Research Article Open Access
Anti-Inflammatory and Regenerative Potential of Probiotics to Combat Inflammatory Bowel Disease (IBD)
Rintu Das, Bhaswati Trafadar, Prosenjit Das, Srabani Kar, Shinjini Mitra, Garima Hore, Silpak Biswas and Ena Ray Banerjee*Department of Zoology, Immunology and Regenerative Medicine Research Laboratory, University of Calcutta, 35, Ballygunge Circular Road, Kolkata- 700019, West Bengal, India
- Corresponding Author:
- Ena Ray Banerjee
University of Calcutta, Zoology
35 Ballygunge Circular Road, Kolkata, West Bengal 700019, India
Tel: 91-33-24615445
Fax: 91-33-24614849
E-mail: enarb1@gmail.com
Received date:: May 06 2015; Accepted date:: May 20, 2015; Published date:: May 28, 2015
Citation: Das R, Trafadar B, Das P, Kar S, Mitra S, et al. (2015) Anti-Inflammatory and Regenerative Potential of Probiotics to Combat Inflammatory Bowel Disease (IBD). J Biotechnol Biomater 5:181. doi:10.4172/2155-952X.1000181
Copyright: © 2015 Das R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Inflammatory Bowel Diseases (IBD) is a chronic disorder of the gastrointestinal (GI) tract characterized by body weight loss, hemorrhage, lower abdominal pain and diarrhea. Indiscriminate use of antibiotics and modern life style disrupts microbial ecosystem of the human GI tract which leads to IBD. No disease modifying treatment exists for IBD which are both inflammatory and degenerative in nature. This study aimed at evaluating anti-inflammatory and pro-regenerative potential of probiotics in preclinical IBD. For in vitro study, inflammation was induced on RAW264.7 cell treated with 3% DSS (w/v), 1μg/ml LPS and 600ng/ml PMA. Novel combinations of probiotics were administrated on inflamed cell to measure their anti-inflammatory role. Cell viability and NO release assay were also done. For in vivo study, composite IBD phenotype was developed by 3% DSS by oral gavage (40μl) for 7 days and our probiotic combo was administered over 7 days (at 108 CFU/200μl) of DSS induction in Balb/c mice. Body weight, NO and ascorbic acid production by cells harvested from key lymphoid organs post-mortem, and histological studies were done to assess inflammation. We found cell viability increased from 30% to 92.5 % and NO concentration was reduced 2.22 fold after administration of probiotics. Mouse body weight (BW) reduced by 30.2% and 35.4% on 7th and 14th day respectively following DSS induction. Oral probiotics increased mouse BW in by 47%. NO concentration decreased 1.19 fold and 2.17 fold in colon and spleen tissue and ascorbic acid concentration increased 1.82 fold and 5.50 fold in colon and spleen tissue respectively. Clonogenic potential of intestine and spleen was decreased 2.38 fold and 2.34 fold in DSS treated mouse on 7th and 14th day respectively but it was increased 1.29 fold and 1.36 fold on 7th and 14th day respectively after probiotics administration in DSS treated mice. This indicates greater clonogenicity in intestine and spleen of probiotic treated mice as opposed to the reduced colony count of progenitor’s post-DSS only mice. Overall, orally administered probiotics showed anti-inflammatory and pro-regenerative action to revive the cells and tissues of DSS treated mice in which all symptoms of IBD were detected earlier. This study validates the use of combination of probiotic microbial strains supplemented in food (curd, yoghurt) as nutraceuticals and possible therapeutic as well as prophylactic to combat inflammation and degeneration in IBD.
Keywords
Inflammatory bowel disease; Inflammation; Regeneration; Probiotics; Nutraceutical; Therapeutics
Introduction
Inflammatory bowel diseases (IBDs), principally ulcerative colitis (UC) and Crohn’s disease (CD), are inflammatory disorders of the gastrointestinal tract (GI) caused by multiple genetic and environmental factors [1,2]. IBD is characterized by chronic, uncontrolled inflammation in the gastrointestinal tract, affecting millions of people worldwide, with a corresponding economic burden [3-7]. Crohn’s disease inflammation occurs anywhere in the gastrointestinal tract, whereas ulcerative colitis inflammation starts in the rectum and is restricted to the colon [8,9]. The clinical features of CD include pain, diarrhea, and narrowing of the gut lumen which causes strictures and fistulization of the skin that lead to bowel obstruction [10]. The clinical features of UC include an increasing loss of peristaltic function; diarrhea, blood loss, and stool with blood stain [11]. The peak age of onset for IBD is 15-30 years, but it may occur at any age. About 10% of cases have their onset before the age of 18, with CD being more frequent in girls, while ulcerative colitis is more common in boys [12]. The disease is one of the most prevalent gastrointestinal disease burdens in Western Countries and it has become more widespread; the incidence has been reported in all age groups including early childhood [13].
The pathogenesis of these diseases has not been fully elucidated, however, it is generally accepted that disease develops in genetically susceptible individuals that have hyper-immune responsiveness to their intestinal microbiota [14-16]. In IBD patients many pro-inflammatory cytokines, such as Tumor Necrosis Factor (TNF), interferon gamma (INFγ), interleukin (IL)-6, IL-17 and members of the IL-12 family, are produced in excess in response to the translocated intestinal microbiota and these responses have been shown to be instrumental to the progression of disease [17-19]. TNF is a pleiotropic cytokine, considered to be a master regulator of cytokine production. This cytokine is elevated in both the serum and mucosa of IBD patients [20-23]. The current, and arguably one of the most effective treatments for CD, is the use of TNF functional inhibitor drugs; however, this treatment can cause adverse reactions [24-27]. The bacterial genus that is significantly higher in adult and pediatric IBD patients is the Escherichia-Shigella group [28,29].
