Research Article Open Access
Antimicrobial Susceptibility Patterns of Methicillin-Resistant Staphylococcus aureus Isolates Collected from Healthcare and Community Facilities in Libya Show a High Level of Resistance to Fusidic Acid
| Wareg SE1,2, Foster HA1* and Daw MA2 | ||
| 1Centre for Parasitology and Disease Research, School of Environment and Life Sciences, University of Salford, Lancs M5 4WT, UK | ||
| 2Department of Medical Microbiology, Faculty of Medicine, University of Tripoli, Libya | ||
| Corresponding Author : | Howard A Foster Centre for Parasitology and Disease Research School of Environment and Life Sciences University of Salford,Salford, M5 4WT, UK Tel: + 44 161 295 3832 Fax: + 44 161 295 5015 E-mail: h.a.foster@salford.ac.uk |
|
| Received August 27, 2014; Accepted November 28, 2014; Published December 08, 2014 | ||
| Citation: Wareg SE, Foster HA, Daw MA (2014) Antimicrobial Susceptibility Patterns of Methicillin-Resistant Staphylococcus aureus Isolates Collected from Healthcare and Community Facilities in Libya Show a High Level of Resistance to Fusidic Acid. J Infect Dis Ther 2:189. doi:10.4172/2332-0877.1000189 | ||
| Copyright: © 2014 Wareg SE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Staphylococcus aureus is an important human pathogen and is implicated in a wide variety of infections in the healthcare and community settings. The organism is often subcategorized as community-associated MRSA (CAMRSA) or health care-associated MRSA (HA-MRSA). Five hundred and eleven S. aureus strains were isolated from clinical specimens submitted to the microbiology laboratories at Tripoli Central Hospital, Tripoli Trauma/ Accident Hospital, Tripoli Medical Centre and Tripoli Burn Hospital between October 2009 and November 2010. MRSA was detected using cefoxitin (30 μg) disc and antibiotic susceptibility pattern was determined using the Kirby and Bauer disc diffusion susceptibility testing method and confirmed for fusidic acid and vancomycin by determination of minimum inhibitory concentration The prevalence of Inpatient Healthcare Associated MRSA (IP-MRSA), outpatient-Healthcare Associated MRSA (OP-MRSA) and community carried MRSA (CC-MRSA) was 43%, 37% and 34% respectively. The isolates of MRSA displayed resistance to fusidic acid and multiple drug resistance (MDR) to 2-9 antibiotics for IP-MRSA, 2-7 antibiotics for OP-MRSA and 2-6 antibiotics for CC-MRSA. The most frequent MDR was resistance to fusidic acid, ciprofloxacin, streptomycin and clindamycin. This study has shown that MRSA is prevalent with similar rates for IP-MRSA, OP-MRSA and CC-MRSA strains. Lack of controls on supply of antibiotics may be responsible for the fusidic acid resistance.
| Keywords | |
| Antibiotic resistance; Fusidic acid; Libya; MRSA; Staphylococcus aureus; Methicillin resistance | |
| Abbreviations | |
| BSAC: British Society for Antimicrobial Chemotherapy; CA-MRSA: Community acquired MRSA; CC-MRSA: Community Carried MRSA; CLSI: Clinical Laboratory Standards Institute; HA-MRSA: Healthcare Acquired MRSA; IP: Inpatients; IPMRSA: MRSA Isolated from Inpaitients; MIC: Minimum Inhibitory Concentration; Mlsbi: Macrolide, Lincosamide and Group B Streptogramin Resistance; MRSA: Methicillin Resistant Staphylococcus Aureus; NB, Nutrient Broth; OP: Outpatients; OP-MRSA: MRSA Isolated From Outpatients | |
| Introduction | |
