Review Article Open Access
Anti-Hyperlipidemia Activity on Neonates and Perinatals
Swaroop HU*
Department of Pharmacology, JNTU University, Telangana, India
- *Corresponding Author:
- Swaroop HU
Department of Pharmacology
JNTU University, Telangana, India
Tel: +91 9160548770
E-mail: hemanth_s@outlook.com
Received Date: April 04, 2016; Accepted Date: June 14, 2016; Published Date: June 21, 2016
Citation: Swaroop HU (2016) Anti-Hyperlipidemia Activity on Neonates and Perinatals. Neonat Pediatr Med 2: 113. doi: 10.4172/2572-4983.1000113
Copyright: © 2016 Swaroop HU. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Neonatal and Pediatric Medicine
Abstract
The review is to show the pharmacological activity of lipid lowering drugs on the perinate, when a type-1 diabetes mellitus pregnant woman takes high amount of anti hyperlipedimic drugs during the gestation period
Keywords
Pharmacological; Anti-hyperlipidemia activity
Hyperlipidemia
The disease condition where the increased levels of unsaturated lipids in the blood. Lipids are fats they help the body to produce hormones and acts as an energy sources when proper diet is not available.
There are different types of lipids available in the body like fats, monoglycerides, diglycerides, triglycerides, phospholipids and others. The lipid levels in the blood is defined based on the type of meal taken so the lipid levels in the body may be fluctuated majorly for pregnant woman as they are recommended to take high cholesterol to make the steroid hormones like progesterone and estrogen, during the second trimester Cholesterol levels increase naturally, and reaches peak during the third trimester and typically return to normal about four weeks after delivery [1-5].
Anti-hyperlipedimic drugs
The drugs which are used lower the low density lipoproteins (LDL) in the blood by preventing the conversion of them from high density lipoproteins by interrupting the various steps during lipolysis [6-11].
Lipolysis
Lipolysis is a process of breaking down of triglycerides to glycerol and fatty acids, the process is done by hydrolysis by inducing some hormones like epinephrine, nor-epinephrine, cortisol, testosterone, etc. to release the compounds like High density lipoproteins (HDL), Low density lipoproteins (LDL) and Very low density lipoproteins (VLDL) [12-18].
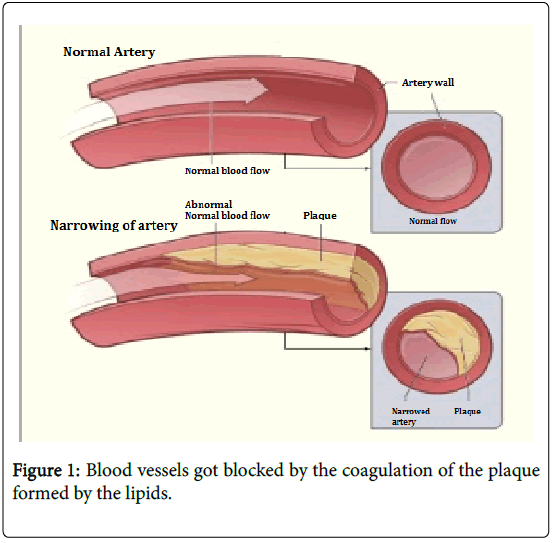
These triglycerides transports through blood to reach the muscle or tissue, while transporting the LDL and VLDL accumulate on the walls of the blood vessels creating plaques which narrows the blood vessel and also generates fat deposits in the body [19].
Lipid lowering drugs
By inhibiting the production of LDL and VLDL the risk factors for the hyperlipidemia can be controlled, Bile acid sequestrants like Cholestyramine, Colestipol and Colesevelam are used to regulate high serum levels by binding the bile acid which is secreting from liver to metabolize the triglycerides [20-24].
HMG-CoA Reductase inhibitors like statins (atorvastatin, lovastatin, simvastatin) acts on the cells which absorbs the lipoproteins by inhibiting the enzyme (HMG CoA reductase) which regulates the cholesterol.
Review
In pregnancy as mentioned earlier the lipid concentration in blood increases, if the concentration of the lipids in the blood is not controlled it results in the increase in the concentration of the lipid in the perinatals and may be leads obesity and/or hyperlipidemia [25-31]. As the blood vessels are blocked in the excess coagulation of the plaques results in choking and heart failure in the time of birth (Figure 1).
Like hypertension, there is a scope of diabetics that happens in pregnancy including prior type 1 and type 2 diabetes mellitus (DM) and gestational diabetes [32-39].
