Research Article Open Access
Antidepressant-like Activity of Andrographis paniculata in Type-2 Diabetic Rats
| Ajit Kumar Thakur1, Shyam Sunder Chatterjee2 and Vikas Kumar1* | |
| 1Neuropharmacology Research Laboratory, Department of Pharmaceutics, Indian Institute of Technology (Banaras Hindu University), Varanasi, India | |
| 2Retired Head of Pharmacology Research Laboratories, Dr. Willmar Schwabe GmbH & Co KG, Karlsruhe, Germany | |
| Corresponding Author : | Vikas Kumar Neuropharmacology Research Laboratory, Department of Pharmaceutics Indian Institute of Technology (Banaras Hindu University), Varanasi, India Tel: +91-542-6702742 Fax: +91-542-2368428 E-mail:vikas.phe@iitbhu.ac.in |
| Received February 13, 2014; Accepted March 20, 2014; Published March 22, 2014 | |
| Citation: Thakur AK, Chatterjee SS, Kumar V (2014) Antidepressant-like Activity of andrographolide in Type-2 Diabetic Rats. Clin Pharmacol Biopharm S2: 003. doi:10.4172/2167-065X.S2-003 | |
| Copyright: © 2014 Thakur AK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Pharmacological observations suggesting antidepressants-like efficacy of a medicinally used Andrographis paniculata extract (AP) in type-2 diabetic are presented in this communication. Efficacies of 10 daily oral doses of 50, 100 and 200 mg/kg/day AP and 15 mg/kg/day imipramine were compared in behavioral despair and learned helplessness tests using type-2 diabetic rats, and bio- and neuro-chemical alterations in the brain tissue of treated animals subjected to learned helplessness test were quantified. Significant imipramine like antidepressant activity of AP was observed even after its lowest daily tested (50 mg/kg/day) in both behavioral tests used, and such efficacy of the extract dose dependently increased with its increasing dose. Imipramine like effects of AP in elevating lower hippocampal levels of norepinephrine, dopamine, and serotonin observed in diabetic rats towards normal values were also observed after its 50 mg/kg/day doses and such efficacy of the extract increased also with its increasing daily doses. Levels of all the three monoamines quantified in 100 mg/kg/day AP treated diabetic rats were significantly higher than those of the non-diabetic animals. Imipramine had no significant effects on body weight losses, hyperglycemia, insulin deficiency, lower catalase and superoxide dismutase activities and higher lipid peroxides in the frontal cortex, and mitochondrial monoamine oxidase activities observed in diabetic animals. All such quantified biochemical and other pathologies observed in diabetic were significantly antagonized even by 50 mg/kg daily oral doses of AP, and its efficacies always increased with its increasing daily doses. These observations strongly suggest that AP could be an herbal alternative for treatments of diabesity-associated depression resistant to imipramine like antidepressants, and that antidepressants like efficacy of the extract is most probably due to its inhibitory effects on brain mitochondrial monoamine oxidase activities. The observed beneficial of AP on brain oxidative status could be indicative of its neuro-protective potentials as well. In any case its minimal effective doses for all such efficacies should be below or around 50 mg/kg/day.
| Keywords |
| Andrographis paniculata; Antioxidant; Depression; Diabetes; Monoamines |
| Introduction |
| Andrographis paniculata (Burm. F.) Wall. Ex Nees (Family: Acanthaceae), commonly known as Kalmegh or ‘‘King of Bitters”, is one of the many traditionally known medicinal plants listed in WHO herbal Monograph [1]. It is a rich natural source of bioactive labdane diterpinoids like andrographolide, neoandrographolide, isoandrographanolide, andrograpanin, etc. Amongst them andrographolide is quantitatively the major easily extractable secondary metabolite of the plant [2]. Diverse types of andrographolide containing andrographolide extracts have been reported to possess broad spectrums of pharmacological activity profiles potentially useful for treatments of diabetes and obesity [3-5], liver disorders [6], inflammatory diseases [7] etc. Beneficial effects of some such extracts for symptomatic relief of upper respiratory tract infections [8] and other disorders have also been observed in clinical trials. Reports revealing brain functions modulating, immunostimulating, and nootropics like efficacies of such extracts suggest that andrographolide could as well be another adaptogenic herb potentially useful for combating mental health problems commonly associated with chronic diseases. In a very first report indicating such possibilities sedative and some other unspecific brain function altering activities of fairly high acute intra-peritoneal doses of an extract of the plant in rodent models were described [9], and in another more recent report appearing after more than a decade the effects of daily doses of another such extracts as immunostimulant, cerebroprotective and nootropic agents in normal and type-2 diabetic rats was described [10]. However, no analytical data or standardization protocols for the extracts used for the studies were mentioned in both these reports, and no attempts were made to detect their potential antidepressants or anxiolytics like therapeutic potentials. Similar, or analogous, have also been the cases for numerous other reports describing diverse therapeutic potentials of different types of extracts obtained from this traditionally known medicinal plant. More recent efforts made in our laboratories to define the psychopharmacological activity profile of an analytically well standardizes and therapeutically used andrographolide extract (AP), highly enriched in andrographolide (>30% w/w), were the very first ones revealing its therapeutic potentials for treatments of depression, anxiety, and other psychopathologies characterized by exaggerated symptoms of both anxiety and depression [11,12]. |