| Staphylococcus aureus is an important pathogen that causes a wide range of diseases, from mild superficial skin infection to life-threatening diseases such as bacteraemia, pneumonia, infective endocarditis, deep-seated abscess and toxic-shock syndrome [1]. The organism is often subcategorized as community-associated MRSA (CA-MRSA) or health care-associated MRSA (HA-MRSA). Most studies distinguish CA-MRSA from HA-MRSA based on whether infection is present or diagnosed within 72 h of admission [2]). Only a few studies on staphylococcal infections and methicillin-resistant S. aureus (MRSA) in particular in Libya have been reported. Toxic shock syndrome toxin of S. aureus from Tripoli (Libya) was detected by El-Godban et al. [3]. Approx. 75% of strains originating from food were resistant to penicillin but none of the strains were resistant to methicillin or vancomycin. Inducible clindamycin resistance among staphylococci isolated from burn patients in Tripoli, Libya was studied by Zorgani et al. [4] and 65/120 (54%) of S. aureus isolates were MRSA. In a study by Buzaid et al. [5], MRSA was found in 31% of S. aureus isolates examined in a tertiary surgical and trauma hospital in Bengazi, Libya. They also reported the antimicrobial resistance patterns of MRSA to vancomycin, ciprofloxacin, chloramphenicol and erythromycin as 17.7%, 33.9%, 38.7% and 46.8% of cases respectively. A study of 169 clinical samples showed that 32% of S. aureus isolates were MRSA and a further 5% carried the MecA gene but did not express resistance [6]. Screening of 569 doctors and nurses from four main hospitals in Tripoli, Libya for MRSA showed a carriage rate of 19% [7]. This contrasts with an earlier study by Zorgani et al. [8] which showed a carriage rate of 37% in Libyan healthcare workers. These studies suggest that MRSA prevalence was high in many hospitals in Libya. The aim of present study was to identify and verify the extent of the spread of both community and healthcare-associated MRSA infections. Antibiotic resistance was detected using the disc diffusion method. Preliminary results showed a high prevalence of fusidic acid resistance and strains apparently resistant to vancomycin were isolated from outpatients. The resistance was confirmed by determination of minimum inhibitory concentration (MIC). | |
| Materials and Methods | |
| Study area and sampling technique | |
| Inpatient samples (IP; 735) from wound/pus swabs, urine, swabs of catheters and blood culture from patients in the Tripoli Central Hospital, Tripoli Trauma/Accident Hospital, Tripoli Medical Centre and Tripoli Burn Hospital, outpatient samples from surgery out-patient departments (503 wound swabs, skin and soft tissue swabs) were sampled between October 2009 and November 2010. Clinical samples were sub-cultured onto Columbia agar base containing 5% sheep blood agar (Oxoid) and incubated at 37°C for 18-24 h. Cultures were screened for colonies typical of S. aureus and a total of five-hundred and eleven strains were isolated at random from those samples where S. aureus was the main infectious agent. S. aureus was similarly isolated from 344 nasal swabs from university students and the general public at random. | |
| Bacterial identification | |
| Clinical samples were streaked to single colonies onto Columbia agar base containing 5% sheep blood agar (Oxoid) and incubated at 37°C for 18-24 h. Colonies consistent with S. aureus were streaked to single colonies and identified and confirmed according to their colony morphology, Gram stain, biochemical and microbiological tests including: catalase test using hydrogen peroxide, coagulase test (both slide test and tube test) using human plasma (Liofilchem-Italy), growth in mannitol salt agar (Liofilchem-Italy) and Deoxyribonuclease test (EMD-Germany). S. aureus (ATCC 25923) was used as a quality control organism. | |
| Antimicrobial susceptibility testing | |
