Research Article Open Access
Antidiabetic Potential and its Relationship with Polyphenol and Degree of Polymerization in Acalypha indica Linn Leaves
| Prakash R Itankar1*, Akash P Jaiswal2, Prashant R Verma2, Sumit K Arora2 and Arun T Patil1 | |
| 1Assistant Professor, Department of Pharmaceutical Sciences, Pharmacognosy and Phytochemistry Division, Rashtrasant Tukadoji Maharaj Nagpur University, Amravati Road, Nagpur- 440 033, Maharashtra, India | |
| 2Department of Pharmaceutical Sciences, Pharmacognosy and Phytochemistry Division, Rashtrasant Tukadoji Maharaj Nagpur University, Amravati Road, Nagpur- 440 033, Maharashtra, India | |
| Corresponding Author : | Prakash R Itankar Department of Pharmaceutical Sciences Pharmacognosy and Phytochemistry Division Rashtrasant Tukadoji Maharaj Nagpur University Amravati Road, Nagpur- 440 033, Maharashtra, India Tel: +91-712-2550324 Fax: +91-712-2500355 E-mail: pri_200672@rediffmail.com |
| Received November 21, 2011; Accepted December 20, 2011; Published December 23, 2011 | |
| Citation: Itankar PR, Jaiswal AP, Verma PR, Arora SK, Patil AT (2012) Antidiabetic Potential and its Relationship with Polyphenol and Degree of Polymerization in Acalypha indica Linn Leaves. J Homeopat Ayurv Med 1:102. doi: 10.4172/2167-1206.1000102 | |
| Copyright: © 2012 Itankar PR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
Acalypha indica commonly known as Kuppi belongs to family Euphorbiaceae. Traditionally the plant used as a laxative, anthelmintic, cathartic and the juice is used as a speedy emetic for children and expectorant. It is used by tribal areas of Maharashtra State as rheumatic arthritis and as antihyperglycemic. However, no scientific data is available to validate the ethnobotanical claim. Keeping the above information in view, the present study has been designed to evaluate its antidiabetic activity. In the present study, hydroalcoholic extract of leaves and its fractions were studied for its anti diabetic potential in alloxan induced diabetic rats. The poly phenolic, flavonoid and flavanone contents of hydroalcoholic extract and its fractions were also determined and correlated with its anti diabetic activity.
The experimental data indicated that the hydroalcoholic extract and its chloroform soluble fraction has significantly lowered the elevated blood glucose levels by 36.82% and 61.52% respectively at dose level of 400 mg/kg per oral after 7 days as compared to diabetic control. The poly phenolic and flavonoid content of hydro alcoholic extract and its chloroform soluble fraction were found to be 7.9 ± 0.20 mg and 1.14 ± 0.21mg (gallic acid equivalent/ g extract) and flavonoid content 2.02 ± 0 .50 mg and 0.56 ± 0.003mg (rutin equivalent/g extract) respectively.
The increased anti diabetic potential of chloroform fraction over hydro alcoholic extract is due to its partial purification achieved by fractionation which resulted in increase in degree of polymerization and segregation of secondary metabolites.