| One medical condition commonly associated with such comorbidities is diabetes, i.e. an endocrine disorder resulting from inadequate release and/or reduced insulin sensitivity. Diabetes and depression are two major chronic diseases with bidirectional relationship [13], and both of them are spreading like epidemics in almost all countries around the global. Co-occurrence of these two pathologies in same patients has strong negative impacts on their quality of life, and shortens their life span [14,15]. Depression has been found also to be associated with alterations in diverse other diabetes related psychological and physiological processes [16,17], and it has been reported that prevalence of depression in diabetics is higher than prevalence of depression in normal population [18]. Numerous structural, behavioral, and biochemical alterations of the central nervous system are observed in diabetic patients, and diverse such alterations are observed also in rodent models of diabetes where exaggerated symptoms of depression, anxiety, and cognitive deficits are observed also [19-24]. Although complex interactions of physical, psychological, and genetic factors that contribute to such associations still remain to be properly defined, available evidence strongly suggest that depression could as well a consequence of persistent metabolic abnormalities [25,26]. However, it has been reported also that depression actually doubles the risk of type-2 diabetes, and that depression could as well be an independent risk factor for type-2 diabetes [27-29]. |
| Unfortunately, most currently available antidepressant and other psychoactive drugs do not meet the therapeutic demands of diabetic patients and many of them are even contraindicated for patients with diabetes [30-34]. Therefore, efforts are now being in our laboratories to identify an adaptogenic herb that could be used as a starting material for identifying novel therapeutic strategies for treatments of diabetes associated depression. In view of the observed antihyperglycemic activity in type-2 diabetic animals [3,4] and antidepressants-like efficacies of AP in non-diabetic animals [11,12], it was of interest to experimentally verify the possibility whether it could also be useful for suppressing the exaggerated depressive state in diabetic animals. Results of the experiments to experimentally verify this possibility are described and discussed in this communication. |
| Materials and Methods |
| Animals |
| Adult Charles Foster albino rats (males with 150 ± 10 g body weights), were acquired from the Central Animal House of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. The animals were housed in groups of six in polypropylene cages at an ambient temperature of 25 ± 1°C and 45-55% relative humidity, with a 12:12 h light/dark cycle. Except stated otherwise, they were always provided with commercial food pellets (Amrut Laboratory Animal Feed; Pranav Agro Industries Ltd., Sangali, India) and water ad libitum, and were acclimatized to the laboratory environment for at least one week before using them for the experiments. Behavioral experiments were conducted between 09.00 and 14.00 h, and “Principles of laboratory animal care” (NIH publication number 85-23, revised in 1985) guidelines were always followed. Prior approval from the Central Animal Ethical Committee of the University (CAECU) was taken for the study protocol used (Dean/11-12/CAEC/325, dated 30-11-2011). |
| Plant extract |
| The same andrographolide extract (AP) used in our earlier studies and generously supplied by Natural Remedies Pvt. Ltd., Bangalore, India was used in this study as well. The extraction procedure used to prepare the analytically well characterized extract has been described in details elsewhere [2]. The extract is rich in andrographolide (>30%, w/w), and some of its other quantified constituents were: isoandrographolide (>0.3%, w/w), neoandrographolide (>1.0%, w/w), andrograpanin (>0.3%, w/w), and 14-deoxy-11,12 didehydroandrographolide (<5.0%, w/w). |
| Induction of type-2 diabetes |
| As described by Husain et al. [35], type-2 diabetes was induced in overnight fasted animals by a single intra peritoneal (i.p.) injection of 65 mg/kg streptozotocin (STZ; HiMedia Mumbai, India), 15 min after the i.p. administration of 120 mg/kg nicotinamide (SD Fine- Chemical Ltd., Mumbai, India). Animals were returned to their cages and provided normal food and 10% sucrose water to minimize hypoglycemic shock. The elevated glucose level in the blood confirmed hyperglycemia, quantified 72 hour and 7th day after STZ injections. Plasma glucose levels were quantified by the method described later. Only preselected diabetic animals with blood glucose levels higher than 250 mg/dl were used as diabetic animals for the behavioral studies. |
| Animal grouping and drug administration |
| For each series of experiments, six experimental groups consisting of 6 animals each were used, whereupon the animals were randomly allotted to different experimental groups. The non-diabetic and diabetic control groups were orally treated with 0.3% CMC (Carboxymethylcellulose, Central Drug House, New Delhi, India) for ten consecutive days. For oral treatments AP was suspended in 0.3% CMC, and 50, 100 and 200 mg/kg/day doses of the extract were administered daily for ten consecutive days. Choices of these doses and treatment regimen were based on observations made earlier in our laboratories with the same extract sample [4,11]. A reference or positive control group of diabetic animals treated similarly with imipramine (Sun Pharmaceutical Industries Ltd., Mumbai, India; 15 mg/kg/ day, p.o.) was always run in parallel in each set of experiments. The treatment groups used were as follow: Group I- Non-diabetic control (Vehicle); Group II- Diabetic control (Vehicle); Group III- Diabetic + AP 50 mg/kg; Group IV- Diabetic + AP 100 mg/kg; Group V- Diabetic + AP 200 mg/kg; and Group VI- Diabetic + Imipramine 15 mg/kg. |