| S. aureus ATCC25923 and isolates were inoculated into Nutrient Broth (NB; Oxoid) and incubated at 37º C for 18-24 h. The cultures were diluted with fresh NB to give a turbidity equivalent to 0.05 McFarland standard absorbance at 625nm. Susceptibility tests were performed by the disc diffusion method of Bauer et al. [9] as described by the Clinical Laboratory Standard Institute (Anon., 2013) using Mueller- Hinton agar (Difco) supplemented with 20gl-1 NaCl. Antibiotic discs (Oxoid UK) were cefoxitin (FOX) 30 μg, vancomycin (VAN) 30 μg, chloramphenicol (CHL) 30 μg, gentamicin (GEN)10 μg, fusidic acid (FUS)10 μg, erythromycin (ERY) 15 μg, streptomycin (STR) 10 μg, cefotaxime (CTX) 30 μg, clindamycin (CLI) 2 μg, ciprofloxacin (CIP) 5 μg. Zones of inhibition were measured after 18 and 24 h incubation at 35°C. Cefoxitin was used as an indicator of methicillin susceptibility disks and an inhibition zone diameter of ≤ 14mm was reported as methicillin resistant, 15-17mm as intermediate and ≥ 18mm was considered as methicillin sensitive. S. aureus ATCC25923 was used in every run. The isolates were reported as sensitive, intermediate and resistant based on the Clinical and Laboratory Standards Institute (CLSI) guidelines [10]. Determination of sensitivity for vancomycin on media containing 6.5% NaCl can give unreliable results [11] and these results were checked by determining the MIC. Interpretative zones of inhibition for fusidic acid are not stated in the CLSI guidelines. Values used (resistant ≤29 mm and susceptible ≥30 mm) were as according to the British Society for Antimicrobial Chemotherapy (BSAC) guidelines [12]. Minimum inhibitory concentrations were determined for fusidic acid and vancomycin using dilutions of 128-0.25 mgl-1 in Isosensitest agar (Oxoid). Plates were inoculated with culture suspension (1 μl) and incubated for 24 h at 35°C. MIC was determined as the lowest concentration that inhibited growth. | |
| Ethical considerations | |
| Ethical permission was obtained from the main management of the hospitals under investigation in Tripoli and the University of Tripoli. | |
| Results | |
| MRSA isolates and antibiotic resistance | |
| A total of 511 S. aureus isolates were obtained (Table 1). Twohundred and forty-three isolates were from inpatients (IP; 164 infected wound/pus swabs, 42 urine samples, 16 from swabs of catheters and 12 blood culture). The prevalence of S. aureus infection was higher for wound swabs at 42.6% compared to 31-32% for all the other samples. One-hundred and sixty-six strains were isolated from outpatients (OP; 88 from wound swabs, 28 from skin swabs and 50 from soft tissue swabs). The prevalence of S. aureus infections in wound swabs was similar to that for inpatients at 32-33%. In addition, 111 community carried (CC) strains were isolated from 344 nasal swabs giving a carriage rate of 32.3%. | |
| The isolates were screened for resistance to cefitoxin as an indicator for methicillin resistance. The prevalence of MRSA was 43%, 37% and 34% in the inpatients (IP-MRSA), outpatients (OP-MRSA) and community carried isolates (CC-MRSA) respectively (Table 1). | |
| The age and sex distribution of the MRSA isolates are shown in Table 2. There was a slightly higher percentage of males than females in the inpatients and outpatients but the reverse was true of the community. Most patients were aged between 30 and 49 but most of the CC-MRSA strains came from those aged below 30, reflecting the number of university students screened. | |
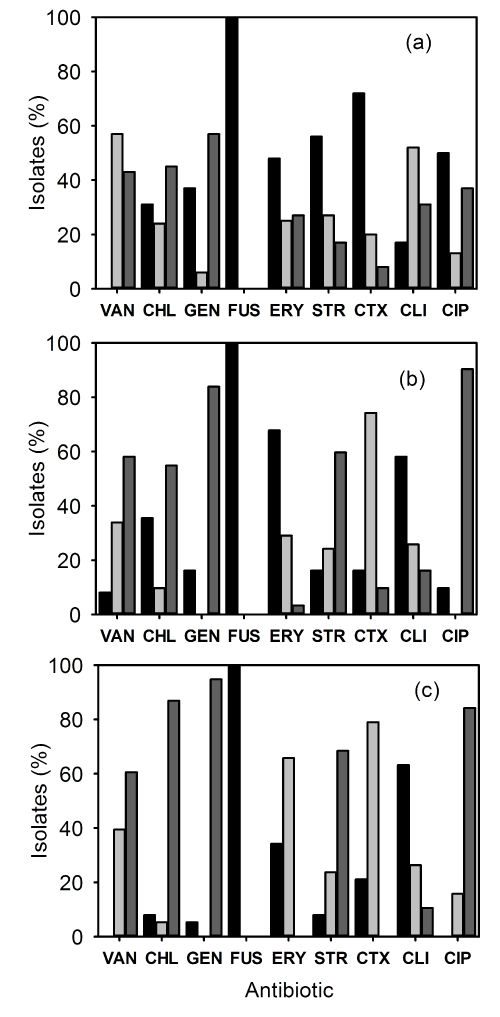
| The MRSA isolates were subjected to antibiotic susceptibility test against 9 antimicrobial agents. The resistance of the IP-MRSA strains is shown in Figure 1a. All of the strains were resistant to fusidic acid. Resistance to vancomycin was 0%, chloramphenicol 31%, gentamicin 37%, erythromycin 48%, streptomycin 56%, cefotaxime 72%, clindamycin 17% and ciprofloxacin 56% (Figure 1a). The resistance pattern of OP-MRSA strains is shown in Figure 1b. Again no strains were susceptible to fusidic acid. Resistance to vancomycin was 8%, chloramphenicol 35%, gentamicin 16%, erythromycin 68%, streptomycin 16%, cefotaxime 16%, clindamycin 58% and ciprofloxacin 10% (Figure 1b). The susceptibility pattern of CC-MRSA strains (Figure 1c) showed that resistance to fusidic acid was 100%. Resistance to vancomycin was 0%, chloramphenicol 8%, gentamicin 5%, erythromycin 34%, streptomycin 8%, cefotaxime 21%, clindamycin 63% and ciprofloxacin 0% (Figure 1c). | |