Pregnant women will have the metabolic risk factors like obesity, hypertension, and poor glycemic levels. In type 1 DM the increase in the triglycerides level and decrease in the HDL levels in the first trimester shows physical changes in, then after the pregnancy the TG levels decreases.
There are unpretentious contrasts in type 1 DM and unusual states of unclear clinical conditions [40-47]. For instance, renal brokenness and type 1 DM is connected with higher TC and LDL portions while ladies with poor glycemic control and type 1 DM have higher TG levels and lower HDL-C without huge changes in LDL parts from typical. So also, ladies with prior type 2 DM have higher TG and lower HDL-C levels amid the primary trimester without critical change in LDL-C and Lp (a) levels in contrast with ordinary [48-54]. Ladies with gestational diabetes may have expanded to unaltered TG and TC levels and stable LDL portions all through growth despite the fact that these outcomes have been dubious.
Maternal obesity, then again, with or without plain gestational diabetes, is connected with atherogenic lipid profiles and adverse pregnancy results, due to inflammation and endothelial dysfunction [55-62]. Pregnant obese women are frequently associated with increased triglycerides level and dense LDL fractions with low HDL-C. Newborns born to obese mothers also tend to be large for gestational age, and may have increased risk of cardiovascular events later in life [63-65].
Clinical complications
As the clinical trials are not allowed to be performed on pregnant women the effect of anti-hyperlipidemia drugs are not demonstrated well [66,67]. Using of Omega-3 fatty acids during pregnancy are have shown no effect to the mother and child, In other hand the lipid lowering substances like Niacin (nicotinic acid), fibrates, HMG-CoA reductase inhibitors and statins have reported teratogenicity, congenital malfunction and in some cased abortion so they are not recommended as the lipid lowering drugs during pregnancy [68,69].
Unlike the babied for the Type 1 DM and obese women the antihyperlipidemia drugs therapy also effects the neonatal when they are taken in high amount of food rich in cholesterol, physical inactivity, smoking, etc. in the time of pregnancy [70]. As the drugs like statins, fibrates, HMG-CoA reductase inhibitors lowers the breakdown of the HDL to LDL and VLDL they may create the dependence and also the malabsorption of the food to the fetus [71,72].
The major complications is risk of heart problems, if the pregnant women is under the anti-lipidemic drug therapy the dependence to the drug will be more, as the 50% of the pregnancies are unplanned, if there is an abnormal increase in the triglycerides level in the, the risk of heart diseases in newborn like coronary artery disease, CHF, etc.
Conclusion
When the LDL and VLDL% in blood increases there will be a high risk of heart diseases, as of some recent studies, the abnormal increase in lipid concentration for pregnant women during the second and third trimester, to maintain the regular hormonal functioning and a gradual decrease will be seen 4 month after delivery.
In the case of type-1 DM the increase in the triglycerides level and decrease in the HDL levels are seen and they reaches to peak during the pregnancy, so that high concentration of lipid lowering drugs have to be taken, which in turn shows teratogenic activity.
References
- Lin J, Yang R, Tarr PT (2005) Hyperlipidemic effects of dietary saturated fats mediated through PGC–1ß coactivation of SREBP. Cell 120: 261-273.
- Sharett AR, Ballantyne M, Coady SA (2001) Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A–I and B, and HDL density subfractions. The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 104: 1108-1113.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third Report of the National Cholesterol Education Program (NCEP).
- O’Keefe JH Jr, Cordain L, Harris WH, Moe RM, Vogel R (2004) Optimal low–density lipoprotein is 50 to 70 mg/dL: lower is better and physiologically normal. J Am Coll Cardiol 43: 2142-2146.
- Grundy SM, Cleeman JI, Merz CNB (2004) Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation 110: 227-239.
- Hata Y, Nakajima K (2000) Life–style and serum lipids and lipoproteins. J Atheroscler Thromb 7: 177-197.
- Cullen P (2000) Evidence that triglycerides are an independent coronary heart disease risk factor. Am J Cardiol 86: 943-949.
- Hunninghake DB, Stein EA, Dujovne CA (1993) The efficacy of intensive dietary therapy alone or combined with lovastatin in outpatients with hypercholesterolemia. N Engl J Med 328: 1213-1219.
- Ornish D, Scherwitz LW, Billings JH (1998) Intensive lifestyle changes for reversal of coronary heart disease. JAMA 280: 2001-2007.
- Barnard ND, Scialli AR, Bertron P, Hurlock D, Edmonds K, et al. (2000) Effectiveness of a low–fat vegetarian diet in altering serum lipids in healthy premenopausal women. Am J Cardiol 85: 969-972.