| Rats behavioral despair test |
| The method described by Willner was followed [36]. In short, a rat was individually placed in a cylinder (45 × 20 cm) containing 38 cm water (25 ± 2°C), so that it could not touch the bottom of the cylinder with its hind limb or tail, or climb over the edge of the chamber. Two swim sessions were given to each rat; an initial 15 min pre-test session on day 9 of drugs treatment followed by a 5 min test session on the next day (i.e. on day 10 of the experiment). Period of immobility (i.e. the total time the animal remained floating in water without struggling and making only those movements necessary to keep its head above water) during the 5 min test period was recorded. |
| Rats learned helplessness test |
| The experimental procedure of Sherman et al. [37] was used with some modification, and has been described earlier in details elsewhere [38]. Briefly, the two parts of the test procedure were: |
| (a) Inescapable shock pretreatment: One hour after oral treatments on the 7th day, electric foot shocks were delivered to a given rat placed in a 20×10×10 cm plexiglass chamber with cover, and with a steel grid floor for delivering foot shocks. A constant current shocker delivering 60 scrambled, randomized inescapable shocks (15 sec duration, 0.8 mA, every min) was used. |
| (b) Conditioned avoidance training: Avoidance training was initiated 24 h after inescapable shock pretreatment in a jumping box. The jumping box were divided into two equal chambers (27×29×25 cm) by a plexiglass partition with a gate providing access to the adjacent compartment through a 14×17 cm open space in the partition. An individual animal was placed in one of the chambers of the jumping box and was allowed to habituate to the test environment for 5 min (for the first session only) and then was subjected to 30 avoidance trials (inter-trial intervals being 30 sec). During the first 3 sec of each trial, a light signal (conditioned stimulus) was presented, allowing the animals to avoid shocks. If a response did not occur within this period, a 0.8 mA shock (3 sec duration; unconditioned stimulus) was applied via the grid floor. In case no escape response occurred within this period, shock and light conditioned stimulus were terminated. Avoidance sessions performed for 3 consecutive days (days 8-10), and the number of escape failures (referred as no crossing response during shock delivery) were recorded. |
| Blood glucose and insulin estimation |
| Blood sample from rats was collected by the retro-orbital venous plexus sampling method on day 10 after performing behavior activity in learned helplessness test. Plasma was prepared by cold centrifugation (5°C) at 3000 rpm (845×g) for 5 minute (Compufuge CPR-30 Plus, with Rotor No. 8; REMI, India) and stored in a freezer (-20°C) till used for biochemical estimation. Plasma glucose levels were quantified by using a glucose test kit, based on glucose oxidase-peroxidase (GODPOD) method (Autospan Glucose test kit; Beacon Diagnostic Pvt. Ltd., Navasari, India). Plasma insulin levels were estimated by using an Enzyme Linked Immunosorbent Assay (ELISA) test kit (DRG Instruments GmbH, Germany). Both glucose and insulin estimations were performed by using absorbance micro-plate reader (iMarkTMBio- Rad Laboratories, California) according to instruction manual of the enzyme test kits used. |
| Brain tissues sample |
| On the 10th day after the learned helplessness test, rats were sacrificed by cerebral dislocation. Brain cortex and hippocampus was dissected out using the protocol described by Spijker [39]. They were weighted and stored in laboratory deep freezer at -80°C until use. Hippocampus part of brain was used for brain monoamines level and monoamine oxidase enzyme assays, and its frontal cortex was used for assaying antioxidant status. |
| Monoamines levels |
| Monoamine levels in hippocampus were quantified by the spectrofluorometric method described by Welch and Welch [40]. Briefly, hippocampus part of a brain was homogenized in 1.5 ml icecold 0.01 N HCl to which 0.1 ml 10% EDTA had been added. The homogenate was added to 25 ml n-butanol in 60 ml glass-stoppered bottle containing 4 gm NaCl. After centrifugation, 24 ml butanol was decanted in a bottle containing 40 ml n-hepatne and 1.5 ml of phosphate buffer and then centrifuged. Aqueous layer was transferred to a clean 30 ml bottle and was acidified with 3 N HCl to pH 3.5-4. After adding 20 ml of peroxide free ether the bottles were shaken for 10 min and centrifuged. The acid-aqueous layer was taken directly from the bottom of the ether extraction bottles and was refrigerated and analyzed later for NE (400/510 nm), DA (335/380 nm) and 5-HT (295/535 nm) in spectrofluorometer (RF 1501 Spectrofluorometer; Shimadzu, Tokyo, Japan) and compared with the standard calibration curves prepared from respective standard amines (Sigma-Aldrich, Co., St. Louis, MO). |
| Monoamine oxidase assay |
| Hippocampus mitochondrial fraction was prepared for estimating monoamine oxidase (MAO) activity [41]. Briefly, the mitochondrial fraction suspended in 10 volumes (1:10 w/v) of cold sodium phosphate buffer (10 mM, pH 7.4, and containing 320 mM sucrose), was mixed at 5°C for 20 min. The mixture was centrifuged at 15000×g for 30 min and the pellets were re├ó┬?┬Ésuspended in the same buffer. The MAO-A and MAO-B activity was assessed spectrophotometrically as described previously [42]. Briefly, the assay mixtures contained 4 mM 5├ó┬?┬ÉHT and 2 mM β├ó┬?┬ÉPEA as specific substrates for MAO-A and B, respectively, 250 μl solution of the mitochondrial fraction and 100 mM sodium phosphate buffer (pH 7.4) up to a final volume of 1 ml. The reaction was allowed to proceed at 37°C for 20 min, and stopped by adding 1M HCl (200 μl), the reaction product was extracted with 5 ml of butyl acetate (for MAO-A assay) and cyclohexane (for MAO-B assay), respectively. The organic phases were measured at a wavelength of 280 nm for MAO-A and 242 nm for MAO-B, respectively using Shimadzu UV/visible spectrophotometer. Blank samples was prepared by adding 1M HCl (200 μl) prior to reaction, and worked up as for the test samples. |