Various models of experimental IBD have been developed to investigate pathogenesis and to improve treatment options. Most commonly, experimental colitis is induced by the heparin-like polysaccharide DSS (Dextran Sodium Sulfate); this model is simple and affords a high degree of uniformity and reproducibility [30,31]. DSS is often used to induce a form of mouse colitis that mimics the clinical and histological features of IBDs that have characteristics of UC. The typical features of colitis appear on day 3 and are maximally expressed by day 7 [32]. Feeding mice for several days with DSS polymers induces a very reproducible acute colitis characterized by bloody diarrhea, ulcerations and infiltrations with granulocytes [33,34]. It is believed that DSS is directly toxic to gut epithelial cells of the basal crypts and therefore affects the integrity of the mucosal barrier [35]. The DSS model has also been shown to be suitable to study epithelial repair mechanisms [36].
Till date no definitive therapies are available for this inflammatory disorder. Conventional treatments for IBD rely on salazosulfamide, glucocorticoids and immunosuppressive agents. However, these therapies are not always effective and are often complicated by significant adverse effects, indicating the need for new therapeutics with lower side-effect risk [37]. Therefore, there is an increasing interest in developing new therapeutic approaches such as probiotics. Probiotics are defined as ‘live microorganisms which, when administered in adequate amounts, confer a health benefit on the host’ according to the consensus of group of scientists convened in 2001 by the Food and Agriculture Organization of the United Nations (FAO) [38]. Certain numbers of living microorganisms provide a desired and beneficial effect on human health beyond inherent basic nutrition. It has long been acknowledged the potential benefit of probiotics in health maintenance and disease prevention. Indiscriminate use of antibiotics and modern life style disrupts microbial ecosystem of the human GI tract which leads to IBD. Recently, some kinds of probiotics have been applied and shown to be significantly effective to IBD [39]. The bacteria most commonly associated with probiotic activity are Lactobacilli, Bifidobacteria, and Streptococci, but other, non-pathogenic bacteria (e.g. some strains of E. coli) and nonbacterial organisms (e.g. the yeast Saccharomyces boulardii) have also been used [40]. Enteric bacteria and their products have been found within the inflamed mucosa of patients with Crohn’s disease [41]. The composition of the enteric flora is altered in patients with IBD, increased numbers of aggressive bacteria, such as Bacteroides, adherent/invasive Escherichia coli, Enterococci, and decreased numbers of protective Lactobacilli and Bifidobacteria have been observed [42]. No disease modifying treatment exists for IBD or related syndromes of the GI tract which are both inflammatory and degenerative in nature [43]. There has been growing interest in using probiotics as an adjunct to standard anti-inflammatory and immune suppressing therapy.
The aim of this study was to evaluate the anti-inflammatory and pro-regenerative potential of combined probiotic microbial strains supplemented in food (like curd, yoghurt) as nutraceuticals and possible therapeutic as well as prophylactic to combat inflammation and degeneration in IBD.
Materials and Methods
Mice
Balb/c mice (6 weeks of age) were purchased from NIN, Hyderabad, India. All animals were maintained under SPF (Specific Pathogen Free) condition in the animal facility of the University of Calcutta following strict guidelines laid down by CPCSEA. Animals were randomly distributed to various groups based on their gender and body weight. They were divided into four experimental groups with three animals in control (Group-1) and four animals each in DSS treated (Group-2), DSS+Probiotics treated (Group-3), DSS+Fisetin+Probiotics treated (Group-4) groups, housed in clean filter top cages under standard conditions in a 12 hr dark/12 hr light cycle and fed with standard mouse chow.
Preparation of probiotics
Milk was boiled for 1-2 minutes, cooled to 42º- 45ºC. Little powder culture of probiotics (ABT Culture, supplied from K.C. Das) was added to the cooled milk in sterilized cotton plugged conical flask, and incubated at 42º- 45ºC in incubator for 4-5 hours.
Treatment of mice
Treatment was performed with adult Balb/c mice. Control group was devoid of any experimental treatment, Group-2 was administered with 40μl of 3% DSS Sicco Pvt. Ltd., India (3gm DSS in 100ml autoclaved water) by oral gavage on day 0 and day 5 to induce colitis. Group 3 was treated with 3% DSS similar to Group-2 for induction of colitis, in addition 200μl of whey water containing 1x108 CFU (approx.) of probiotics (ABT) was administered on day 5, day 7 and day 10 of treatment to ameliorate the inflammation caused by DSS treatment. Control group was administered equal volume of autoclaved water by gavage at the same point in time when experimental groups received intervention. Group 2 was also administered buffer at the same points in time when Group 3 was administered probiotics. All 3 groups of mice were sacrificed on day 14. Organ such as bone marrow, intestine, colon, spleen, Peyer’s patch, lung, kidney and peripheral blood was collected.
Assessment of daily weight of mice
Weight of each experimental group was taken daily with the help of a weighing balance and compared with control.
Total cell count (TC) by using haemocytometer
Tissues-Colon, Intestine, Lung, Spleen, Blood, Bone marrow. After dissection, the tissues except blood were kept in IMDM (Iscove’s Modified Dulbecco’s Medium). Blood collected was kept in RBC lysis buffer and mixed well. The sample was centrifuged and then the supernatant was discarded. PBS was added in its place. For bone marrow, the femur was taken into the Biosafety cabinet and flushed with PBS until the bone turns white. The cell suspension was then kept in PBS and centrifuged at 5000 rpm for 10 minutes. Total cell count was measured by taking 10μl of sample+10μl of Trypan blue dye, mixing it well and loading it into haemocytometer chambers for cell count to determine cell viability and total number of cells present.
Histology
Histopathological assessment was done for control and treated groups. Tissues taken were colon, intestine, spleen, lung and Peyer’s patch. Briefly, after dissection, the tissues were immediately fixed in 10% neutral buffered formalin solution (1ml formalin stock solution+9 ml 1%PBS), hematoxylin and eosin staining was done.
