 |
| Figure 1 | Figure 2a | Figure 2b | Figure 2c | Figure 3a | Figure 3b |
 |
 |
 |
 |
 |
| Figure 3c | Figure 3d | Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 109974
- [From(publication date):
April-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 105389
- PDF downloads : 4585