| Brain anti-oxidative status |
| The brain tissue (frontal cortex) was homogenized in ten volumes (1:10 w/v) of 20 mM sodium phosphate buffer (pH 7.4) containing 140 mM KCl using a Teflon-glass homogenizer. The homogenates were centrifuged at 750×g for 10 min at 4°C. Lipid peroxidation levels (LPO), and superoxide dismutase (SOD) and catalase (CAT) activities were quantified in the supernatants of tissue homogenates in duplicate by using microplate absorbance reader (iMark-Bio-Rad Laboratories, Hercules, CA). For LPO levels, lipid peroxidation was quantified by measuring the level of malondialdehyde (MDA) and expressed as nmol MDA/mg protein according to the method of Ohkawa et al. [43]. For SOD activity, the method described by Kakkar et al. [44] was followed, and the results were expressed as units of SOD activity/mg protein. For CAT activities the standard method described elsewhere was used and expressed as μmol H2O2 decomposed/min/mg protein [45]. Protein estimation was performed by the method of Lowry et al. [46]. |
| Statistical Analysis |
| Mean ± standard error of mean (SEM) was calculated for the observed values in each experimental group (n=6). Statistical analysis was performed by ordinary one way analysis of variance (ANOVA) followed by Student-Newman-Keuls multiple comparison test. GraphPad Prism 6 was used for statistical analysis (GraphPad Software Inc., San Diego, CA). P value less than 0.05 was always considered as statistically significant. |
| Results |
| Behavioral despair test |
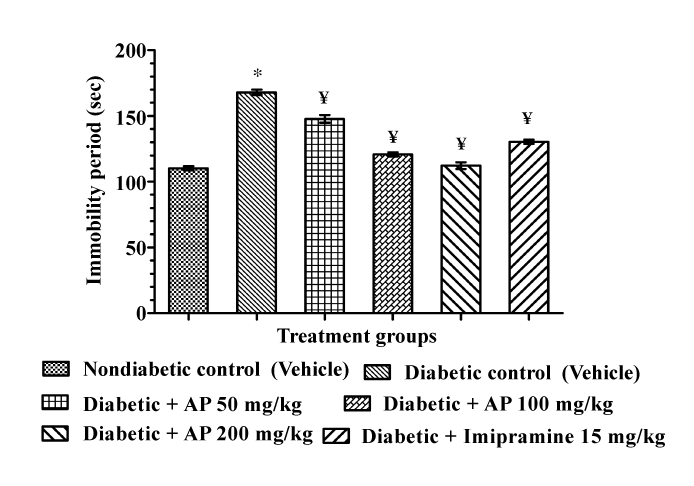
| Effects of AP and imipramine treatments on the duration of immobility in the diabetic rat behavioral despair test are summarized in Figure 1. Mean immobility period of the diabetic control group was significantly higher than that of the non-diabetic control group. Ten daily AP treatments to diabetic rats at doses of 50, 100 and 200 mg/ kg/day significantly and dose-dependently decreased the duration of immobility [F (5, 30)=115.8, P<0.05]. Numerically, the mean immobility period of 200 mg/kg/day AP treated group was somewhat lower than that of the group treated with 15 mg/kg/day imipramine. However, efficacies of the two higher AP doses tested were almost equal in magnitude to that of the standard antidepressant drug imipramine. |
| Learned helplessness test |
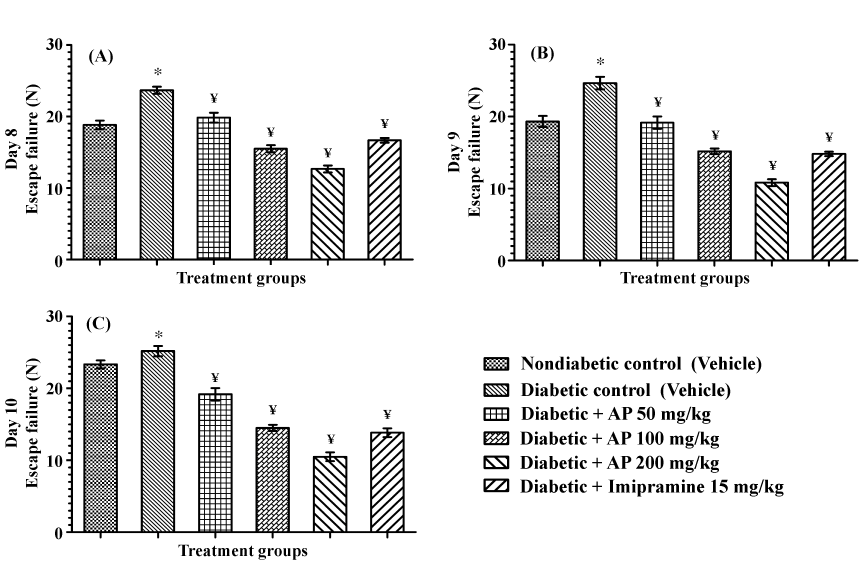
| Mean numbers of escape failures of the vehicle treated diabetic control group in this test on all the three observational days were significantly higher, than those observed for the non-diabetic control group (Figure 2). AP treatments dose-dependently reduced the escape failures of diabetic rats on all test days, and its efficacy increased somewhat during the three days [F (5, 30)=51.51, P<0.05; F (5, 30)=52.91, P<0.05; and F (5, 30)=80.34, P<0.05 for day 8, 9 and 10 respectively]. Efficacy of 100 mg/kg/day AP was similar in magnitude to that of 15 mg/kg/day of the standard antidepressant imipramine, and that of the 200 mg/kg/day AP treated group was somewhat higher than that of the standard antidepressant. |
| Body weight |
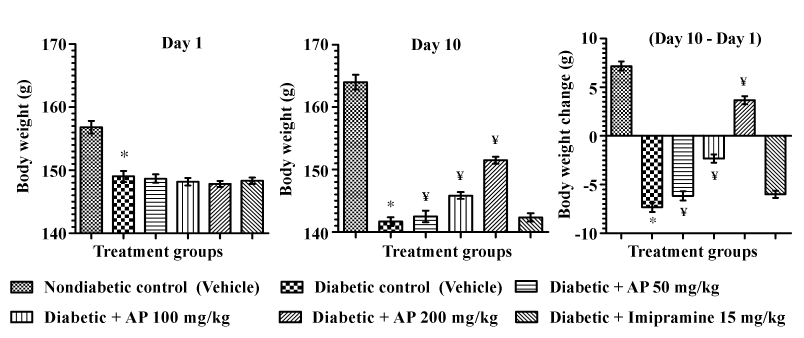
| Unlike in the non-diabetic control group, the mean body weights of the vehicle treated diabetic control group during the treatment period decreased considerably. Such body weight losses were less severe in the 50 and 100 mg/kg/day AP treated diabetic group, and 200 mg/kg/day AP treated diabetic animals gained some body weights during the 10 days of the treatment [F (5, 30)=179.4, P<0.05]. Imipramine treatments had no significant effects on the body weight losses of diabetic animals. These results are summarized in Figure 3. |
| Blood glucose and insulin level |
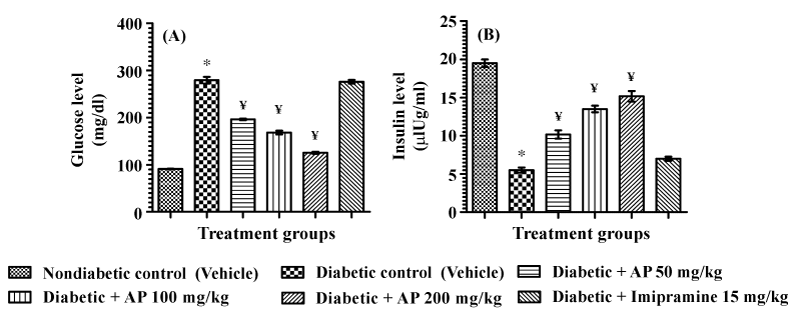
| Mean blood glucose and insulin levels of the different test groups quantified just after completion of the learned helplessness test are summarized in Figure 4. Ten daily imipramine treatments had no effects on hyperglycemia or on insulin deficiency observed in diabetic animals. AP treatments dose-dependently decreased the blood glucose levels of diabetic animals [F (5, 30)=245.3, P<0.05], and the blood insulin levels diabetic rats were also significantly and dose-dependently increased by AP treatments [F (5,30)=143.9, P<0.05]. |
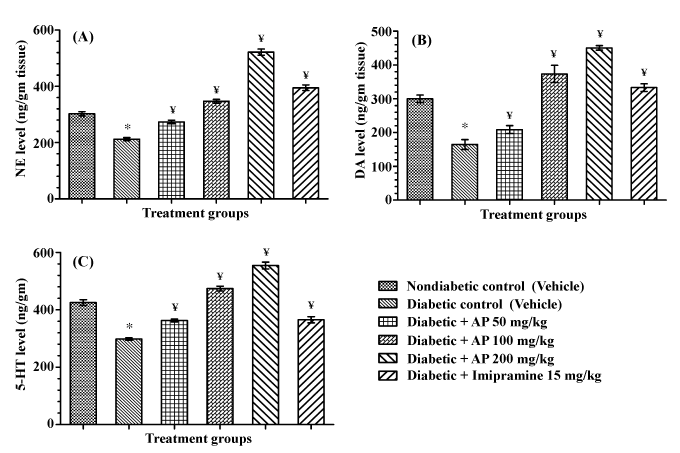
| Monoamines level in hippocampus |
| Hippocampal levels of all the three monoamine quantified (NE, DA, and 5-HT) in vehicle treated diabetic control rats were lower than those observed in non-diabetic control rats. AP (50, 100 and 200 mg/ kg) treatments to diabetic rats significantly and dose-dependently increased the levels of all the three monoamines in diabetic animals [F (5,30)=177.7, P<0.05; F(5, 30)=52.03, P<0.05; and F(5, 30)=106.3, P<0.05 for NE, DA, and 5-HT respectively], and their levels observed in the 100 and 200 mg/kg/day AP treated diabetic groups rats were higher than those of the non-diabetic control group. Such efficacies of imipramine (15 mg/kg/day) in diabetic rats were somewhat lower than those observed after the intermediate dose of AP (100 mg/kg/day) treated diabetic group. These results are summarized in Figure 5. |
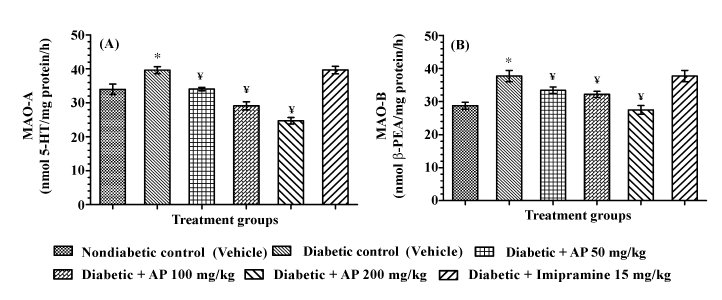
| Monoamine oxidase activity in hippocampus |
| Results of the MAO-A and MAO-B assays conducted with mitochondria preparations from rat hippocampus of different groups of animals used in the learned helplessness test are summarized in Figure 6. Mean enzymatic activities observed in the diabetic control group were significantly higher than those of the non-diabetic control group. Imipramine treatment had no significant effects on these enzymatic activities in diabetic animals. AP treatments dosedependently decreased enzymatic activities of both MAO-A [F (5, 30) = 28.62, P < 0.05] and MAO-B [F (5, 30) =10.83, P <0.05] in diabetic animals, and the MAO-A activity levels quantified in the 100 and 200 mg/kg/day AP treated groups were even lower than that estimated in the vehicle treated non-diabetic control group. |