| Zone of inhibition of fusidic acid for IP-MRSA, OP-MRSA and CC-MRSA | |
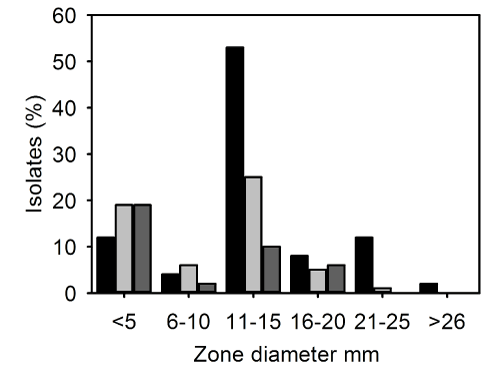
| The zone sizes for fusidic acid were heterogenous (Figure 2). The majority of strains had a zone size in the range 11-15mm for IP-MRSA and CC-MRSA (53/100, 53% and 10/38, 26% respectively) whereas most OP strains had zones of <5 mm (12/62; 20%). A smaller numbers of strains had zone sizes in the range 26-30 mm which were 2/100 (2%), 0/62 (0%) and 0/38 (0%) respectively for IP-MRSA, OP-MRSA and CCMRSA respectively (Figure 2). | |
| Determination of MIC | |
| MIC’s were determined for vancomycin and fusidic acid and the results are shown in Table 3. For fusidic acid the breakpoint is 2 mgl- 1 and all the strains were resistant with three IP- and 4 OP-MRSA isolates showing a high level of resistance of ≥64 mgl-1 (Table 3). For vancomycin resistance is defined as an MIC of >16 mgl-1 and no strains were resistant, even in the outpatient isolates in contrast to the results of the disk diffusion assay. | |
| Multi-drug resistance in MRSA isolates | |
| The highest number of resistance phenotypes were shown by the IP-MRSA followed by the OP-MRSA with the CC-MRSA showing the lowest incidence of multiple antibiotic resistance (Table 4). There were no common resistance patterns between the three grouos of isolates. The highest MDR pattern (23%) was observed for two antibiotics with the pattern fusidic acid/streptomycin. The maximum number of antibiotics resisted by one or two isolates was eight antibiotics with MDR pattern fusidic acid/gentamicin/chloramphenicol/ streptomycin/ erythromycin/cefotaxime/clindamycin and ciprofloxacin. | |
| The most frequent MDR pattern (42%) for OP-MRSA strains was observed for five antibiotics. The maximum number of antibiotics resisted by single isolate was six antibiotics with the MDR pattern fusidic acid/streptomycin/erythromycin /gentamicin/ciprofloxacin/ cefotaxime, whereas the highest MDR pattern (34%) for CC-MRSA strains was observed for four antibiotics with the pattern fusidic acid/ vancomycin/ clindamycin/erythromycin. The maximum number of antibiotics resisted by single isolate or two isolates was five antibiotics with the pattern fusidic acid/ vancomycin/erythromycin/streptomycin/ clindamycin (Table 4). | |
| Discussion | |
| This study has shown the prevalence of MRSA in S. aureus infections in Libya as IP-MRSA, 43% OP-MRSA, 37% and CC-MRSA, 34% respectively. This is rather higher than a similar study of MRSA in a tertiary surgical and Trauma hospital in Bengazi, Libya which showed that that MRSA were 31% of S. aureus isolates [5]. Staphylococcal carriage rate was 32.3% which is slightly higher than in previous studies [11] but may reflect the restricted sample size. MRSA is an increasing important cause of morbidity and can easily spread from hospital to another hospital or community based facility and even spreading from country to another [13]. In this study no vancomycin resistance was found in either IP-MRSA or CC-MRSA strains while 5% of OP-MRSA strains were resistant by disc diffusion assay but the MIC values for these strains indicated sensitivity to vancomycin. This result suggests that vancomycin can be used to treat MRSA infections. Clindamycin resistance represented 17% for IP-MRSA, OP-MRSA, 58% and CCMRSA, 63% which is higher than in previous studies in Libya [4]. This suggests that clindamycin can be used to treat IPH-MRSA infection but should be used with caution in OP and CC-MRSA infections. A study on detection of inducible clindamycin resistance to) phenotype among MRSA from Libya was reported and of the 128 MRSA isolates collected 24.2% were resistant to