Research Article Open Access
Antinociceptive Activity of Methanolic Stem Bark Extracts of Harrisonia Abyssinica Oliv and Landolphia Buchananii (Hallier F.) Stapf in Mice Model
Nthiga PM*, Kamau JK, Safari VZ, Ngugi MP and Mburu DN
Department of Biochemistry and Biotechnology, School of Pure and Applied Sciences, Kenyatta University, Kenya
- Corresponding Author:
- Peter Mwangi Nthiga
Department of Biochemistry and Biotechnology
School of Pure and Applied Sciences
Kenyatta University, P.O. Box 43844-00100, Nairobi, Kenya
Tel: 254-701-227-995
E-mail: nthigapete@gmail.com
Received Date: May 24, 2016; Accepted Date: June 21, 2016; Published Date: June 24, 2016
Citation: Nthiga PM, Kamau JK, Safari VZ, Ngugi MP, Mburu DN (2016) Antinociceptive Activity of Methanolic Stem Bark Extracts of Harrisonia Abyssinica Oliv and Landolphia Buchananii (Hallier F.) Stapf in Mice Model. J Pain Relief 5:253.doi:10.4172/2167-0846.1000253
Copyright: © 2016 Mbamalu ON, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pain & Relief
Abstract
Pain is an unpleasant sensation attributed to an interplay of sensory and cognitive mechanisms and thus a source of burden to the individual and society. Since pain acts as a source of discomfort, it requires medical suppression. There is a growing interest in the use of herbal remedies as an attractive healthcare alternative to synthetic drugs. This is due to conventional medications being expensive and arguably associated with various adverse effects, hence the necessity for herbal agents that are effective, safe and relatively cheap. Harrisonia abyssinica and Landolphia buchananii are herbs that have been utilized to manage various ailments afflicting people especially in tropical Africa. However, despite their wide folklore use, extensive literature research reveals limited evaluation on the pharmacological activities of their described effects on pain. Thus, the aim of the current study was to evaluate the antinociceptive effects of their methanolic extracts. Experimental mice were divided into a normal control group, a negative control group, a reference group and three dosage groups. The dosage groups were treated with stem bark extracts at concentration of 50 mg/kg, 100 mg/kg and 150 mg/kg. The formalin paw licking test was used to determine the antinociceptive potential. Evaluation of the antinociceptive activities was compared with diclofenac as the reference drug. The H. abyssinica extract reduced pain by 39.73%-81.13% (in the early phase) and 15.92%-69.84% (in the late phase) while L. buchananii extract reduced it by between 35.35%-47.72% (in the early phase) and 20.57-55.17% (in the late phase). In the early phase, reduction of pain by diclofenac was by 19.97%-46.50% and 76.77-74.80% (in the late phase). Several phytochemicals were observed to be present. The traditional medicinal use of the aforementioned plants in the suppression of pain has thus been confirmed by the study results.