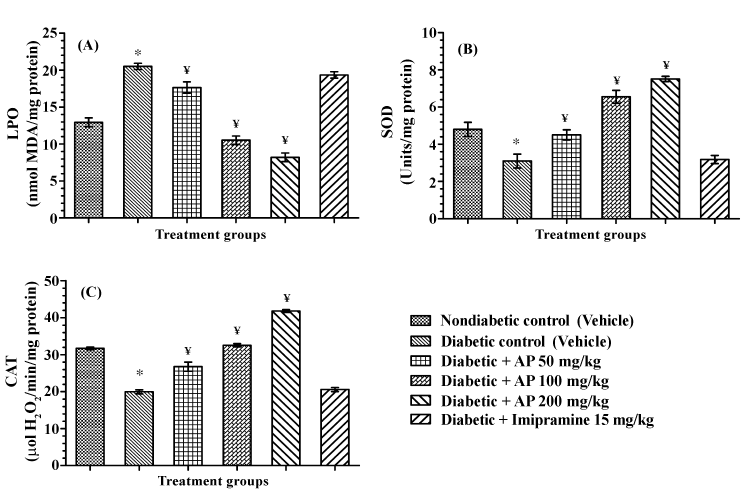
| Antioxidative status in frontal cortex |
| The results summarized in Figure 7 revealed that in comparison to the non-diabetic control group, mean LPO level and SOD and CAT activities in frontal cortex of the diabetic control group were significantly altered. Imipramine treatments had no significant effects on any of these assayed parameters. AP treatments dose-dependently and significantly lowered LPO level in diabetic animals [F (5, 30)=77.27, P<0.05] and the SOD and CAT activities of AP treated diabetic animals [F (5, 30) =34.99, P<0.05; and F (5, 30)=158.1, P<0.05 respectively for SOD and CAT] were significantly higher than those of the diabetic control group. Mean LPO value of the higher two AP dose (100 and 200 mg/kg/day) treated diabetic groups was lower than that of the non-diabetic control group, and the SOD and CAT activities of these two AP treated groups were either higher or equal to those of the nondiabetic control one. |
| Discussion |
| The behavioral despair and learned helplessness tests are two wellknown and commonly used behavioral test for assessing depressive state of animals, and they are often used for identifying therapeutic leads against clinical depression. In the present study the depressive state of vehicle treated type-2 diabetic rats in both the tests were more pronounced than non-diabetic ones, and imipramine like antidepressant activity of AP in diabetic rats were also observed in both the tests. Even 50 mg/kg/day dose of AP completely antagonized the exaggerated depressive behavior of diabetic rats in the behavioral despair test and its antidepressants like efficacies observed in this test after its 100 or 200 mg/kg/day doses were quantitatively almost equal to that of the tested imipramine dose (15 mg/kg/day). Analogous were also the behavioral observations made on the first test day in the learned helplessness test. Efficacy of AP in this test increased somewhat on the subsequent two testing days, and efficacies of 200 mg/kg/day dose of AP were always higher than that of imipramine on all the three test days. These differences could as well be due the difference in the modes of actions of imipramine and AP. |
| The behavioral despair test is more specific for imipramine and other inhibitors of synaptic monoamine reuptake, whereas the learned helplessness test is sensitive also to diverse other psychoactive agents with antidepressant, anxiolytics, cognitive function modulating, or stress response modulating agents [47,48]. Earlier neuropsychopharmacological observations made in our laboratories have revealed that even 25 mg/kg daily oral doses of AP completely blocks the handling and mild foot shock stress triggered physiological responses and that it also possess anxiolytics like efficacies in other behavioral tests using in non-diabetic rodents [11]. Thus it seems reasonable to assume that AP is a stress response modulating or adaptogenic agent with a broader spectrum of therapeutically interesting psychopharmacological activity profile than synaptic monoamine reuptake inhibitors. |
| That such is indeed the case is evident also from several other observations made during this study. These include the observed beneficial effects of AP against body weight losses, hyperglycemia, hypo-insulinemia in diabetic rats and also against worsened oxidative status and effectively reduced MAO activities in their brain regions studied. Clear dose-dependent effects of AP on all these parameters were observed, whereas no significant effects of the tested antidepressant dose of imipramine were observed. However, the lower hippocampal levels of all three monoamines quantified in the type-2 diabetic control rats were antagonized by both AP and imipramine. Although qualitatively these observed effects of AP were quite analogous to those of the antidepressant, here again the efficacy of the highest AP dose tested was higher than that observed for imipramine. Thus, it seems reasonable to assume that although AP is not an imipramine like psychoactive agent, its observed antidepressant like efficacy in animal models are due to its modulating effects on central monoaminergic neurotransmitter systems. Such effects of the extract seem to be due its suppressive effects on brain mitochondrial monoamine oxidase activities. |
| Taken together with other reports on therapeutically interesting bioactivities of andrographolide extracts, our observations strongly suggest that the behavioral effects of AP in diabetic animals is due to its beneficial effects against oxidative damages caused by hyperglycemia and insulin deficiency. Both anti-hyperglycemic and anti-oxidative effects of andrographolide extracts and andrographolide in diabetic animals have often been reported [49], and more recently a report revealing cerebroprotective and nootropics like efficacy of an andrographolide extract in type-2 diabetic rats has appeared also [10]. However, in this later mentioned and diverse other preclinical reports the tested extracts were administered intraperitoneally, and as yet no reports on the effects of andrographolide extracts and their constituents on brain monoamine levels, oxidative status, and mitochondrial monoamine oxidase activities, or on the depressive state of diabetic and other animals, have appeared. The observed bio- and neuro-chemical alterations reported in this communication revealed that even the lowest oral AP dose tested (50 mg/kg/day for ten days) was effective in reversing the altered enzymatic activities of both oxidative (MAO-A and MAO-B) as well as anti-oxidative (SOD and catalase) enzymes and lipid peroxide levels in the brain samples of diabetic animals to those observed in non-diabetic ones. This dose of AP was also effective in partially reversing the lower hippocampal levels of the three quantified monoamines (NE, DA, and 5-HT) in diabetic rats. |