Colony Forming Unit (CFU-c) assay- assessment of clonogenic potential of tissue resident progenitors
For quantification of committed progenitors of all lineages, Colony Forming Units in culture (CFU-c) were performed using standard protocol. Briefly, after dissection, the tissues (Spleen, Lung, and Intestine) were immediately kept in IMDM (Himedia, India). For Bone marrow samples, the bone was taken into the Biosafety cabinet and flushed with PBS until the bone turns white. The cell suspension was then kept in IMDM. The tissues were minced and the cell suspension was collected with the help of a nylon mesh. Spleen and lung samples were centrifuged at 5000rpm for 5 mins. Bone marrow was centrifuged at 5000rpm for 10 mins. Cell count was taken. Number of cells per well taken was 1x106. For bone marrow and peripheral blood samples, 1x106 cells were taken per well. CFU-c media was prepared using IMDM, supplemented with 30% FBS (Himedia, India), 10% BSA (Biosera), 1%Penicillin-Streptomycin (Himedia, India) and 5ng/ml murine SCF (Biovision). Lastly, 1.5% methylcellulose (in powdered form purchased from Himedia, India) was added into the concoction.1ml CFU-c assay media and 500μl cell suspension was plated in each 24 well cell culture plate. The 24 well plate (NEST Biotech Co. Ltd.) was kept in CO2 incubator at5% CO2 and 37°C for 14 days. All colony types were counted using Floid Cell Imaging Station (Life Technologies, India) and pooled to get total CFU-c.
Biochemical assays
Cell Viability Assay (MTT assay) after DSS and probiotics induction: Seeded RAW 264.7 cells in two 96-well plates (cell concentration 40X104 and dilutions). Incubated the cells at 37°C in CO2 incubator for 24 hours and 48 hours respectively. After 24 hours and 48 hours incubation, 10μl of 5mg/ml MTT added to each well. Again incubated the 96 well plates at 37°C in CO2 incubator for 3 hours. The media was removed carefully from each well and 100 μl DMSO was added to each well. After 15 minutes, the OD was measured at 570 nm (background wavelength is 620 nm). This was done to check the viability of the cells. The cell viability assay after addition of probiotics was done by adding10 μl of probiotic (ABT culture) whey water (CFU-1x10^8) of 4 hours of incubation (Pro 1) and another batch of overnight incubation (Pro 2) to RAW cells (concentration-5x10^4) and then MTT assay was performed for 1 hour, 20 hours of incubation as mentioned before. DSS (3%, 0.3%) were used to induce inflammation in separate wells and 10 μl of probiotic whey water (CFU-1x10^8) was added as anti-inflammatory agent and cell viability was assessed.
Nitric oxide (NO) estimation assay: Samples were taken from Colon and Spleen from Control, DSS treated and DSS+Probiotics treated mice. The sample was first centrifuged at 3000 rpm for 10 minutes, and then the supernatant were collected in one tube. 200 μl sample + 30 μl NaOH (10%) + 300 μl (Tris-HCL) + 470 μl Griess reagent added. Incubated in dark for 30 minutes. The OD was read at 540 nm.
Nitric Oxide assay was also done in RAW 264.7 cells before and after addition of probiotics. The Nitric Oxide released by the RAW cells into the medium is converted to several nitrogen derivatives, from which only nitrite is stable, being easily measured by Griess reagents. After 1 hour and 20 hours DSS (0.3%, 3%), 100 μL of culture medium supernatant was mixed with the same volume of Griess reagent, during 10 min, at room temperature. The nitrite produced was determined by measuring the optical density at 540 nm, in a microplate reader (Shimadzu). Similarly, 10 μl of probiotic whey water (CFU-1x10^8) of 4 hours of incubation and another batch of overnight incubated probiotics was added to each experimental set and NO assay was performed.
Ascorbic acid estimation assay: Ascorbic acid has been considered to act as a scavenger of free radicals generated in the cell after oxidative stress. Ascorbic acid estimation is done with tissue samples to measure the amount of oxidative stress (ROS). The sample was first centrifuged at 3000rpm for 10minutes, and then the supernatant were collected in one tube.100μl sample + 10 μl Thio urea + 10 μl DNPH was added (DNPH should be made 1 day before the experiment and it should be kept in a cool, dark place). It was shaken well and heated at boiling water bath at 85°C for 20minutes. Immediately placed in ice and 200 μl 85% sulfuric acid was added. The OD was read at 515nm.
Statistical analysis
The comparisons between two groups were performed using unpaired two-tailed Student's t-tests. The statistical analysis was performed with GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA). A P value less than 0.05 were considered statistically significant. The significant results are marked with “ ” for DSS and “
” for DSS and “ ” for probiotics.
” for probiotics.
Results
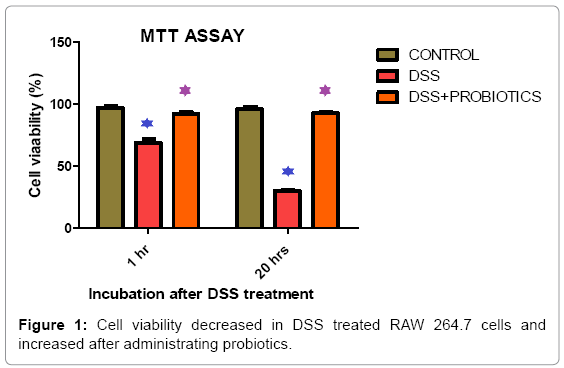
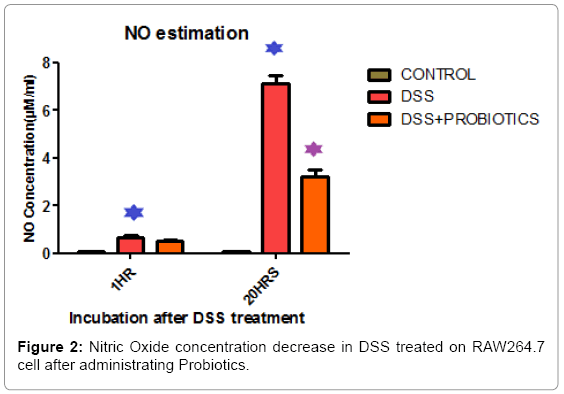
We found cell viability decreased by 24.4% and 67.22% respectively in 1 hour and 20 hours of incubation in DSS treated RAW 264.7 cells. It increased by 24.08% and 34.05% after probiotics treatment under same experimental condition (Figure 1). In vitro screening of inflammation and NO production was done by DSS on Raw 264.7 cell line. Figure 2 explaining the fact that, Nitric Oxide (NO) concentration in DSS induced RAW264.7 cell was increased 7.82 fold and 85.71 fold in 1 hour and 20 hour respectively. After probiotics administration, NO concentration was decreased 1.28 fold and 2.22 fold respectively (Figure 2).