- Pelkman CL, Fishell VK, Maddox DH, Pearson TA, Mauger DT, et al.(2004) Effects of moderate–fat (from monounsaturated fat) and low–fat weight–loss diets on the serum lipid profile in overweight and obese men and women. Am J Clin Nutr 79: 204-212.
- Peters JC (2003) Dietary fat and body weight control. Lipids 38: 123-127.
- Ornish D, Brown SE, Scherwitz LW (1990) Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet 336: 129-133.
- Brown L, Rosner B, Willett WW, Sacks FM (1999) Cholesterol–lowering effects of dietary fiber: a meta–analysis. Am J Clin Nutr 69: 30-42.
- Jenkins DJ, Kendall CW, Vuksan V (2002) Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am J Clin Nutr 75: 834-839.
- Jenkins DJ, Wolever TM, Kalmusky J (1987) Low–glycemic index diet in hyperlipidemia: use of traditional starchy foods. Am J Clin Nutr 46: 66-71.
- Anderson JW, Johnstone BM, Cook–Newell ME (1995) Meta–analysis of the effects of soy protein intake on serum lipids. N Eng J Med 333: 276-282.
- Kris–Etherton PM, Yu–Poth S, Sabate J, Ratcliffe HE, Zhao G, et al. (1999) Nuts and their bioactive constituents: effects on serum lipids and other factors that affect disease risk. Am J Clin Nutr 70: 504S-511S.
- Hu FB, Manson JE, Willett WC (2001) Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr 20: 5-19.
- Katan MB, Grundy SM, Jones P (2003) Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 78: 965-978.
- Jenkins DJ, Kendall CW, Marchie A (2003) Effects of a dietary portfolio of cholesterol–lowering foods vs lovastatin on serum lipids and C–reactive protein. JAMA 290: 502-510.
- Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ (1999) Moderate alcohol intake and lower risk of coronary heart disease: meta–analysis of effects on lipids and haemostatic factors. BMJ 319: 1523-1528.
- Malloy MJ, Kane JP (2001) A risk factor for atherosclerosis: triglyceride–rich lipoproteins. Adv Intern Med 47: 111-136.
- Kamshoushy A, Mahdy N (2012) Evaluation of the Efficacy of Injection Lipolysis using Phosphatidylcholine/Deoxycholate Versus Deoxycholate Alone in Treatment of Localized Fat Deposits. Journal of Clinical & Experimental Dermatology Research.
- Jansen EH, Beekhof PK, Schenk E (2014) Long term stability of parameters of lipid metabolism in frozen human serum: Triglycerides, free fatty acids, total-, HDL-and LDL-cholesterol, apolipoprotein-A1 and B. Journal of Molecular Biomarkers & Diagnosis.
- Yang S, Liu M, Wu T (2015) Magnitude of the Difference between Fasting and Non-fasting Triglycerides, and Its Dependent Factors. J Community Med Health Educ 5: 2161-0711.
- Zayed MA, Harring SD, Abendschein DR, Vemuri C, Lu D, et al. (2016) Natriuretic Peptide Receptor-C is Up-Regulated in the Intima of Advanced Carotid Artery Atherosclerosis. Journal of medical & surgical pathology 1: 3.
- Alam MA (2016) Editorial: Misguided Macrophage and Risk of Coronary Atherosclerosis. J Vasc Med Surg 4: e118.
- Merlo S (2016) Polymorphisms rs699 and rs4762 of the Angiotensinogen Gene and Progression of Carotid Atherosclerosis in Patients with Type 2 Diabetes Mellitus. J Diabetic Complications Med 1: 107.
- Afroz R, Tanvir EM, Little PJ (2016) Honey-derived Flavonoids: Natural Products for the Prevention of Atherosclerosis and Cardiovascular Diseases. Clin Exp Pharmacol 6: 208.
- Domenico PEM, Marta V, Luca N, Fabio B, Antonella A (2016) The Complex Role of Leptin in SLE: Is Leptin A Key Link Between Metabolic Syndrome, Accelerated Atherosclerosis and Autoimmunity?. Lupus Open Access 1: 107.
- Hariane C, Ana MO (2015) Endothelial Dysfunction Induced by Chronic Psychological Stress: A Risk Factor for Atherosclerosis. Cardiovasc Pharm Open Access 4: 5.
- Zapolska DD, Bryk D, Olejarz W (2015) Trans Fatty Acids and Atherosclerosis-effects on Inflammation and Endothelial Function. J Nutr Food Sci 5: 426.