Keywords
Harrisonia abyssinica; Landolphia buchananii; Antinociceptive
Introduction
Pain, as a sub-modality of somatic sensation, describes a complex array of unpleasant experiences associated with actual or perceived tissue damage and exhibited by specific independent, psychological, and behavioral responses [1]. It is an intricate experience comprising of physiological response to a noxious stimulus, which in some cases is followed by an emotional response [2]. Formalin induction of pain is biphasic; an early phase, which comprises of the first to fifth minute and a late phase, which occurs fifteen to thirty minutes after pain induction. The initial acute response (early phase), indicating a neurogenic type of pain, develops immediately after formalin injection for 5 min (0-5 min), and then a sustained response (late phase), measured 15-30 min after formalin injection [3]. The early phase results from a direct effect of formalin on nociceptors [4], predominantly afferent C fibers and partly Aδ fibers [5]. The early phase can be inhibited by opioid agonists such as morphine, fentanyl, bradykinin B1 and B2 receptor antagonists [6].The late phase is a tonic response whereby inflammatory processes are involved and is associated with activation of the neurons in the dorsal horns of the spinal cord [4]. Both phases have been proven to be inhibited by centrally acting drugs, such as opiods while those that act peripherally, like steroids and nonsteroidal anti-inflammatory drugs like aspirin, inhibit only the late phase of the test [7]. To relieve pain in many disorders, nonsteroidal anti-inflammatory drugs (NSAIDs) are prescribed routinely worldwide [8]. However, longterm administration of NSAIDs may cause severe complications such as gastric ulcers, renal damage and cardiac abnormalities because of their non-selective inhibition of cyclooxygenases (COX) enzymes [9]. Interest in herbal medicine is on the rise as they are arguably believed to be more effective and possess fewer adverse effects [10]. Harrisonia abyssinica and Landolphia buchananii have been used by the Ameru and Embu communities in Kenya to alleviate various ailments but there is no scientifically investigated report of their described effects on pain. Therefore, the study mainly aimed to evaluate the antinociceptive potential of the aforementioned plants as preliminary drug screening towards production of more efficient plant-derived analgesic agents. The study also compared antinociceptive activities between these extracts as well as with diclofenac and also aimed to determine qualitative composition of the extracts.
Materials and Methods
Collection and preparation of plant samples
The fresh stem bark samples of Harrisonia abyssinica and Landolphia buchananii were collected with the help of local herbalists from their natural habitat in Mbeere, Embu County, Kenya. An acknowledged taxonomist botanically authenticated the plant samples and sample voucher deposited at the Kenyatta University herbarium. The plant samples were transported in clean burlap sacks to Kenyatta University for further processing. They were sorted, cleaned and rinsed with distilled water. They were then air dried at room temperature after being chopped separately into small pieces. The dried samples were then ground into fine powder by an electric mill.
Extraction
A volume of 400 grams of powder for each sample was soaked separately in 1500 ml of methanol and left to stand for 24 hours. The filtration of the extracts was done using Whatmann No.1 filter papers. A rotary evaporator set at 65°C concentrated the filtrate to dryness under reduced pressure and vacuum. The resultant concentrates were packaged separately in airtight containers and stored at 4°C awaiting bioassay studies.
Experimental design
Laboratory animals: Healthy strains of Swiss albino mice (Mus musculus) of either sex weighing 20 to 30 g were employed for this study. The animal breeding colony was bred in the Animal House, Department of Biochemistry and Biotechnology at Kenyatta University. The mice were acclimatized for 48 hours before use in the experiment. Standard ethical procedures were adhered to when handling the mice.
Determination of antinociceptive activities
The antinociceptive activities of the plant extracts were determined through the formalin paw licking test. The mice were divided into six groups (n=5) and then treated as shown on (Table 1). Half an hour after administering the treatments, the formalin test was performed using a known protocol [6]. The plant extracts were prepared on the day of the experiment and were administered by intraperitoneal injection. The induction of nociceptive response (licking and biting) was performed by subcutaneous injection of 0.1 ml of 2.50% formalin in the subplantar region of the right hind paw [4,11].
| Group | Status | Treatment |
|---|---|---|
| I | Normal control | 10% DMSO only |
| II | Negative control | 2.5% Formalin+10% DMSO |
| III | Positive control | 2.5% Formalin+15 mg/kg Diclofenac+10% DMSO |
| IV | Experimental group A | 2.5% Formalin+50 mg/kg extract+10% DMSO |
| V | Experimental group B | 2.5% Formalin+100 mg/kg extract+10% DMSO |
| VI` | Experimental group C | 2.5% Formalin+150 mg/kg extract+10% DMSO |
Table 1: Evaluation protocol of antinociceptive activities of methanolic extracts of Harrisonia abyssinica and Landolphia buchananii in mice
The mice were then individually placed in a transparent glass chamber for observation. The time that the mice spent licking or biting the injected paw was scored [4]. The pain scores were identified for two periods of nociceptive behavior (licking/biting) and recorded separately. The first period was recorded 1-5 minutes after injecting formalin and the second period recorded 15-30 minutes after formalin injection. The percentage licking inhibition was then calculated using the formula;
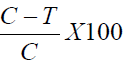
Where;
C- The vehicle-treated control group value for the each phase
T-The treated group value for each phase
Qualitative phytochemical screening
Qualitative phytochemical screening was done on the extracts to identify the presence or absence of selected phytochemical constituents using known protocols [12,13]. The secondary metabolites tested for included flavonoids, terpernoids, alkaloids, sterols, saponins, phenolics, and cardiac glycosides as these are generally associated with the antinociceptive effects.