| Keywords |
| Antidiabetic; Acalypha indica; Flavonoid; Flavanone; Polymerization |
| Introduction |
| Diabetes mellitus (DM) is a major endocrine disorder, affecting approximately 5% of the world’s population. Diabetes is characterized by abnormalities in carbohydrate, lipid and lipoprotein metabolisms, which not only lead to hyperglycemia but also cause many complications such as hyperlipidemia, hyperinsulinemia, hypertension and atherosclerosis [1,2] |
| Epidemiological studies and clinical trials strongly support the notion that insulin deficiency results in hyperglycaemia and long lasting hyperglycaemia eventually leading to coronary artery disease, cerebrovascular disease, renal failure and blindness [3]. In diabetic patients, the blood glucose concentration is controlled by multiple injection of insulin, a principal hormone regulating glucose metabolism and administrations of oral hypoglycaemic agents in type II diabetes. When therapy with oral hypoglycaemic agents is ineffective, insulin can also be used to treat type II diabetes [4]. |
| Acalypha indica commonly known as Kuppi belongs to family Euphorbiaceae. Acalypha indica is an annual herb up to 60 cm tall or a little more, with a few ascending branches, angled and pubescent; leaves broadly ovate sub deltoid rather coarsely toothed, on petioles as long as or longer than 3-5 cm long blades; nerves 3-5 from base, thereafter innately arranged; stipulous minute; flowers sessile on erect auxiliary spikes longer than the leaf. The plant is native and common throughout the hotter part of India [5]. |
| Traditionally the plant used as a laxative, anthelmintic, cathartic and the juice is used as a speedy emetic for children and expectorant. The whole plant is useful in treating chronic bronchitis, asthma, scabies and other skin diseases, rheumatism, congestive headache. |
| In Ayurveda, Leaves possess laxative properties, in smaller doses it is expectorant, and is useful in chronic bronchitis, asthma. The decoction of leaves is employed in ear-ache. Mixed with garlic they are used as Anthelmintic in worms, in congestive headache. An infusion of the root or the root bruised in water, acts as a cathartic [5]. It is used by tribal areas of Nagpur, Chandrapur and Gadchiroli districts of Maharashtra State as rheumatic arthritis and as anti hyperglycemic. However, no scientific data is available to validate the ethno botanical claim. Keeping the above information in view, the present study has been designed to evaluate its anti diabetic activity. |
| Materials and Methods |
| Animals |
| Swiss albino rats of Sprague-Dawley strain (200-250 g) of either sex obtained from animal house of our institute were used. The animals were fed a standard pellet diet and water ad libitum. They were maintained in a controlled environment and temperature (22±5°C with 12-h of light/dark cycle). All experimental protocols were approved by the Institutional Animal Ethical Committee (13/2010/CPCSEA). |
| Chemicals and standard drugs |
| Aluminum trichloride hexahydrate, glacial acetic acid, anhydrous sodium carbonate, Folin-Ciocalteu phenol reagent, naringin, gallic acid, rutin (hydrate, min 95%), sodium acetate and 2,4-Dinitrophenylhydrazine were of analytical grade obtained from S. D. Fine Chemicals Pvt., Ltd. (Vadodra, India.). |
| Plant material, preparation of extract and its fractionation |
| The Plant was collected locally, authenticated by the Department of Botany, R. T. M. Nagpur University Campus, Nagpur. A voucher specimen has been deposited in the Herbarium of Department of Botany, with collection number 9554. |
| The Leaves were dried under shade and pulverized to a coarse powder. The powdered crude material was defatted with petroleum ether and then extracted with 50% alcohol. The hydroalcoholic extract (AIHA) was concentrated in a rotary vacuum evaporator to yield a dark brown mass (yield: 26.67 % w/w). For fractionation, hydroalcoholic extract was triturated with silica (1:3), loaded to Soxhlet assembly and extracted by chloroform to yield chloroform soluble fraction (AICS; yield: 40.80 % w/w) [6]. The chloroform insoluble portion was further extracted with n-butanol to yield n-butanol soluble fraction (AIBS; yield: 18.40 % w/w) and n-butanol insoluble fraction (AIBIS; yield: 40.80 % w/w). The hydroalcoholic extract (AIHA) and these three broad fractions i.e, AICS, AIBS and AIBIS were subjected to phytochemical and pharmacological screening. |
| Phytochemical screening |
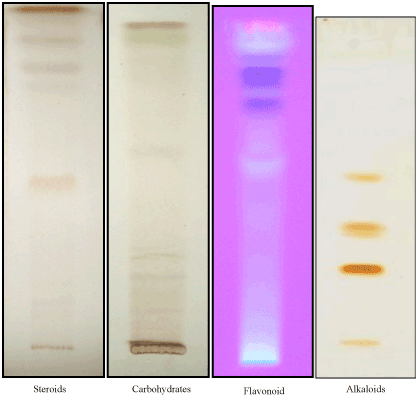