| Thus, the observations reported in this communication reveal not only that antidepressants like efficacy of AP is maintained in type-2 diabetic rats, but also that its minimal effective psychoactive oral doses are not higher than 50 mg/kg/day. Moreover, they add further experimental to the convictions that modulating effects of the extract on brain functions are also involved in its clinically observed symptomatic relief after treatments with this and other andrographolide extracts [50], and that AP could as well be a therapeutic herbal alternative for treatments of depression and diverse other psychopathologies commonly associated with or caused by type- 2 diabetes and other metabolic disorders. Since currently available antidepressants and all other psychoactive drugs do not properly meet the therapeutic demands of such patients, and are often contraindicated for such purposes, further efforts to more precisely define its sites and modes of actions and its bioactive principles, are now being made in our laboratories. |
| Observation made to date to identify the antidepressant and adaptogenic components of AP have revealed that andrographolide is indeed is the quantitatively major such component of the extract (manuscript under preparation). Although the efficacy of pure andrographolide have not yet been tested in a type-2 diabetic animals, it has been reported that even very low daily oral doses (1.5 mg/kg/ day) of pure andrographolide possess anti-hyperglycemic activity in diabetic but not in normal rats [51]. Therefore, it seems reasonable to assume that the observed effects of AP on brain monoamines and oxidative status of diabetic animals is primarily due to its effects on some biological processes and mechanisms involved in deregulation of glucose homeostasis. Information on available on oral bioavailability of pure andrographolide [52] and other bioactivity constituents of andrographolide extracts [53,54] strongly suggest that the primary sites of actions andrographolide and other bioactive constituents of AP most probably is not the brain tissue itself. It is now well recognized that gut microbial ecology and the so called microbiota-gut-brain axis are involved in physiological regulation of brain functions and metabolic processes [55-59]. Therefore, it seems reasonable to assume that high efficacies of AP and andrographolide observed in our studies could as well be due to its regulatory effects on gut microbial ecology and enteric nervous system. |
| That such could indeed be the case is suggested also by the fact that andrographolide and other constituents of possess antibacterial and antiviral activities and that andrographolide and its structural analogues present in AP are extremely bitter substance with high affinity to specific bitter receptors present also in the entire gastrointestinal tract and other organs [60,61]. It has been reported also that andrographolide forms strong covalent bonds with endogenous thiols and macromolecules involved in regulation of oxidative and other processes leading to metabolic disturbances [62]. Therefore, it can be expected that the pharmacologically pleiotropic or polyvalent and therapeutically interesting bioactivities of AP and andrographolide observed in experimental animals after their daily oral doses are due to their irreversible interactions with biologically important macromolecules within the gastrointestinal tract. Efforts to identify such macromolecules and their biological functions could eventually lead to identification of novel pharmacological targets involved in the therapeutically interesting activity profile of AP reported in this and our earlier reports on this extract. |
| Thus, the observations reported in this communication have revealed not only a novel therapeutic potential of AP for prevention or cure of diabesity associated depression and other psychopathologies, but also have supplied us with a tool potentially useful for identifying novel pharmacological targets potentially useful for discovering novel therapeutic leads against co-morbid psychopathologies commonly associated with metabolic disorders and other chronic diseases. Since we have been successful in identifying andrographolide as quantitatively major psychoactive constituent of AP, we are now concentrating our efforts to identify its primary pharmacological site of action involved in its observed brain function modulating activities only. It must be mentioned though, that like all other plant extracts AP also contains other phytochemicals with known brain function modulating and hyperglycaemic activities worth following further not only for drug discovery purposes, but also for better understanding of the biological processes involved in the modes of actions of the traditionally known and still widely used medicinal plant andrographolide. |
| Conclusion |
| Andrographis paniculata extracts rich in andrographolide could be a herbal alternatives for treatments of diabetes associated depressive disorders and other co-morbidities. Further efforts necessary for conducting properly controlled clinical trials for such purposes can be warranted. |
| Conflict of Interest |
| The authors declare that there are no financial and other conflicts of interest in this study. |
| Acknowledgements |
| Ajit Kumar Thakur graciously acknowledged the Department of Science and Technology, Government of India, New Delhi for awarding INSPIRE Fellowship (IF110595). Authors would like to thank Natural Remedies Pvt. Ltd., Bangalore for generously providing the analytically well characterized extract of andrographolide. |
| References |
References
- World Health Organization (2004) Monographs on selected medicinal plants. Volume 2, 358.