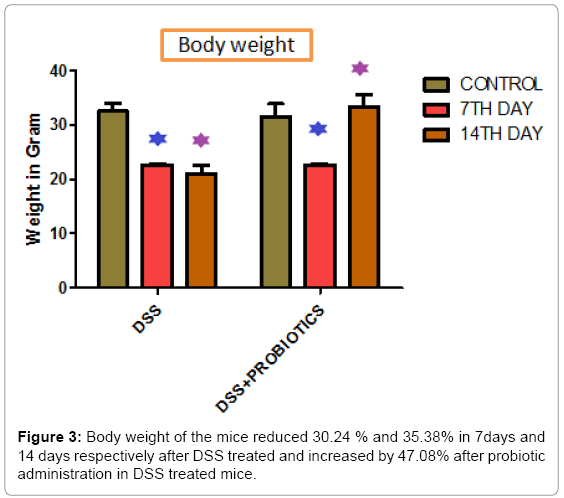
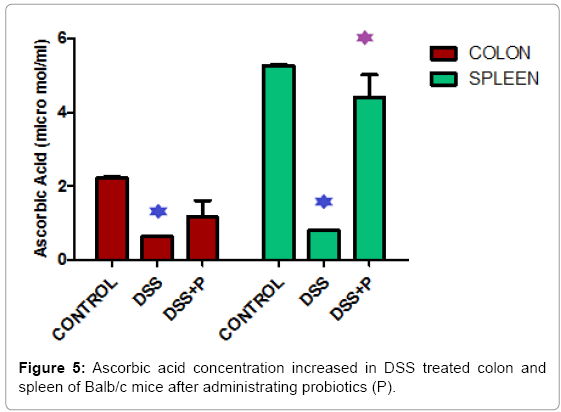
Body weight of the mice reduced 30.24 % and 35.38% in respect to control in 7 days and 14 days respectively after DSS treated and increased by 47.08% after probiotic administration in DSS treated mice (Figure 3). Figure 4 demonstrated that, Nitric Oxide concentration increased 1.36 fold in colon and 2.05 fold in spleen tissue in DSS challenged mice. But after probiotics administration in DSS treated mice, NO concentration was decreased 1.19 fold and 2.17 fold in colon and spleen respectively. In another assay, ascorbic acid concentration decreased 3.57 fold and 6.66 fold in colon and spleen tissue after treatment of DSS and it increased 1.82 fold and 5.5 fold after probiotics administration (Figure 5).
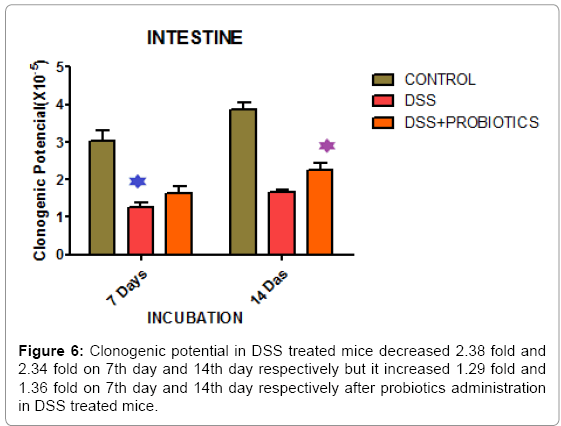
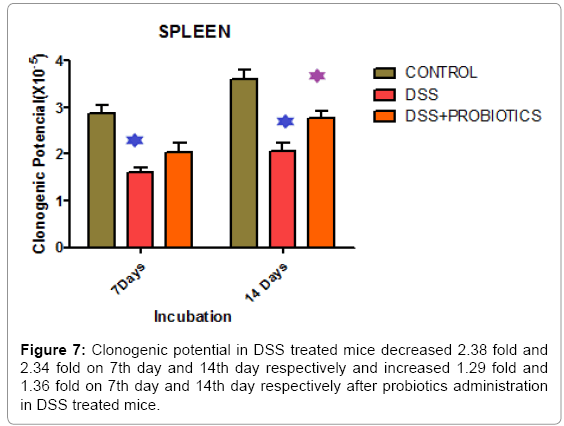
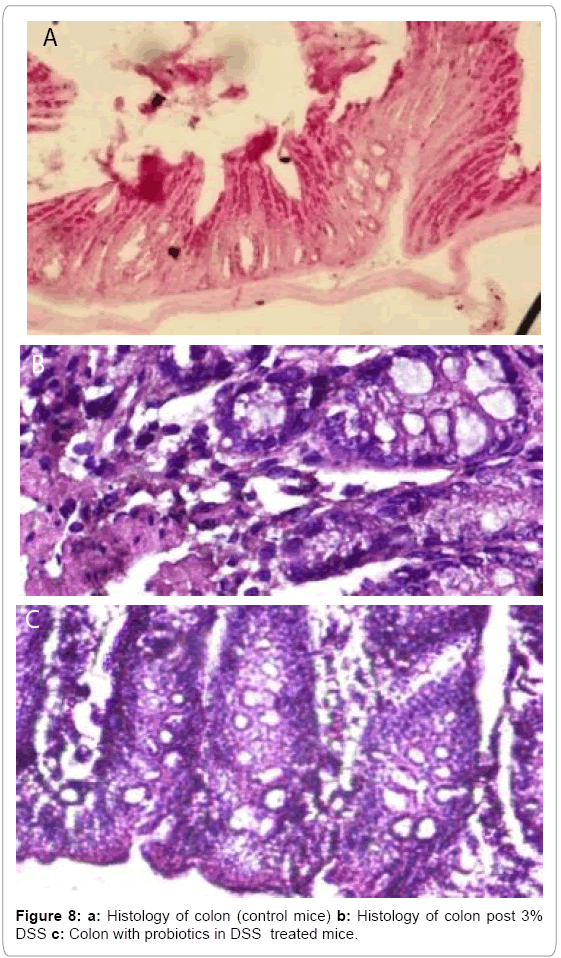
In our study, we also found that clonogenic potential on intestine decreased 2.38 fold and 2.34 fold on 7th day and 14th day respectively but after administration of probiotics the clonogenic potential increased 1.29 fold and 1.36 fold on 7th day and 14th day respectively which is well demonstrated in Figure 6. In another experiment, Figure 7 describing that, clonogenic potential of spleen decreased 2.38 fold and 2.34 fold on 7th day and 14th day respectively but it increased 1.29 fold and 1.36 fold on 7th day and 14th day respectively after probiotics administration. Tissue histology of colon showed regenerative properties of our novel combinatorial probiotics when compared with the control mice, DSS treated mice; and with probiotics administered in DSS treated mice (Figure 8).