| The AIHA extract and its broad fractions were screened for the presence of tannins, saponins, unsaturated sterols, triterpenes, alkaloids, flavonoids, lactones/esters, protein/amino acids and carbohydrates and/or glycosides with thin layer chromatography (TLC). Thin layer plates precoated with silica gel G (Merck, 0.25mm thickness) were used. Development was carried out with different solvent systems such as ethyl acetate: methanol : water (100:13.5:10, v/v/v), ethyl acetate: formic acid : acetic acid : water (100:11:11:26, v/v/v/v), chloroform : methanol : water (70:30:4, v/v/v), toluene : ethyl acetate : diethylamine (70:20:10, v/v/v) and ethyl acetate : methanol : water : acetic acid (65:15:15:10, v/v/v/v). After development of chromatogram in the solvents the plates were dried and sprayed with Dragendorff’s, AlCl3, hydroxylamine-ferric chloride, ninhydrin and antimony trichloride for the detection of alkaloids, flavonoids, lactones/esters, protein/amino acids, unsaturated sterols and triterpenes respectively. While detection of anthraquinones, saponins, tannins, carbohydrate and/or glycosides is carried out using KOH, anisaldehyde-sulphuric acid reagent, ferric chloride and naphthoresorcinol reagent respectively and visualization is carried out under visible and UV light (λ: 366 nm) [7-9]. |
| AIHA extract and its fractions were also quantified for presence of important secondary metabolites such as total polyphenol, flavonoid and flavonone compounds using following spectroscopic methods. |
| Determination of total polyphenol compounds (TP), flavonoids (TFA), total flavanones (TFO) |
| Total polyphenol content was measured using Folin–Ciocalteu colorimetric method [10,11]. Gallic acid was used as a reference for constructing the standard curve. The results were expressed as mg of gallic acid equivalents (GAE) / g of extract. |
| Flavonoid content was determined by the aluminium chloride method [12,13]. While the modified 2, 4-dinitrophenylhydrazine method was used for determination of flavanones [14] Rutin and naringin were used as a reference standard and results were expressed as mg of rutin equivalents (RE)/g and as mg of naringin equivalents (NE)/g of extract respectively |
| Glucose tolerance test |
| The oral glucose tolerance test was performed in overnight fasted (18 h) normal rats. Rats were divided into ten groups, each consisting of five rats. Group I was administered with 0.9% w/v saline; group II received metformin (50 mg/kg i.p.); Group III–X received AIHA extract and its fractions in dose range of 200 and 400 mg/kg per oral. Glucose (3 g/kg) was fed 30 min after the administration of test samples. Blood was withdrawn from tail-tip at 0, 30, 60 and 120 min of glucose administration and glucose levels were estimated using glucose oxidase–peroxidase reactive strips and a glucometer (Accuchek, Roche Diagnostics, USA). |
| Induction of experimental diabetes |
| Diabetes was induced in overnight fasted rats by the intraperitoneal (i.p.) injection of alloxan monohydrate 130 mg/kg dissolved in 0.1M cold citrate buffer (pH 4.5). The animals were allowed to drink 5% glucose solution overnight to overcome the drug induced hypoglycemia. Seventy-two hours and then on day 7 after the injection, blood was withdrawn from overnight fasted animals and blood glucose level was assessed by glucometer. The rats with a blood glucose level above 200 mg/dl were selected for the experiment as diabetic rats [15,16]. Control animals were injected with normal saline alone. |
| Experimental |
| The diabetic rats were fasted overnight and divided randomly into ten groups (I–X) of five rats (n = 5) each as follows, namely: Group I-Received vehicle (distilled water 10 ml/kg; p.o.); Group IIReceived insulin (4 IU /kg; i.p.); Group III–X received AIHA extract and its fractions in dose range of 200 and 400 mg/kg per oral. After this administration, antidiabetic activity was assessed by withdrawing blood samples at 0, 1, 3, 5 and 7 days respectively and reported the results as mg/dl [17]. |
| Acute toxicity studies |
| Rats were divided into test and control groups (n = 5). The test group was given an increasing oral dose (1, 2 and 4 g/kg) of AIHA and its fractions. The rats were allowed food and water ad libitum and were kept under regular observation for symptoms of mortality and behavioral changes for the period of 48 h [18,19]. |
| Statistical analysis |
| The results were expressed as mean±SD. Comparison between the groups were made by analysis of variance (ANOVA), followed by Dunnett’s test as per suitability. p < 0.05 was considered significant. |
| Results and Discussion |
| Phytochemical screening |