Data management and statistical analysis
Experimental raw data on licking time/latency of pain response was tabulated on Ms Excel spreadsheet program. This data was then entered to the Minitab statistical software for descriptive statistics. The results were expressed as mean ± standard error of mean (SEM) for analysis. Then one-way analysis of variance (ANOVA) was performed to compare the means of the groups, subsequently followed by Tukey’s post hoc test for pair-wise mean separations and comparisons to obtain the specific significant differences among the different groups. The comparison of the mean activities of H. abyssinica and L. buchananii extracts against pain was performed using unpaired student t-test. The values of P ≤ 0.05 were considered statistically significant. The data on licking time/latency was presented using tables and graphs.
Results
Antinociceptive activity of methanolic stem bark extracts of Harrisonia abyssinica in mice
In general, the administration of methanolic stem bark extract of Harrisonia abyssinica appreciably reduced the formalin-induced pain in both phases which was indicated by reduction in paw licking time (Table 2).
| Group | Treatment | Phase I (seconds) | Phase II (seconds) |
|---|---|---|---|
| Normal control | DMSO only | 00.00 ± 0.00c(100.00) | 00.00 ± 0.00c(100.00) |
| Negative control | Formalin+DMSOc | 113.80 ± 13.9a(00.00) | 143.80 ± 8.14a(00.00) |
| Positive control | Formalin+DMSO+Diclofenac | 55.40± 4.72b(46.50) | 33.20 ± 5.89b(76.77) |
| Methanolic Stem bark extracts |
50mg/kg+Formalin+DMSO | 66.80± 7.14b(39.73) | 119.6 ± 4.01a(15.92) |
| 100mg/kg+Formalin+DMSO | 34.60± 9.44bc(69.73) | 50.40 ± 7.32b(63.80) | |
| 150mg/kg+Formalin+DMSO | 20.40± 5.03c(81.13) | 41.60 ± 10.5b(69.84) |
Table 2: Antinociceptive effects of methanolic stem bark extract of Harrisonia abyssinica Oliv. on formalin-induced pain in mice
In the early phase, the percent inhibition of paw licking time upon administration of stem bark extracts of H. abyssinica at the three dose levels (50,100 and 150 mg/kg body weight) was 39.73%, 69.73% and 81.13% respectively and exhibited a dose dependent trend (Table 2). The treatment of mice with methanolic stem bark extract of H. abyssinica at the dose levels of 50 and 100 mg/kg body weight was not significantly different from that of positive control with the reference drug, diclofenac (p ≥ 0.05; Table 2). However, treatment of mice with the extract at dose level of 150 mg/kg produced a stronger antinociceptive effect compared to the positive control group (p ≤ 0.05; Table 2). All the dose levels (50,100 and 150 mg/kg body weights) of methanolic stem bark extract of H. abyssinica significantly reduced paw licking time compared to negative control (p ≤ 0.05; Table 2).