Discussion
The human gut is the largest reservoir of microbes in the body. Many studies have indicated that the gut microbiota plays an active and integral role in maintaining host health [43,44]. In humans, studies are confounded by environmental and behavioral variables (e.g., smoking, antibiotic use), thus model animal studies are best suited to examine the interactions between the gut microbiota and disease in order to elucidate the potential role these microbes play in IBD pathogenesis. The conventional approach to managing active inflammatory bowel diseases (IBD) has been based on progressive intensification of therapy as disease worsens [45]. For at least two decades, inflammatory bowel disease has been the focus of intense attention at the basic science, translational, and clinical level.
ABT, is a combination culture with the individual components in fixed ratios. S. thermophilus is a component. The culture of probiotics used contains multiple microbes of which one is indeed of species thermophilus which explains the thermoresistance and activation temperature being high. The crux of the finding is possibly an inducer (soluble protein) from this novel combination culture involving quorum sensing. This is however beyond the scope of this paper. I maintain that the data was generated out of 23 independent experiments in multiple laboratories, in independent and blinded experiments. Some research work may be cited to support that probiotics retain their biological activity at high temperature [46,47].
In this research study, we found cell viability decreased in 1 hour and 20 hours of incubation in DSS treated RAW 264.7 cells. Interestingly, the cell viability found increased after probiotics treatment under same experimental condition and this incident was explained in Figure 1. The combo culture used is a novel combination of probiotics and shall remain confidential pending patent grant. Suffice it to say at this point and in this communication, the RAW macrophages were incubated with DSS in the mentioned dosage and the data by MTT assay is self-explanatory as to the cytotoxicity generated and the amount reversed by probiotics treatment. DSS did not kill the cells within an hour nor the probiotics as the cells were incubated with DSS and then washed to remove DSS but not the damage done. So presence of the combination community of probiotics purportedly secreted factors or initiated healing mechanism which reversed DSS induced damage and post proliferation, cells were healthy. It may be noted that MTT assay measures NADPH dependent cellular oxidoreductase enzymes that reflect cell viability. After 5 hours, number of viable cells increased by factor(s) induced by the presence of probiotics. Around 10% proliferation is seen in our passages (3-5) of RAW macrophages. It follows that such recovery seen in number of viable cells is plausible. It may be noted that when incubated for 12h (overnight) with probiotics, number of viable cells by MTT assay was actually less than when done for 1h.The mechanism of such recovery is under study and beyond the scope of this paper. Probiotics do not have any toxic effect on the growth of the cells (data not presented). Indeed, they have a positive effect on the cells post prolonged stress by DSS. Some research work on effects of DSS ON raw macrophages may be cited in this connection [48-50].
In another experimental assay, in vitro screening of inflammation and NO production was done by DSS on Raw 264.7 cell line. Figure 2 describing that Nitric Oxide (NO) concentration in DSS induced RAW264.7 cell was increased 7.82 fold and 85.71 fold in 1 hour and 20 hour respectively and after probiotics administration, NO concentration was decreased accordingly.
In this connection it may be mentioned that a number of research groups have employed RAW 264.7 macrophage cell line for preliminary screening of anti-inflmamatory activities by induction of inflammation by LPS, so we used the same strategy to determine dose and intensity of various nuances of inflammation [51,52].
When treated with DSS, body weight of the mice reduced significantly in respect to the control animal in 7days and 14 days. Interestingly, we found that, the body weight increased by 47.08% after probiotic administration in DSS treated mice (Figure 3). Figure 4 explained that, the Nitric Oxide (NO) concentration increased 1.36 fold in colon and 2.05 fold in spleen tissue in DSS treated mice and after probiotics administration in DSS challenged mice, NO concentration decreased 1.19 fold and 2.17 fold in colon and spleen respectively. In another significant assay, ascorbic acid concentration decreased in colon and spleen tissue after treatment with DSS and the concentration increased significantly after probiotics administration (Figure 5). Ascorbic acid’s antioxidant buffering capacity decreases the capacity of the inflamed mucosa to prevent oxidative tissue damage and hinder recovery of the inflamed mucosa. Probiotics have anti-inflammatory properties related to their inhibition of NO production, increased ascorbic acid concentration & cell viability in IBD.
We also found that clonogenic potential of intestine decreased in DSS treated mice but it increased after probiotics administration (Figure 6). Much of the recent progress in the understanding of mucosal immunity has been achieved by the study of new experimental animal models of intestinal inflammation [53,54]. These models are valuable tools for studying many important disease aspects that are difficult to address in humans, such as the pathophysiological mechanisms in early phases of colitis and the effect of emerging therapeutic strategies. In another experimental study, we demonstrated that, clonogenic potential of spleen decreased in DSS treated animals and it increased significantly after probiotics administration (Figure 7). Therefore, this study indicates greater clonogenicity in intestine and spleen of probiotic treated mice as opposed to the reduced colony count of progenitor’s post-DSS only mice.