| The phytochemical screening of AIHA extract revealed the presence of alkaloids, carbohydrates, cyanogenetic glycoside, flavonoids and saponins. AICS fraction has shown the presence of carbohydrates, cyanogenic glycoside, flavonoids and steroids. AIBS fraction showed the presence of alkaloids, carbohydrates, cyanogenic glycoside, flavonoids, proteins, steroids and saponins. While, AIBIS fraction showed the presence of carbohydrates, cyanogenetic glycoside, flavonoids and tannins (Figure 1). |
| Determination of total polyphenol, flavonoid and flavanones |
| The total polyphenol content (mg/g) determined by Folin– Ciocalteu colorimetric method was found to be 7.9 ± 0.20, 1.14 ±0.21, 6.7 ± 0.23 and 0.58±0.023mg (GAE mg/g of extract) for AIHA, AICS, AIBS and AIBIS respectively. Polyphenol content was determined from linear regression equation of Gallic acid and expressed as GAE of extract (y = 0.007x + 0.068, r2= 0.994). |
| The flavonoid content determined by aluminum chloride method was found to be 2.02 ± 0.50, 0.0156 ± 0.0030, 3.21 ± 0.84 and 0.96 ± 0.04 QE mg/g of extracts for AIHA, AICS, AIBS and AIBIS respectively. Flavonoid content was determined from linear regression equation of quercetin (y = 0.005x - 0.002, r2 = 0.992). |
| The flavanone content determined by 2, 4 -dinitrophenylhydrazine method was found to be 0.80 ± 0.15, 0.097 ± 0.0021, 0.61 ± 0.06 and 2.56 ± 0.0034 (HE mg/g of extract) for AIHA, AICS, AIBS and AIBIS respectively. Flavanone content was determined from linear regression equation of hesperidin (y = 0.005x + 0.020, r2 = 0.994). |
| As suggested by Chang et al., the flavones, flavonols and isoflavones formed complexes only with aluminum chloride, while flavanones strongly reacted only with 2, 4- dinitrophenylhydrazine, so the contents determined by the two methods were added up to obtain the total flavonoid content [20]. |
| The flavonoid and flavanone content represented 25.57% (w/w) and 10.12% (w/w) of the TP in AIHA extract respectively and similar pattern was observed in all its fraction, suggesting that the extracts are very complex, and contain many other polyphenols such as flavanones, isoflavones, phenolic acids and tannins, and the degree of polymerization of the polyphenols present in the samples is high. Degree of polymerization can be estimated by the ratio between the TP and TFA contents [21]. The highest polymerization is observed in AICS fraction and it varies from 3.76, 4.50, 0.57 and 1.612 for AIHA, AICS, AIBS and AIBIS respectively. |
| Antihyperglycemic effect |
| Acute toxicity studies revealed the non-toxic nature of AIHA extract and its fractions. There were no lethality or toxic reactions found at any doses selected. The AIHA extract, AICS, AIBS and AIBIS fractions showed a significant reduction in blood glucose levels from 30 min onwards in oral glucose tolerance test (table 1). |
| While comparing with the diabetic control, amongst the extract and its fractions, AICS fraction have significantly lowered the elevated fasting blood glucose levels after treatment with 7 days. The most prominent antidiabetic activity was observed by AICS fraction (61.52%) at dose level of 400 mg/kg, per oral after 7 day. While the AIBS fraction marginally lowered the blood glucose level in both the selected doses (table 2) and AIBIS fraction was found to be inactive at the dose level of 400mg/kg of body weight. |
| Results obtained from experiment revealed that extract and its fractions have shown dose dependant antidiabetic activity in decreasing order of AICS > AIHA > AIBS > AIBIS. In conclusion, the increased antidiabetic potential of AICS fraction over AIBS fraction even though rich in flavonoids and polyphenols was due to partial purification achieved by fractionation of extract which in turn resulted in increase in degree of polymerization and segregation of secondary metabolites such as steroids and complex polyphenols present in the AICS extract. These results are in agreement with previous studies that, increase in degree of polymerization of extract, cytotoxic, anti-inflammatory and immunomodulatory activity increases [22-24]. |
| Suggesting that the degree of polymerization along with others secondary metabolites such as steroids and polyphenol present in AICS fraction may play important role in its antidiabetic potential. Further study is needed to ascertain the exact mechanism of antidiabetic activity and effect of degree of polymerization on antidiabetic potential. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 6479
- [From(publication date):
February-2012 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 1810
- PDF downloads : 4669