In the late phase, the methanolic stem bark extract of H. abyssinica at the three dose levels (50,100 and 150 mg/kg body weight) reduced formalin induced pain in mice in a dose dependent manner by 15.92%, 63.80% and 69.84% respectively. The dose levels (100 and 150 mg/kg body weight) of methanolic stem bark extract of H. abyssinica significantly reduced paw licking time compared to negative control (p ≤ 0.05; Table 2). However, the treatment of mice with the extract at the dose level of 50 mg/kg body weight was statistically insignificant when compared with the negative control group (p ≥ 0.05; Table 2). The treatment of mice with methanolic stem bark extract of H. abyssinica at the dose levels of 100 and 150 mg/kg body weight was comparable with that of positive control with the reference drug, diclofenac (p ≥ 0.05; Table 2).
Antinociceptive activity of methanolic stem bark extracts of Landolphia buchahanii in mice
On the other hand, the administration of methanolic stem bark extract of L. buchananii also generally reduced the formalin-induced pain in both phases. In the early phase, methanolic stem bark extract of L. buchananii, at the three dose levels (50,100 and 150 mg/kg body weight), reduced formalin-induced pain by 35.35%, 40.80 and 47.72% respectively in a dose dependent fashion (Table 3).
| Group | Treatment | Phase I(seconds) | Phase II(seconds) |
|---|---|---|---|
| Normal control | DMSO only | 00.00± 0.00d (100.00) |
00.00 ± 0.00e (100.00) |
| Negative control | Formalin+DMSO | 154.10 ± 13.90a (00.00) |
156.40 ± 7.76a (00.00) |
| Positive control | Formalin+DMSO+Diclofenac | 119.80± 6.91ab (19.97) |
40.40 ±16.30de (74.80) |
| Methanolic Stem bark extracts |
50mg/kg+Formalin+DMSO | 96.40 ± 4.88bc (35.35) |
69.60± 5.58cd (55.17) |
| 100mg/kg+Formalin+DMSO | 93.20± 14.10bc (40.80) |
95.11 ± 11.70bc (39.14) |
|
| 150mg/kg+Formalin+DMSO | 77.40± 11.30c (47.72) |
125.00 ± 12.50ab (20.57) |
Table 3: Antinociceptive effects of methanolic stem bark extract of Landolphia buchananii (Hallier f.)Stapf on formalin-induced pain in mice
Treatments with the extract at all the dose levels (50,100 and 150 mg/kg body weights) significantly inhibited the paw licking response time in mice compared to the negative control group (p ≤ 0.05; Table 3). The antinociceptive effectiveness of the doses at the three dose levels (50, 100 and 150 mg/kg body weights) was statistically insignificant from each other (p ≥ 0.05; Table 3). In this phase, the methanolic stem bark extract of L. buchananii at the dose levels of 50 mg/kg and 100 mg/kg body weight exhibited a decrease in pain that was comparable to diclofenac (reference drug) (p ≥ 0.05; Table 3). However, the antinociceptive effect of the extract at the dose level of 150 mg/kg body weight was stronger with a 47.72% licking inhibition when compared to the positive control group which had a 19.97% licking inhibition (p ≤ 0.05; Table 3).
In the late phase, the methanolic stem bark extract of L. buchananii at the dose levels (50, and 100 mg/kg body weights) also reduced formalin-induced pain in mice (Table 3). However, the paw licking reduction activity of the extract at the dose level of 150 mg/kg body weight was statistically insignificant compared to the negative control group (p ≥ 0.05; Table 3). The percent paw licking inhibitions of the stem bark extract at the three dose levels were 55.17%, 39.14% and 20.57% respectively (Table 3). The treatment at the dose level of 50 mg/kg bodyweight was comparable to that of positive control with the reference drug, diclofenac (p ≥ 0.05; Table 3). However, treatments at the dose levels of 50 and 100 mg/kg body weights were not statistically significant from one another (p ≥ 0.05; Table 3).