As more and more sophisticated IBD models become available researchers can exploit the unique potential of each model to ask specific questions. Studies with animal models have improved our understanding of the complex field of human IBD and allowed the molecular dissection of pathophysiological mechanisms that are presumably responsible for disease initiation and progression. Although the etiology of IBD is still unclear, promising biological therapeutic strategies on the basis of this improved mechanistic understanding of the gut immune system are emerging.
Miyazawa et al. [48] showed that DSS caused disruption of biological mechanisms (such as inhibitory effects on reverse transcriptase activities that affect major cellular functions), competing with poly (U) to this end [55]. Previously, it was shown that dextran sulfate inhibited ribonuclease action [56]. Other natural and synthetic polyanionic polymers play important roles in establishing the association of mRNA with ribosomes and can disturb mRNA translation [56]. Other studies have shown that DSS induced significant macrophage infiltration into the epithelium of the colon [57]. Previously, it has been shown that short chain fatty acids, like butyrate, attenuate inflammation in DSS-induced colitis [58-60]. During DSS treatment, inflammation is enhanced. Colonic inflammation is also characterized by severe lesions throughout the mucosa, alteration of epithelial structure, highlevel neutrophil and lymphocyte infiltration into the mucosal and submucosal areas, and loss of crypts.
Probiotics are preparations utilizing live bacteria that can be beneficial to human health. Several reports have shown the efficacy of various probiotic bacteria for IBD [61-63]. Probiotic therapy can be improved through combination with a prebiotic (a nondigestible oligosaccharide that is absorbed in the upper gut). Previous works demonstrated that probiotic microorganisms are able to induce a gut mucosal immune response which requires the bacteria to interact with the epithelial and immune cells in the gut to induce the network of signals involved in an immune response [64]. In a double-blinded randomized controlled trial, Furrie et al. [65] demonstrated that the administration of a synbiotic, for a period of one month to patients with active UC, improved the full clinical appearance of chronic inflammation. The proinflammatory cytokines TNF-α and IL-1α were significantly reduced after treatment [66-68]. Our study demonstrated that, there is obviously considerable potential for the benefits of probiotics over a wide range of clinical conditions such as ulcerative colitis and Crohn’s disease.
Among the various model of colitis experimental colitis is induced by the heparin-like polysaccharide DSS; this model is simple and affords a high degree of uniformity and reproducibility of most lesions in the distal colon [69].
By first interfering with intestinal barrier function, and then stimulating local inflammation, DSS is often used to induce a form of mouse colitis that mimics the clinical and histological features of IBDs that have characteristics of Ulcerative colitis. Expression of proinflammatory cytokines and chemokines (IL-1, IL-6, KC, TNF-α, and Interferon-γ) are upregulated, whereas synthesis of anti-inflammatory cytokines, such as IL-10, is downregulated.
Miyazawa et al. [60] showed that DSS caused disruption of biological mechanisms (such as inhibitory effects on reverse transcriptase activities that affect major cellular functions), competing with poly (U) to this end. Previously, it was shown that dextran sulfate inhibited ribonuclease action. Other natural and synthetic polyanionic polymers play important roles in establishing the association of mRNA with ribosomes and can disturb mRNA translation. But the mechanism of how DSS penetrates the cell is unknown as it could be through passive or active uptake by the cell via a specific receptor, or DSS could penetrate the cell after complexation with another molecular form (such as polycationic forms). Other studies have shown that DSS induced significant macrophage infiltration into the epithelium of the colon.
More detailed mechanistic studies on the effectiveness of probiotics in IBD are necessary to determine their potential beneficial effects. Therefore, more clinical trials with the use of appropriate molecular tools are necessary to determine which main outcomes and additional immune- and inflammation-associated variables are clearly influenced, and particularly the cause of these changes in the development of IBD. Major clinical trials should also study the mechanisms of action of probiotics using new molecular tools such as the study of the microbiota changes using massive parallel sequencing (MPS), metabolomics, transcriptomics, and proteomics analyses of biopsies.
In in vitro data, DSS was washed away from the cell culture before introduction of probiotics and in in vivo experiments, DSS was discontinued after mice were introduced to probiotics which was continued till the day of sacrifice so the obvious conclusion will be that pathophysiology of colitis induced due to various factors- by interfering with the barrier functions, by breaching commensal biofilm and inducing inflammation by also modulating macrophage functions, is “healed” or the healing process is initiated. Probiotics never see the DSS so that question is unnecessary and irrelevant. The probiotics themselves through some innate interaction or some paracrine mechanism initiatiated by their inducible factors reverse the tissue damage and cell destruction in the inflammatory and degenerative situation created by DSS treatment [70].
In conclusion, our study will help researcher and clinicians to understand the beneficial role of probiotics or living microorganisms in the diseased models and its promising therapeutic effect against inflammatory bowel diseases. In the future, biological therapies for both CD and UC will be used selectively based on personalised benefit/risk assessment and will be optimised throughout the course of treatment. Choice of therapy will depend on individual patient profiles, determined through reliable biomarkers and tissue signatures. The specific knowledge of the mechanisms of action of probiotics would be a helpful tool to design an efficient and specific therapy to improve the specific disease symptoms in IBD.
Contribution of Authors
PD did most of the preliminary experiments of the in vitro model and BT performed most of the preliminary in vivo preclinical model experiments. CFU data was generated by GH while SK performed all microbiological experiments. SM collated all preliminary data and perfomed statistical analyses and created the graphical representations. RD collated and performed detailed analyses of all data and performed all confirmatory experiments of both in vivo and in vitro experiments. SB contributed intellectually and participated in writing the manuscript. ERB conceptualized the project, designed all experiments and analyzed the data and wrote this manuscript.