clindamycin, 63.2% isolates were resistant to erythromycin and 12 isolates (9.3%) exhibited the MLSBi phenotype [14]. The authors also emphasised that clindamycin could still be used to treat MRSA infection in Libyan hospitals. The findings from the present study suggest that sensitivity to clindamycin should be confirmed. Ciprofloxacin had a resistance of 0% in the CC-MRSA strains which means that it can be used to treat CC-MRSA infections successfully. Gentamicin resistance was 5% in CC-MRSA and 16% for OPH-MRSA strains which indicates that gentamicin could also be used to successfully to treat CC-MRSA and OPH-MRSA infections. These results agree with other studies in Libya by Shebani et al. [15]. | |
| There are no established standard international interpretation criteria for zone size for fusidic acid. Early studies on zone size interpretation indicated that the range ≤ 19 ≥ 21 mm as susceptible [16,17]. Interpretive zone diameter breakpoints for S. aureus according to the Swedish Reference Group for Antibiotics (SRGA) for fusidic acid using 50 μg disc were sensitive ≥ 30 mm and resistant ≤ 26 mm [18]. According to these criteria all the MRSA isolates were resistant. The latest BSAC 2013 version guidelines indicate that a zone size of 26-30 mm should be considered as susceptible. In this study BSAC–version 2013 guidelines [11] were used for zone size interpretation for fusidic acid which is not stated in the CLSI guidelines. Even if interpretation of zone size was based on the previous investigators only 12% of IPHA-MRSA, 1.6% of OPHA-MRSA and 0% of CC-MRSA strains were susceptible which still shows a high rate of fusidic acid resistance. Resistance was confirmed by determination of MIC. The results confirmed the disk diffusion results and showed that most of the resistance was low level and that only 7 of the strains, all from inpatients or outpatients had high level resistance. Such resistance may be due to clonal spread of resistant strains as has been shown in Europe [19] and Taiwan [20]. Fusidic acid has been used widely in Libyan healthcare and community facilities for many years and is still currently used as a topical treatment for skin and soft tissue infection. In particular it is widely used and freely available to the general public and widely used for even minor skin infections. High usage of fusidic acid has been associated with increased levels of resistance elsewhere [21,22]. | |
| These results also demonstrated high rates of multi-resistant strains of MRSA in healthcare facilities. If MRSA are not properly identified, clinicians are forced to use different types of antimicrobials where many would have no beneficial effect on the patient and increase the pool of multi-drug resistant organisms such as MRSA. The availability of nearly all antimicrobials on an over-the counter basis in Libyan community settings in particular, the absence of prescription control policies or guidelines further complicates the problem. Further investigation to examine the presence of resistance genetic determinants (i.e., Fus B, Fus C and FusE; [23]) and mutations in Fus A are required. A surveillance study has shown evidence of wider mis-use of antimicrobials in Libyan healthcare and community facilities and will be reported elsewhere. | |
References
- Tenover FC, Vaughn RR, McDougal LK, Fosheim GE, Jr. McGowan (2007) Multiple-locus Variable-Number Tandem-Repeat Assay Analysis of Methicillin-Resistant Staphylococcus aureus strains. J ClinMicrobiol 45: 2215-2219.
- Harmsen D, Claus H, Witte W, Rothanger J, Turnwald D, et al. (2003) Typing of Methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J ClinMicrobiol 41: 5442-8.
- El-Ghodban A, Ghenghesh KS, Márialigeti K, Esahli H, Tawil A (2006) PCR detection of toxic shock syndrome toxin of Staphylococcus aureus from Tripoli, Libya.J Med Microbiol 55: 179-182.