Comparison between the antinociceptive activity of Harrisonia abyssinica and Landolphia buchananii
In comparison, the methanolic stem bark extract of H. abyssinica exhibited more effective antinociceptive effect at the dose levels of 100 and 150 mg/kg body weight than methanolic stem bark extract of L. buchananii during the early phase at p values of 0.01 and 0.02 respectively (Figure 1). However, in this phase, the comparison of the antinociceptive effectiveness at the dose level of 50 mg/kg body weight of the two extracts was not significantly different from each other (p = 0.60; Figure 1).
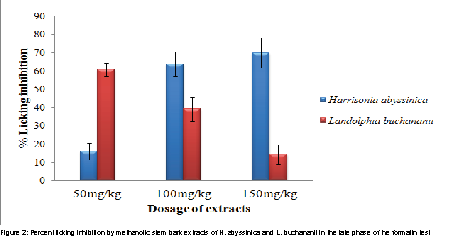
In the late phase, the antinociceptive effect of methanolic stem bark extract of H. abyssinica was more efficacious at the dose levels of 100 and 150 mg/kg body weights than that of L. buchananii at p values of 0.034 and 0.002 respectively (Figure 2). However, L. buchananii exhibited more antinociceptive activity than H. abyssinica at the dose level of 50 mg/kg body weight (p=0.00; Figure 2).
Qualitative Phytochemical Screening
Qualititative phytochemical screening revealed the presence of flavonoids, terpernoids, alkaloids, sterols, saponins, phenolics and cardiac glycosides (Table 4).
| Phytochemicals | H.abyssinica stem bark extract | L.buchananii stem bark extract |
|---|---|---|
| Alkaloids | + | + |
| Flavonoids | + | + |
| Steroids | + | + (trace) |
| Saponins | + (trace) | + |
| Cardiac glycosides | - | + |
| Phenolics | + | + |
| Terpernoids | + | + |
Table 4: Phytochemical composition of methanolic stem bark extracts of Harrisonia abyssinica Oliv and Landolphia buchananii (Hallier f.) Stapf
Discussion
Clinically, several broad processes are associated with pain: nociception, pain perception and some secondary consequences including suffering and pain behavior [14]. The detection and perception of noxious stimuli is referred to as nociception [15]. Nociceptors exhibit impressive plasticity which can intensify tissue signaling and nociceptors can be classified into two; myelinated Aδ fibers of medium diameter that mediate acute, well-localized fast pain and differ substantially from the larger Aβ fibers that respond to innocuous mechanical stimulation. The other class comprises smaller unmyelinated C fibers that transmit poorly localized slow pain [16].
Tissue injury or inflammation results in the release of sensitizing and algogenic agents including bradykinin, histamine, prostaglandins, interleukin-1β (IL-1β) and tumor necrosis factor (TNF) [17]. These substances result in amplified firing of the neurons as they lower the activation threshold of nociceptors and enhance neuronal excitability. Increased activity of nociceptors is manifest as hyperalgesia and allodynia [18].
The evaluation of antinociceptive activity of methanolic stem bark extracts of H. abyssinica and L. buchananii was performed using the formalin paw licking test. The formalin paw licking test is a credible and dependable model for analgesic activity. It produces a definite biphasic response and various analgesics may act differently in early and late phases of this test. Thus, the test can be used to resolve the possible mechanism of the antinociceptive effect of a proposed analgesic [19].
In this study, 2.5% formalin was used to induce nociception. A formalin concentration of 2.5-5% is a better dose than 10% because it evokes maximal responses [20]. In fact, the 10% formalin is not recommended for a pain test. This is because higher formalin concentrations induce other behavioral responses that may hamper the primary behavior [21]. The rising environmental temperature increases pain response of formalin hence the formalin test was performed at room temperature (22-24°C), during the daytime, to give mice time for familiarization with the environment to minimize other factors that may lead to compromised pain scores [4].