Acknowledgement
The authors wish to acknowledge West Bengal Department of Science and technology for the research grant that funded the entire research. Fellowship for BT and PD were paid from a research grant by Department of Biotechnology, Govt. of India. RD was paid fellowship from WB DST grant, SK from a grant from SERB, GOI, GH was paid fellowship from a WB DBT grant, while SB was paid fellowship from a grant from the Tata Education Trust. ERB is the Principal Investigator on all the above grants except UGC SM’s who is a NET scholar. The authors also acknowledge K.C. Das for providing some of the probiotic strains.
References
- Lowe AM, Roy PO, B-Poulin M, Michel P, Bitton A, et al. (2009) Epidemiology of Crohn's disease in Québec, Canada. Inflamm Bowel Dis 15: 429-435.
- Brant SR (2011) Update on the heritability of inflammatory bowel disease: the importance of twin studies. Inflamm Bowel Dis 17: 1-5.
- Gunnarsson C, Chen J, Rizzo JA, Ladapo JA, Lofland JH (2012) Direct health care insurer and out-of-pocket expenditures of inflammatory bowel disease: evidence from a US national survey. Dig Dis Sci 57: 3080-3091.
- Heaton PC, Tundia NL, Schmidt N, Wigle PR, Kelton CM (2012) National burden of pediatric hospitalizations for inflammatory bowel disease: results from the 2006 Kids' Inpatient Database. J PediatrGastroenterolNutr 54: 477-485.
- Wan GJ, Kozma CM, Slaton TL, Olson WH, Feagan BG (2014) Inflammatory bowel disease: healthcare costs for patients who are adherent or non-adherent with infliximab therapy. J Med Econ 17: 384-393.
- Park KT, Bass D (2011) Inflammatory bowel disease-attributable costs and cost-effective strategies in the United States: a review. Inflamm Bowel Dis 17: 1603-1609.
- Chami B, Yeung AW, van Vreden C, King NJ, Bao S (2014) The role of CXCR3 in DSS-induced colitis. PLoS One 9: e101622.
- Mulder DJ, Noble AJ, Justinich CJ, Duffin JM (2014) A tale of two diseases: the history of inflammatory bowel disease. J Crohns Colitis 8: 341-348.
- Wijmenga C (2005) Expressing the differences between Crohn disease and ulcerative colitis. PLoS Med 2: e230.
- Loftus EV Jr, Sandborn WJ (2002) Epidemiology of inflammatory bowel disease. GastroenterolClin North Am 31: 1-20.
- Bouma G1, Strober W (2003) The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 3: 521-533.
- Loftus EV Jr (2004) Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 126: 1504-1517.
- Duricova D, Burisch J, Jess T, Gower-Rousseau C, Lakatos PL; ECCO-EpiCom (2014) Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis 8: 1351-1361.
- Xavier RJ1, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427-434.
- Blumberg RS (2009) Inflammation in the intestinal tract: pathogenesis and treatment. Dig Dis 27: 455-464.
- Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347: 417-429.
- Ardizzone S, Bianchi Porro G (2005) Biologic therapy for inflammatory bowel disease. Drugs 65: 2253-2286.
- Liu ZJ, Yadav PK, Su JL, Wang JS, Fei K (2009) Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol 15: 5784-5788.
- MonteleoneI, Pallone F, Monteleone G (2009) Interleukin-23 and Th17 cells in the control of gut inflammation. Mediators Inflamm 2009: 297645.
- Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT (1992) Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 339: 89-91.
- Masuda H, Iwai S, Tanaka T, Hayakawa S (1995) Expression of IL-8, TNF-alpha and IFN-gamma m-RNA in ulcerative colitis, particularly in patients with inactive phase. J Clin Lab Immunol 46: 111-123.
- Reimund JM, Wittersheim C, Dumont S, Muller CD, Baumann R, et al. (1996) Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn's disease. J ClinImmunol 16: 144-150.
- van Deventer SJ (1997) Review article: Chemokine production by intestinal epithelial cells: a therapeutic target in inflammatory bowel disease? Aliment PharmacolTher 11 Suppl 3: 116-120.
- Thukral C, Travassos WJ, Peppercorn MA (2005) The Role of Antibiotics in Inflammatory Bowel Disease. Curr Treat Options Gastroenterol 8: 223-228.
- Colombel JF, Loftus EV Jr, Tremaine WJ, Egan LJ, Harmsen WS, et al. (2004) The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology 126: 19-31.
- Jain VV, Evans T, Peterson MW (2006) Reactivation histoplasmosis after treatment with anti-tumor necrosis factor alpha in a patient from a nonendemic area. Respir Med 100: 1291-1293.
- Wood KL, Hage CA, Knox KS, Kleiman MB, Sannuti A, et al. (2003) Histoplasmosis after treatment with anti-tumor necrosis factor-alpha therapy. Am J RespirCrit Care Med 167: 1279-1282.
- Papa E, Docktor M, Smillie C, Weber S, Preheim SP, et al. (2012) Non-invasive mapping of the gastrointestinal microbiota identifies children with inflammatory bowel disease. PLoS One 7: e39242.
- Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, et al. (2012) Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13: R79.
- Pinho RA, Silveira PC, Silva LA, LuizStreck E, Dal-Pizzol F, et al. (2005)Nacetylcysteine and deferoxamine reduce pulmonary oxidative stress and inflammation in rats after coal dust exposure. Environ Res 99: 355-360.
- Toth G, Murphy FM, Lovas S (2001) Stabilization of local structures by pi-CH and aromatic-backbone amide interactions involving prolyl and aromatic residues. Protein eng 14: 543-547.
- Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, et al. (2009) Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One 4: e6073.
- Mähler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, et al. (1998) Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am J Physiol 274: G544-G551.
- Wirtz S, Neufert C, Weigmann B, Neurath MF (2007) Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541-546.
- Wirtz S, Neurath MF (2007) Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev 59: 1073-1083.
- Williams KL, Fuller CR, Dieleman LA, DaCosta CM, Haldeman KM, et al. (2001) Enhanced survival and mucosal repair after dextran sodium sulfate- induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology 120: 925-937.
- Calafiore A, Gionchetti P, Calabrese C, Tambasco R, Fornarini GS, et al. (2012) Probiotics, prebiotics and antibiotics in the treatment of inflammatory bowel disease. Journal of Gastroenterology and Hepatology Research 1: 97-106.