- Chandrasekaran CV, Thiyagarajan P, Sundarajan K, Goudar KS, Deepak M, et al. (2009) Evaluation of the genotoxic potential and acute oral toxicity of standardized extract of Andrographis paniculata (KalmCold). Food ChemToxicol 47: 1892-1902.
- Thakur AK, Chatterjee SS, Kumar V (2013) Antidiabetic activity of standardised extract of Andrographis paniculata in rodents. Proceedings of the 2nd International Pharma-Nutrition Conference (Elsevier), MAX Atria, Singapore.
- Thakur AK, Chatterjee SS, Kumar V (2014) Therapeutic potential of traditionally used medicinal plant Andrographis paniculata (Burm. F.) against diabesity: An experimental study in rats. TANG: Int J Genuine Tradit Med 4: e7.1-e7.8.
- Zhang XF, Tan BK (2000) Antihyperglycaemic and anti-oxidant properties of Andrographis paniculata in normal and diabetic rats. ClinExpPharmacolPhysiol 27: 358-363.
- Nagalekshmi R, Menon A, Chandrasekharan DK, Nair CK (2011) Hepatoprotective activity of Andrographis paniculata and Swertiachirayita. Food ChemToxicol 49: 3367-3373.
- Sheeja K, Shihab PK, Kuttan G (2006) Antioxidant and anti-inflammatory activities of the plant Andrographis paniculataNees. ImmunopharmacolImmunotoxicol 28: 129-140.
- Saxena RC, Singh R, Kumar P, Yadav SC, Negi MP, et al. (2010) A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomedicine 17: 178-185.
- Mandal SC, Dhara AK, Maiti BC (2001) Studies on psychopharmacological activity of Andrographis paniculata extract. Phytother Res 15: 253-256.
- Radhika P, Annapurna A, Rao SN (2012) Immunostimulant, cerebroprotective&nootropic activities of Andrographis paniculata leaves extract in normal & type 2 diabetic rats. Indian J Med Res 135: 636-641.
- Thakur A, Chatterjee S, Kumar V (2013) Neuropsychopharmacology of a therapeutically used Andrographis paniculata extract: A preclinical study. Oriental Pharm Exp Med.
- Thakur AK, Chatterjee SS, Kumar V (2012) General neuropharmacological screening of standardized extract of Andrographis paniculata in rodents. Proceedings of the XXX Annual Conference of Indian Academy of Neurosciences (IAN) & International Symposium on Translational Neuroscience/Annals of Neurosciences. Amritsar, India.
- Lin EH, Rutter CM, Katon W, Heckbert SR, Ciechanowski P, et al. (2010) Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care 33: 264-269.
- Dirmaier J, Watzke B, Koch U, Schulz H, Lehnert H, et al. (2010) Diabetes in primary care: prospective associations between depression, nonadherence and glycemic control. PsychotherPsychosom 79: 172-178.
- Roy T, Lloyd CE, Pouwer F, Holt RI, Sartorius N (2012) Screening tools used for measuring depression among people with Type 1 and Type 2 diabetes: a systematic review. Diabet Med 29: 164-175.
- de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ (2001) Association of depression and diabetes complications: a meta-analysis. Psychosom Med 63: 619-630.
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, et al. (2000) Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 23: 934-942.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ (2001) The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 24: 1069-1078.
- Hilakivi-Clarke LA, Wozniak KM, Durcan MJ, Linnoila M (1990) Behavior of streptozotocin-diabetic mice in tests of exploration, locomotion, anxiety, depression and aggression. PhysiolBehav 48: 429-433.
- Husain GM, Chatterjee SS, Singh PN, Kumar V (2011) Beneficial effect of Hypericumperforatum on depression and anxiety in a type 2 diabetic rat model. Acta Pol Pharm 68: 913-918.
- Rowland NE, Bellush LL (1989) Diabetes mellitus: stress, neurochemistry and behavior. NeurosciBiobehav Rev 13: 199-206.
- Thakur AK, Chatterjee SS, Kumar V (2013) Beneficial effects of Brassica juncea on cognitive functions in rats. Pharm Biol 51: 1304-1310.
- Thakur AK, Chatterjee SS, Kumar V (2013) Anxiolytic-like activity of leaf extract of traditionally used Indian-mustard(Brassica juncea) in diabetic rats. TANG: Int J GenuinTradit Med 3: 1-7.