In the current study, the methanolic stem bark extracts of H. abyssinica and L. buchananii showed a significant antinociceptive effect by lowering the formalin-induced licking time in both phases. However, the extracts did not inhibit both phases equally The highest analgesic effect by methanolic stem extract of H. abyssinica was by 81.13% and 69.84% in the early and late phases, respectively while by methanolic stem bark extract of L. buchananii was by 47.72% and 55.17% in the early and late phases, respectively. The ability of these herbal extracts to inhibit both phases of the formalin paw licking test suggests their involvement in peripheral and central mediated activity, probably by inhibition of prostaglandin synthesis, as well as central mechanism inhibition. The antinociceptive effect of H. abyssinica at the dose level of 100 and 150 mg/kd body weight (in the early phase ) was more efficacious than that of L. buchananii (Figure 1) might be due to stronger synergetic effects in the H. abyssinica than in L. buchananii.
These results relate with other previous studies on evaluation of antinociceptive effects of herbal extracts. The methanolic stem bark extracts of H. abyssinica and L. buchananii demonstrated a reduction in the paw licking time of formalin-induced pain in both phases which was consistent with a study on antinociceptive activity of alcohol leaf and root bark extracts of Carissa edulis in rats [22]. Similarly, acetone leaf extract of Carissa spinarum demonstrated related antinociceptive effect in formalin paw licking model [23].
The dose ranges applied in this study were equivalent to the dose ranges employed by a study examining the antinociceptive effect of dichloromethane: methanolic extracts of Caesalpinia volkensii in animal models [24]. More so, another study used dose levels of 50, 100, and 200 mg/kg while evaluating antinociceptive activity of the roots extracts of Alafia barteri Oliver (Apocynaceae), and Combretum mucronatum Schumach (Combretaceae) and Capparis on acetic acid, formalin and hot plate induced pain tests [25].
That the findings of the methanolic stem bark extracts of H. abyssinica and L. buchananii (in the early phase) produced dose dependent antinociceptive activity is in agreement with a study on antinociceptive activities of Tecoma stans leaf extract in laboratory animals [26]. The effect of L.buchananii in the second phase was however inversely dose-dependent, whereby the smallest dose of 50 mg/ kg body weight produced greater antinociceptive effect compared to the largest dose of 150 mg/kg body weight. This might be due to declining synergetic effects of some active principles when concentrations are increased.
The antinociceptive effect of H. abyssinica at the dose levels of 150 mg/kg body weight (in the early phase) and L. buchananii at the dose levels of 100 and 150 mg/kg body weight (in the late phase) was greater and statistically significant compared to diclofenac (p ≤ 0.05; Table 1; Table2) which might suggest a better inhibition of algogenic agents such as prostaglandins or a better mimicry of the diclofenac action.
The antinociceptive effects of methanolic stem bark extracts of H. abyssinica and L. buchananii might be due to one or several phytoconstituents present in the extracts. Several studies have shown the antinociceptive activity of such compounds. A number of flavonoids have been reported to produce analgesic activity [27]. Some studies have demonstrated that flavonoids inhibit prostaglandin synthetase [28]. Since prostaglandins are involved in pain perception and flavonoids have inhibitory effects against them, it might be implied that reduced presence of prostaglandins by flavonoids present in H. abyssinica and L. buchananii might be responsible for their antinociceptive effects.
Alkaloids, which were present in both extracts have been documented to possess potent analgesic activities [29] and thus might be contributory to the observed antinociceptive effects of H. abyssinica and L. buchananii. An evaluation of the several alkaloids from Ziziphus oxyphylla has demonstrated antinociception in both phases of the formalin test [30]. Terpenoids have also been linked with antinociceptive activity. For instance, a study on 1, 8-cineole terpenoid has demonstrated antinociceptive effect in the formalin test through a non-opiod mechanism [31]. Phenols, which were also detected in both extracts, have been shown to exert antinociceptive effect in mice in an opiod-mediated action according to a study on the extract of Emilia sonchifolia [32]. Saponins, which were also found in both extracts although in trace amounts in H. abyssinica, have also been associated with analgesic effects [33]. Some studies have also shown sterol constituents possess analgesic actions. For instance, β-sitosterol which has previously been isolated from H. abyssinica extract [34] has been shown to exert analgesic effect according to a study using leaf extracts of Oxalis corniculata [35] and therefore, sterol constituents present in both extracts might also be contributory to the observed antinociceptive effects.