- FAO/WHO (2001) Health and Nutritional Properties of Probiotics in Food including PowderMilk with Live Lactic Acid Bacteria.
- Guarner F, Casellas F, Borruel N, Antolín M, Videla S, et al. (2002) Role of microecology in chronic inflammatory bowel diseases. Eur J ClinNutr 56 Suppl 4: S34-38.
- Neut C, Bulois P, Desreumaux P, Membré JM, Lederman E, et al. (2002) Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn's disease. Am J Gastroenterol 97: 939-946.
- Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, et al. (2006) Reduced diversity of faecalmicrobiota in Crohn's disease revealed by a metagenomic approach. Gut 55: 205-211.
- Jonkers D, Penders J, Masclee A, Pierik M (2012) Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs 72: 803-823.
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, et al. (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature 488: 178-184.
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI (2011) Human nutrition, the gut microbiome and the immune system. Nature 474: 327-336.
- Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, et al. (2012) Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 6: 991-1030.
- Thantsha MS, Labuschagne PW, Mamvura CI (2014) Supercritical CO2 interpolymer complex encapsulation improves heat stability of probiotic bifidobacteria. World J MicrobiolBiotechnol 30: 479-486.
- Gan F, Chen X, Liao SF, Lv C, Ren F, et al. (2014) Selenium-enriched probiotics improve antioxidant status, immune function, and selenoprotein gene expression of piglets raised under high ambient temperature. J Agric Food Chem 62: 4502-4508.
- Lu N, Wang L, Cao H, Liu L, Van Kaer L, et al. (2014) Activation of the epidermal growth factor receptor in macrophages regulates cytokine production and experimental colitis. J Immunol 192: 1013-1023.
- Shifrin H, Nadler-Milbauer M, Shoham S, Weinstock M (2013)Rivastigmine Alleviates Experimentally Induced Colitis in Mice and Rats by Acting at Central and Peripheral Sites to Modulate Immune Responses. PLos one 8: e57668.
- Choi SY, Hur SJ, An CS, Jeon YH, Jeoung YJ, et al. (2010) Anti-inflammatory effects of Inonotusobliquus in colitis induced by dextran sodium sulfate. J Biomed Biotechnol 2010: 943516.
- Kim MJ, Jeong HJ, Kim DW, Sohn EJ, Jo HS, et al. (2014) PEP-1-PON1 protein regulates inflammatory response in raw 264.7 macrophages and ameliorates inflammation in a TPA-induced animal model. PLoS One 9: e86034.
- Moita E, Gil-Izquierdo A, Sousa C, Ferreres F, Silva LR, et al. (2013) Integrated Analysis of COX-2 and iNOS Derived Inflammatory Mediators in LPS-Stimulated RAW Macrophages Pre-Exposed to Echiumplantagineum L. Bee Pollen Extract. PLos one 8: e59131.
- Wirtz S, Neurath MF (2000) Animal models of intestinal inflammation: new insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int J Colorectal Dis 15: 144-160.
- Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, et al. (2005) Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev 206: 260-276.
- Miyazawa F, Olijnyk OR, Tilley CJ, Tamaoki T (1967) Interactions between dextran sulfate and Escherichia coli ribosomes. BiochimBiophysActa 145: 96-104.
- FELLIG J, WILEY CE (1959) The inhibition of pancreatic ribonuclease by anionic polymers. Arch BiochemBiophys 85: 313-316.
- Kim IW, Myung SJ, Do MY, Ryu YM, Kim MJ, et al. (2010) Western-style diets induce macrophage infiltration and contribute to colitis-associated carcinogenesis. J GastroenterolHepatol 25: 1785-1794.
- Venkatraman A, Ramakrishna BS, Pulimood AB, Patra S, Murthy S (2000) Increased permeability in dextran sulphate colitis in rats: time course of development and effect of butyrate. Scand J Gastroenterol 35: 1053-1059.
- Venkatraman A, Ramakrishna BS, Shaji RV, Kumar NS, Pulimood A, et al. Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-kappaB. Am J PhysiolGastrointest Liver Physiol285: G177–184.
- Vieira EL, Leonel AJ, Sad AP, Beltrão NR, Costa TF, et al. (2012) Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J NutrBiochem 23: 430-436.
- Hedin CR, Mullard M, Sharratt E, Jansen C, Sanderson JD, et al. (2010) Probiotic and prebiotic use in patients with inflammatory bowel disease: a case-control study. Inflamm Bowel Dis 16: 2099-2108.
- Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, et al. (2009) Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol 104: 437-443.
- Saggioro A (2004) Probiotics in the treatment of irritable bowel syndrome. J ClinGastroenterol 38: S104-106.
- Kelly D, Conway S, Aminov R (2005) Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol 26: 326-333.
- Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, et al. (2005)Synbiotic therapy (Bifidobacteriumlongum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut 54:242-249.
- Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, et al. Dextran Sodium Sulfate (DSS) Induces Colitis in Mice by Forming Nano-Lipocomplexes with Medium-Chain-Length Fatty Acids in the Colon.PLose one 7: e32084.
- Miyazawa F, Olijnyk OR, Tilley CJ, Tamaoki T (1967) Interactions between dextran sulfate and Escherichia coli ribosomes. BiochimBiophysActa 145: 96-104.
- Sasaki S, Hirata I, Maemura K, Hamamoto N, Murano M, et al. (2000) Prostaglandin E2 inhibits lesion formation in dextran sodium sulphate-induced colitis in rats and reduces the levels of mucosal inflammatory cytokines. Scand J Immunol 51:23-28.
- Matkowskyj KA, Nathaniel R, Prasad R, Weihrauch D, Rao M, et al. (2004)Galanin contributes to the excess colonic fluid secretion observed in dextran sulfate sodium murine colitis. Inflamm Bowel Dis 10:408-416.
- Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, et al. (2012) Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One 7: e32084.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15595
- [From(publication date):
August-2015 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 10927
- PDF downloads : 4668