- Aranjan LK, Abha Gupta (2015) Association between the Number of Chondrocytes of Lumbar Intervertebral Discs and Age, Abdominal Aorta Atherosclerosis and Lumbar Artery Arteriosclerosis Descriptive Cross Sectional Study. Anat Physiol 5: 183.
- Alexander B, Alexander K, Tatyana B, Yulia M, Olena G (2015) The Pattern of Circulating Microparticles in Diabetes Mellitus Patients with Known Subclinical Atherosclerosis. Clin Med Biochemistry Open Access 1: 104.
- Leonardo R, Elmiro SR, Antonio CPC (2016) Perivascular Adipose Tissue can be Considered a Risk Factor for Atherosclerosis?. Atheroscler Open Access 1: e104.
- Leonardo R, Elmiro SR, Giuseppe BZ (2016) Atherosclerosis and Lipid Lowering: is there a Need for New Agents? Atheroscler.
- Fuior EV, Trusca VG, Roman C, Gafencu AV (2015) Enzymatic Targets in Atherosclerosis. J Mol Genet Med 9: 176.
- Mary WF (2015) Premature Atherosclerosis, and Arterial stiffness as a Challenge in Rheumatoid Arthritis. J Arthritis 4: 163.
- Jonathan EF, Jessica LF, Annapoorna SK (2015) The Regression of Atherosclerosis: The Power of Multimodality Imaging. J Clin Exp Cardiolog 6: 369.
- Shimoyama S (2015) Statins Cardiovascular Benefits Outweigh their Diabetogenicity: A Direct Comparison between Number Needed to Treat and Number Needed to Harm. Adv Pharmacoepidemiol Drug Saf 4: 185.
- Krisztina L, Laszlo JB, Nora H, Eva P, Attila JS, et al. (2016) Primary Hyperlipidemia, Acute Pancreatitis and ketoacidosis in an Adolescent with Type 2 Diabetes. J Diabetes Metab 7: 651.
- Niki GP, Antonia D, Panagiota P, Eleftheria RG, George PC, et al. (2015) Low Glycemic Index and Load, Hypo Caloric Diet as an Effective Treatment for Obesity and Hyperlipidemia in Girls with Metabolic Syndrome. Endocrinol Metab Syndr 4: 180.
- Yasir M, Muhammad IA (2015) Ruta chalepensis L Considerable Action against Obesity or Hyperlipidemia in Body. J Bioequiv Available.
- Khulan TS, Ambaga M, Chimedragcha CH (2015) Effect of Honey Bee Venom (Apis mellifera) on Hyperglycemia and Hyperlipidemia in Alloxan Induced Diabetic Rabbits. J Diabetes Metab 6: 507.
- Jhuma KA, Giasuddin ASM, Haq AMM, Huque MM, Mahmood N (2014) Effects of Atorvastatin and Niacin, Alone and in Combination, On Lowering Serum LDL-Cholesterol and Lipoprotein (a) in Hyperlipidemia Patients. J Metabolic Synd 3: 136
- Kadir ST, Kepenekli E, Yasar D (2013) Hyperlipidemia Due to Rectal Phenobarbital Use: Case Report. Pediat Therapeut 3: 165.
- Bassam ARH (2013) Overview on Hyperlipidemia. J Chromat Separation Techniq 4: 113.
- Faruk A, Ahmet AY, Mehmet E, Fatih U (2013) Valvular and Supravalvular Aortic Stenosis Secondary to Familial Hyperlipidemia. J Cardiovasc Dis Diagn 1: 102.
- Maha AH, Hanan AAK, Eman MA, Azra K (2011) Prevalence of hyperlipidemia and associated risk factors among healthy young Saudi females:relationship with waist Circumference and body Mass Index. Endocrinol Metab Syndr S2: 001.
- Makoto S, Tomohiro K, Daigo K, Yayoi M, Yu K, et al. (2016) High Dose Octreotide for the Treatment of Chylothorax in Three Neonates. J Neonatal Biol.
- Ihab AN, Heba AI (2016) Impact of Early and High Doses of Amino Acid Supplement on the Growth and Development of Preterm and Low Birth Weight Neonates. Clin Pediatr 1: e105
- Arnaldo C (2015) Assessment of the Essential Fatty Acids in Neonates at Risk for Atopy. J Biomol Res Ther 4: 4.
- Ikenna KN, Benedict O E, Samuel NU, Josephat CC, Agozie U, et al. (2015) Maternal Risk Factors Associated with Low Birth Weight Neonates: A Multi-Centr. Cross-Sectional Study in a Developing Country. J Neonatal Biol.