It is noteworthy to mention a limitation in this study regarding the use of either sex of mice in performing antinociceptive studies since pain sensitivity is gender sensitive as documented by a recent study [36].
Conclusion
In conclusion, the present study has demonstrated the antinociceptive potential of methanolic stem bark extracts of Harrisonia abyssinica and Landolphia buchananii in mice. The methanolic stem bark extracts of H. abyssinica and L. buchananii were able to inhibit pain sensation of both phases. Thus it might be likely to find opioid analgesics as well as analgesics in H. abyssinica and L. buchananii that act by inhibition of inflammatory pathways responsible for pain. Therefore, the methanolic stem bark extracts of H. abyssinica and L. buchananii extracts might prove useful in managing painful complications and serve as a more effective alternative treatment strategy than the conventional synthetic drugs. The present study, therefore, scientifically confirms the traditional use of Harrisonia abyssinica and Landolphia buchananii for management of painful conditions.
References
- Terman GW, Bonica JJ (2001) Spinal mechanisms and their modulation. Bonica’s Management of Pain. (3rd edn). Baltimore, MD: Lippincott Williams &Wilkins 73-152.
- Fürst S (1999) Transmitters involved in antinociception in the spinal cord. Brain Res Bull 48: 129-141.
- Malmberg AB, Yaksh TL (1992) Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J PharmacolExpTher 263: 136-146.
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992) The formalin test: an evaluation of the method. Pain 51: 5-17.
- Shibata M, Ohkubo T, Takahashi H, Inoki R (1989) Modified formalin test: characteristic biphasic pain response. Pain 38: 347-352.
- Hunskaar S, Hole K (1987) The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30: 103-114.
- Chen YF, Tsai HY, Wu TS (1995) Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med 61: 2-8.
- Wolfe MM, Lichtenstein DR, Singh G (1999) Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 340: 1888-1899.
- Tapiero H, Ba GN, Couvreur P, Tew KD (2002) Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother 56: 215-222.
- Kamboj VP (2000) Herbal medicine. Current Science Bangalore 78: 35-38.
- Heapy CG, Jamieson A, Russell NJW (1987) Afferent C-fiber and A-delta activity in models of inflammation. British Journal of Pharmacology 90: 164.
- Harbone JB (1998) Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. Chapman & Hal Publishers, London, UK 3: 60-66.
- Kotake CK (2000) Practical Pharmacognosy. VallabhPrakashan, New Delhi, India 4: 107-111.
- Loeser JD, Melzack R (1999) Pain: an overview. Lancet 353: 1607-1609.
- Loeser JD, Treede RD (2008) The Kyoto protocol of IASP Basic Pain Terminology. Pain 137: 473-477.
- Meyer RA, RingkampM, Campbell JN, Raja SN (2008) Peripheral mechanisms of cutaneous nociception. Wall and Melzack’s Textbook of Pain, S.B. McMahon and M. Koltzenburg3: 34.
- Costigan M, Scholz J, Woolf CJ (2009) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32: 1-32.
- Merskey H, Thompson EN (2002) Nerve blocks and cognitive therapy: a beneficial failure. Pain Res Manag 7: 175-178.
- Rosland JH, Tjølsen A, Maehle B, Hole K (1990) The formalin test in mice: effect of formalin concentration. Pain 42: 235-242.
- Lee IO, Jeong YS (2002) Effects of different concentrations of formalin on paw edema and pain behaviors in rats. J Korean Med Sci 17: 81-85.