- Thakur AK, Chatterjee SS, Kumar V (2014) Antidepressant-like effects of Brassica juncea leaves in diabetic rodents. Indian J Exp Biol.
- MacKenzie RG, Trulson ME (1978) Effects of insulin and streptozotocin-induced diabetes on brain tryptophan and serotonin metabolism in rats. J Neurochem 30: 205-211.
- Trulson ME, Himmel CD (1985) Effects of insulin and streptozotocin-induced diabetes on brain norepinephrine metabolism in rats. J Neurochem 44: 1873-1876.
- Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE (1996) Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care 19: 1097-1102.
- Kawakami N, Takatsuka N, Shimizu H, Ishibashi H (1999) Depressive symptoms and occurrence of type 2 diabetes among Japanese men. Diabetes Care 22: 1071-1076.
- Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, et al. (2010) Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia 53: 2480-2486.
- Andersohn F, Schade R, Suissa S, Garbe E (2009) Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry 166: 591-598.
- Brown LC, Majumdar SR, Johnson JA (2008) Type of antidepressant therapy and risk of type 2 diabetes in people with depression. Diabetes Res ClinPract 79: 61-67.
- Deuschle M (2013) Effects of antidepressants on glucose metabolism and diabetes mellitus type 2 in adults. CurrOpin Psychiatry 26: 60-65.
- Goodnick PJ (2001) Use of antidepressants in treatment of comorbid diabetes mellitus and depression as well as in diabetic neuropathy. Ann Clin Psychiatry 13: 31-41.
- Goodnick PJ, Henry JH, Buki VM (1995) Treatment of depression in patients with diabetes mellitus. J Clin Psychiatry 56: 128-136.
- Husain GM, Singh PN, Singh RK, Kumar V (2011) Antidiabetic activity of standardized extract of Quassiaamara in nicotinamide-streptozotocin-induced diabetic rats. Phytother Res 25: 1806-1812.
- Willner P (1984) The validity of animal models of depression. Psychopharmacology (Berl) 83: 1-16.
- Sherman AD, Sacquitne JL, Petty F (1982) Specificity of the learned helplessness model of depression. PharmacolBiochemBehav 16: 449-454.
- Kumar V, Singh PN, Jaiswal AK, Bhattacharya SK (1999) Antidepressant activity of Indian Hypericumperforatum Linn in rodents. Indian J ExpBiol 37: 1171-1176.
- Spijker S (2011) Dissection of Rodent Brain Regions. In: Li KW, Neuroproteomics, Part II. New York: Humana Press Inc, 13-26.
- Welch AS, Welch BL (1969) Solvent extraction method for simultaneous determination of norepinephrine, dopamine, serotonin, and 5-hydroxyindoleacetic acid in a single mouse brain. Anal Biochem 30: 161-179.
- Schurr A, Livne A (1976) Differential inhibition of mitochondrial monoamine oxidase from brain by hashish components. BiochemPharmacol 25: 1201-1203.
- Charles M, McEwen J (1977) In: Tabor H, Tabor CW, Methods in Enzymology, XVIIB. New York and London: Academic Press, 692-698.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J BiochemBiophys 21: 130-132.
- Luck H (1963) Methods of enzymatic analysis. In: Bergmeyer HU, editor. VerlagChemie, Weinheim and New York: Academic Press, 885-888.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J BiolChem 193: 265-275.
- Takamori K, Yoshida S, Okuyama S (2001) Availability of learned helplessness test as a model of depression compared to a forced swimming test in rats. Pharmacology 63: 147-153.
- Yan HC, Cao X, Das M, Zhu XH, Gao TM (2010) Behavioral animal models of depression. Neurosci Bull 26: 327-337.
- Chao WW, Lin BF (2010) Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin Med 5: 17.
- Panossian A, Wikman G (2013) Efficacy of Andrographis paniculata in Upper Respiratory Tract Infectious Diseases and the Mechanism of Action. In: Wagner H, Ulrich-Merzenich G, Evidence and Rational Based Research on Chinese Drugs. Vienna: Springer, 137-179.
- Yu BC, Hung CR, Chen WC, Cheng JT (2003) Antihyperglycemic effect of andrographolide in streptozotocin-induced diabetic rats. Planta Med 69: 1075-1079.
- Ye L, Wang T, Tang L, Liu W, Yang Z, et al. (2011) Poor oral bioavailability of a promising anticancer agent andrographolide is due to extensive metabolism and efflux by P-glycoprotein. J Pharm Sci 100: 5007-5017.
- Panossian A, Hovhannisyan A, Mamikonyan G, Abrahamian H, Hambardzumyan E, et al. (2000) Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine 7: 351-364.
- Wang J, Yang W, Wang G, Tang P, Sai Y (2014) Determination of six components of Andrographis paniculata extract and one major metabolite of andrographolide in rat plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B AnalytTechnol Biomed Life Sci 951-952: 78-88.
- Montiel-Castro AJ, González-Cervantes RM, Bravo-Ruiseco G, Pacheco-López G (2013) The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front IntegrNeurosci 7: 70.
- Kelsen JR, Wu GD (2012) The gut microbiota, environment and diseases of modern society. Gut Microbes 3: 374-382.
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, et al. (2012) Host-gut microbiota metabolic interactions. Science 336: 1262-1267.
- McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Lošo T, et al. (2013) Animals in a bacterial world, a new imperative for the life sciences. ProcNatlAcad Sci U S A 110: 3229-3236.
- Thakur AK, Shakya A, Husain GM, Emerald M, Kumar V (2014) Gut-Microbiota and Mental Health: Current and Future Perspectives. J PharmacolClinToxicol 2: 1016.
- Behrens M, Brockhoff A, Batram C, Kuhn C, Appendino G, et al. (2009) The human bitter taste receptor hTAS2R50 is activated by the two natural bitter terpenoidsandrographolide and amarogentin. J Agric Food Chem 57: 9860-9866.
- Clark AA, Liggett SB, Munger SD (2012) Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J 26: 4827-4831.
- Xia YF, Ye BQ, Li YD, Wang JG, He XJ, et al. (2004) Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J Immunol 173: 4207-4217.
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 16099
- [From(publication date):
specialissue-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11401
- PDF downloads : 4698
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