- Bamgboye MA, Abiodun O, Cecilia OC, Victor I (2015) An Appraisal of the Medical Records of Critically Ill Neonates in Lagos, Nigeria. J Infect Dis Ther 2: 196.
- Chetan K, Deepak S, Aakash P (2014) Late Preterm and Early Term Neonates: A New Group of High Risk Newborn in Neonatology with Varied Complications. J Neonatal Biol 3: E112.
- Jummanah J, Soad A, Sharma MC, Bushra MSJ, Mohammad AK (2012) Detection of Glucose-6-Phosphate Dehydrogenase Deficiency in Heterozygous Saudi Female Neonates. Enz Eng 1: 105
- Karel A (2012) Propylene Glycol in Neonates: Never Prescribed, Frequently Administered, Hardly Evaluated. J Clin Toxicol 2: e113
- Gagan A, Sartaj A, Kapil G, Vijay K, Parul G, et al. (2012) Maternal Risk Factors Associated with Low Birth Weight Neonates in a Tertiary Care Hospital, Northern India. J Community Med Health Edu 2: 177.
- Osama M, Yu SP (2012) Plenty of Pain in Neonates: The Mission to Find a Treatment for the Complications of Premature Birth. J Pain Relief 1: e103.
- Suksham J, Manju S, Deepak C (2011) Barriers of Exclusive Breast Feeding in Healthy Term and Late Preterm Neonates. J Community Med Health Edu 1: 113.
- Darshan S, Subhadra N, Gayatri BJ, Sandeep C, Kesheng W, et al. (2011) Pre-Term Exposure Patterns in Neonatal Intensive Care Unit Alters Immunological Outcome in Neonates. J Allergy Ther 2: 106.
- Arindam B, Gayatri M (2016) Maternal Obesity: An Important Contributor to Congenital Anomalies, Infant and Child Mortality with Negative Economic Impact. J Preg Child Health.
- Shoar Z, Zivot AT, Nasiri S, Mandhani N, Kelly BA (2016) Maternal Obesity, Maternal Gestational Diabetes Mellitus, and Maternal and Neonatal Outcomes. J Obes Weight Loss Ther 6: 292.
- Rolando AHF, Lizmery VC, Sonia CH, Araceli LC (2015) Relationship between Maternal Obesity and Congenital Malformation in a Subpopulation of Havana. J Diabetes Metab 6: 498.
- Elham K, Gity S, ARD Motlagh, Mohammad RE, Minoo B (2012) Maternal Obesity and Energy Intake as Risk Factors for Pregnancy Induced Hypertension among Iranian Women. J Women's Health Care 1: 116.
- Mark CA, Elizabeth MS, Yefim M, Danyelle MT, Laura MG (2012) Maternal Obesity and Placental Oxidative Stress in the First Trimester. J Obes Weight Loss Ther.
- Anjum H, Jamil AS, Zafar I, Tahira KS, Khalid S (2012) Maternal Obesity a Global Health Problem and its Implications on Maternal and Fetal Health. Reprod Syst Sex Disord 1: 103.
- Chhabra S, Chopra S (2016) Maternal Hemoglobin, Preterm Pains, Failure of Tocolysis, Preterm Birth, Small for Gestational Age Neonate. J Preg Child Health.
- Abdulmoein Eid, Aisha O, Manal K, Mada I (2016) The Association between Children Born Small for Gestational Age and Short Stature. J Preg Child Health.
- Janardhan Shenoy, Venkat R, Kiran NB (2014) Serum Lipid Profile in Preterm and Term Appropriate for Gestational Age Indian Newborns: A Hospital Based Comparative Study. J Neonatal Biol 3: 156.
- Ritter BC, Nelle M, Steinlin M, Everts R (2013) Influence of Gestational Age and Parental Education on Executive Functions of Children Born Very Preterm. J Neonatal Biol 2: 120.
Relevant Topics
- About the Journal
- Birth Complications
- Breastfeeding
- Bronchopulmonary Dysplasia
- Feeding Disorders
- Gestational diabetes
- Neonatal Anemia
- Neonatal Breastfeeding
- Neonatal Care
- Neonatal Disease
- Neonatal Drugs
- Neonatal Health
- Neonatal Infections
- Neonatal Intensive Care
- Neonatal Seizure
- Neonatal Sepsis
- Neonatal Stroke
- Newborn Jaundice
- Newborns Screening
- Premature Infants
- Sepsis in Neonatal
- Vaccines and Immunity for Newborns
Recommended Journals
Article Tools
Article Usage
- Total views: 13688
- [From(publication date):
December-2016 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 12721
- PDF downloads : 967