- Zorgani A, Shawerf O, Tawil K, El-Turki E, Ghenghesh K (2009) Inducible Clindamycin Resistance among Staphylococci Isolated from Burn Patients.Libyan J Med 4: 104-106.
- Buzaid N, Elzouki AN, Taher I, Ghenghesh KS (2011) Methicillin-resistant Staphylococcus aureus (MRSA) in a tertiary surgical and trauma hospital in Benghazi, Libya.J Infect Dev Ctries 5: 723-726.
- Zarmouh MM, Khan AKS, Nazeerullah R (2012) Studies on Methicillin resistant Staphylococcus aureus (MRSA) prevalence rate in Miserata, Libya. Ind J Nat Sci 10: 734-740.
- Ahmed MO, Elramalli AK, Amri SG, Abuzweda AR, Abouzeed YM (2012) Isolation and screening of methicillin-resistant Staphylococcus aureus from health care workers in Libyan hospitals.East Mediterr Health J 18: 37-42.
- Zorgani A, Elahmer O, Franka E, Grera A, Abudher A, et al. (2009) Detection of meticillin-resistant Staphylococcus aureus among healthcare workers in Libyan hospitals.J Hosp Infect 73: 91-92.
- Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method.Am J ClinPathol 45: 493-496.
- Anon. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; publication M100-S23, Clinical and Laboratory Standards Institute, Wayne, PA, USA 2013.
- Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, et al. (2005) The role of nasal carriage in Staphylococcus aureus infections.Lancet Infect Dis 5: 751-762.
- Henwood CJ, Livermore DM, James D, Warner M; Pseudomonas Study Group (2001) Antimicrobial susceptibility of Pseudomonas aeruginosa: results of a UK survey and evaluation of the British Society for Antimicrobial Chemotherapy disc susceptibility test.J AntimicrobChemother 47: 789-799.
- de Souza V, MacFarlane A Murphy AW, Hanaho Be, Barber A, et al.(2006) Qualitative study of factors influencing antimicrobial prescribing by non-consultant hospital doctors. J AntimicrobChemother 58: 840-843.
- Ahmed MO, Alghazali MH, Abuzweda AR, Amri SG (2010) Detection of inducible Clindamycin resistance (MLSBi) among Methicillin-resistant Staphylococcus aureus (MRSA) from Libya. Libyan J Med 5: 4636. DOI:10.3402/ljm.v5i0.4636.
- Shebani OA, Muftah, MI, Daw MA (2004) Clinical relevance of Methicillin-resistant Staphylococcus aureus isolated from surgical site infections. Jordan Med J 3:46-49.
- Toma E, Barriault D (1995) Antimicrobial activity of fusidic acid and disk diffusion susceptibility testing criteria for gram-positive cocci.J ClinMicrobiol 33: 1712-1715.
- Traub WH, Kleber I (1974) Interpretation of diffusion susceptibility data obtained with 10-mug fucidin (sodium fusidate) disks against clinical isolates of Staphylococcus aureus.Chemotherapy 20: 92-96.
- Kronvall G (2000) MIC determination of fusidic acid and of ciprofloxacin using multidisk diffusion tests.ClinMicrobiol Infect 6: 483-489.
- McLaws FB, Larsen AR, Skov RL, Chopra I, O'Neill AJ (2011) Distribution of fusidic acid resistance determinants in methicillin-resistant Staphylococcus aureus.Antimicrob Agents Chemother 55: 1173-1176.
- Chen HJ, Hung WC, Tseng SP, Tsai JC, Hsueh PR, et al. (2010) Fusidic acid resistance determinants in Staphylococcus aureus clinical isolates.Antimicrob Agents Chemother 54: 4985-4991.
- Ravenscroft JC, Layton A, Barnham M (2000) Observations on high levels of fusidic acid resistant Staphylococcus aureus in Harrogate, North Yorkshire, UK.ClinExpDermatol 25: 327-330.
- Shah M, Mohanraj M (2003) High levels of fusidic acid-resistant Staphylococcus aureus in dermatology patients.Br J Dermatol 148: 1018-1020.
- Lannergård J, Norström T, Hughes D (2009) Genetic determinants of resistance to fusidic acid among clinical bacteremia isolates of Staphylococcus aureus.Antimicrob Agents Chemother 53: 2059-2065.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14579
- [From(publication date):
December-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10078
- PDF downloads : 4501