- Clavelou P, Dallel R, Orliaguet T, WodaA, Raboisson P (1995) The orofacial formalin test in rats: effects of different formalin concentrations. Pain 62: 295-301.
- Maina GS, Kelvin JK, Maina MB, Muriithi NJ, Kiambi MJ, et al. (2015)Antinociceptive properties of dichloromethane: methanolic leaf and root bark extracts of Carissa edulis in rats. The Journal of Phtyopharmacology 4:106-112
- Mworia JK, Gitahi SM, Juma KK, Njagi JM, Mwangi BM (2015) Antinociceptive Activities of Acetone Leaves Extracts of Carissa Spinarum in Mice. Journal of Medicinal and Aromatic Plants 1: 2167-0412.
- Maina MB, Gitahi SM, Njagi JM, Mworia JK, Aliyu U, et al.(2015) Antinociceptive Properties of Dichloromethane: Methanolic Leaf Extracts of Caesalpiniavolkensii and Maytenusobscura in Animal Models. Journal of Pain Relief 4: 191.
- Ishola IO, Agbaje EO, Adeyemi OO,Rakesh S (2014) Analgesic and anti-inflammatory effects of the methanol root extracts of some selected Nigerian medicinal plants. Journal of Pharmaceutical Biology 52: 1208-1216.
- Alguacil LF, de Mera AG, Gomez J, Llinares F, Morales L, et al. (2000)Tecomasambucifolia: anti- inflammatory and antinociceptive activities, and ‘in vitro’toxicity of extracts of the ‘huarumo’of Peruvian Incas. Journal of Ethnopharmacology 70: 227-233.
- Hosseinzadeh H, Ramezani M, Fadishei M, Mahmoudi M (2002) Antinociceptive, anti-inflammatory and acute toxicity effects of Zhumeriamajdae extracts in mice and rats. Phytomedicine 9: 135-141.
- Ramaswamy S, Pillai NP, Gopalakrishnan V, Parmar NS, Ghosh MN (1985) Analgesic effect of O-(beta-hydroxy ethyl)rutoside in mice. Indian J ExpBiol 23: 219-220.
- Radulovic SN, Blagojevic DP, Randjelovic JP, Stojanovic MN (2013)The last decade of antinociceptive alkaloids: structure, synthesis, mechanism of action and prospect. Current topics in medicinal chemistry 13:2134-2170.
- Kaleem WA, Muhammad N, Qayum M, Khan H, Khan A, et al. (2013)Antinociceptive activity of cyclopeptide alkaloids isolated from ZiziphusoxyphyllaEdgew (Rhamnaceae) Fitoterapia91: 154-158.
- Santos FA, Rao VSN (2000) Antiinflammatory and antinociceptive effects of 1, 8-cineole a terpenoid oxide present in many plant essential oils. Phytotherapy Research 14: 240-244.
- Couto VM, Vilela FC, Dias DF, Dos Santos MH, Soncini R, et al. (2011) Antinociceptive effect of extract of Emilia sonchifolia in mice. J Ethnopharmacol 134: 348-353.
- Akkol EK, Tatli II, Akdemir ZS (2007) Antinociceptive and anti-inflammatory effects of saponin and iridoid glycosides from Verbascumpterocalycinum var. mutense Hub.-Mor. Z Naturforsch C 62: 813-820.
- Baldé AM, Apers S, De Bruyne TE, Van den Heuvel H, Claeys M, et al. (2000) Steroids from Harrisoniaabyssinica. Planta Med 66: 67-69.
- Dighe SB, Kuchekar BS, Wankhede SB (2016) Analgesic and anti- inflammatory activity of ß-sitosterol isolated from leaves of Oxalis corniculata. International Journal of Pharmacological Research 6: 109-113.
- Bartley EJ, Fillingim RB (2013) Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111: 52-58.
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 11536
- [From(publication date):
July-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10687
- PDF downloads : 849