Research Article Open Access
Anticholinesterase and Antioxidant Potentials of a Medicinal Plant Abroma augusta: Implications for the Alternative Treatment Therapy of Cognitive Deficits in Alzheimers disease
| Mst. Marium Begum1, Kushal Biswas2, Arjyabrata Sarker3, Tamanna Binte Huq4, Abeer Sarwar4, Md. Belal Hossain5, Hasan Tarek5, Md. Noor-A-Alam5and Asma Rahman6* | |
| 1Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh | |
| 2Department of Pharmacy, University of Rajshahi, Rajshahi-6205, Bangladesh | |
| 3Department of Pharmacy, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh | |
| 4Department of Pharmaceutical Sciences, North South University, Dhaka-1229, Bangladesh | |
| 5Department of Pharmacy, Dhaka International University, Dhaka-1213, Bangladesh | |
| 6Centre for Advanced Research in Sciences (CARS), University of Dhaka, Dhaka-1000, Bangladesh | |
| Corresponding Author : | Asma Rahman Centre for Advanced Research in Sciences (CARS) University of Dhaka, Dhaka-1000, Bangladesh Tel: +8801718617915 E-mail: asmadupharma@gmail.com |
| Received: August 07, 2015; Accepted: October 23, 2015; Published: October 28, 2015 | |
| Citation: Begum MM, Biswas K, Sarker A, Huq TB, Sarwar A, et al. (2015) Anticholinesterase and Antioxidant Potentials of a Medicinal Plant Abroma augusta: Implications for the Alternative Treatment Therapy of Cognitive Deficits in Alzheimer’s disease. Clin Pharmacol Biopharm 4:148. doi:10.4172/2167- 065X.1000148 | |
| Copyright: © 2015 Begum MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Oxidative stress and low level of neurotransmitter (especially acetylcholine) are main characteristics of Alzheimer’s disease (AD), a progressive neurodegenerative disease. Prolonging the function of acetylcholine by inhibiting acetylcholinesterase or butyrylcholinesterase enzyme and reducing oxidative stress with antioxidants are most effective treatment therapy of AD. Abroma augusta is a well-known medicine plant with a variety of medicinal uses. In this study we examine cholinesterase inhibitory activity as well as antioxidant activity of dried fruit extract including crude methyl extract and its sub fractions (Chloroform fraction, Petroleum ether fraction, Ethyl acetate fraction and aqueous fraction) of A. augusta. Both cholinergic inhibitory activity and antioxidant activity of various fractions suggested that, ethyl acetate fraction are most prominent among all fractions and can be used in symptomatic treatment of AD.
| Introduction |
| AD is a progressively developing neurodegenerative cognitive disorder of the brain, primarily found in the elder person with symptoms of memory loss, cognitive and behavioral abnormalities [1-5]. Alzheimer’s Association estimated that one of eight persons in America, after age 65, suffer from AD. According to this estimation the number of AD patients will be 16 million by 2050 [6]. |
| The pathology of AD is complex and multifaceted. Several pathogenic conditions are believed to contribute to the progression of disease. Oxidative stress, cholinergic deficit and senile plaque formation are some major sign of AD [7-9]. Among several causes that play vital role in oxidative stress are beta amyloid, reactive oxygen species and reactive nitrogen species. All of these cause major destruction of brain area like cortex and hippocampus in the early stages of AD [10,11]. Acetylcholinesterase enzyme (AChE) is a prominent marker of AD. Formation of relatively AChE cause more breakdown of ACh in the brain and ultimately cause decrease of ACh in the nervous system [12,13]. Similarly Butyrylcholinestyerase (BChE) enzyme act synergistically with AChE to hydrolyze both ACh and Butyrylcholine in the nerve endings [14,15]. Both AChE and BChE are involved in the breakdown of neurotransmitter. So by inhibiting these enzyme results increase concentration of ACh in the CNS leads to improve cognitive functions [16-19]. β-amyloid associated free radicals can initiate lipid peroxidation, protein oxidation, ROS formation, intracellular and mitochondrial Ca2+ accumulation and eventually lead to death of neurons [20-24]. Therefore, inhibition of Cholinesterase enzyme and prevent oxidative stress devised as a suitable strategy for providing symptomatic treatment to AD as well as other forms of dementia and myasthenia gravis [25,26]. |
| The synthetic drugs approved for AD from FDA such as Tacrine, Rivastigmine and Donepezil are associated with a lot of adverse effects like gastrointestinal disturbance, liver associated problems and other nervous system. Beside these expensiveness further adds limitations of these drugs [27-29] All these limitations prompts an urgent need to look for new lead compound from different sources including plant based natural products. |
| Abroma augusta (Family: Sterculiaceae, Bengali name: Ulatkambal, English name: Devil’s cotton, DC) a species of Abroma, with a dark red flower with an characteristics appearance [30]. Geographically it is widely distributed in Asia (especially India, Bangladesh and Sri Lanka). It is one of the most widely used medicinal plants in the treatment of various ailments. The root parts of the plants were used as a uterine tonic and several urine disorders. Powdered root plants acts as an abortification and fertility agent. Leaves are useful in treating uterine disorder, diabetes, rheumatic pains of the joint infections, headache with sinusitis. Leaves and stems are documented as gonorrhea treating agent [31-37]. |
| Primarily the plant contains alkaloids. The root bark of the plant contain alkaloids (abromine), sterol (friedelin, abromasterol, taraxesteryl acetate, taraxerol β sitosterol) etc [38]. Although A. augusta has important medicinal value no studies have yet examined for its anti-alzheimer’s activities. This study discusses the investigation of dried fruit extract of A. augusta in antioxidant, acetylcholinesterase and butyrylcholinesterase inhibitory point of view relevant to the treatment of Alzheimer’s Disease. |
| Method and Materials |
| Almost all chemicals were used in this study are analytical grade. Acetylthiocholine iodide, Butyryllthiocholine iodide, Donepezil, Galantamine, Folin–Ciocalteu reagent, 2-deoxy-D-ribose, thiobarbituric acid, (+)-catechin, 5, 5’dithio-bis-(2-nitro) benzoic acid, Gallic Acid were brought from Sigma-Aldrich (Tokyo, Japan). Aluminium chloride, Ammonium molybdate, Ascorbic acid (AA), DPPH, Tris-HCl and Triton X-100 were obtained from Wako Pure Chemical Company Ltd. (Osaka, Japan). Solvents are used in this experiment are also analytical grade. |
| Plants materials |
| Fruits of A. augusta are collected from Rajshahi, Bangladesh in January and February 2014. All fruits are authenticated by an expert taxonomist from Botany Department of Rajshahi University. Beside this a voucher specimen were submitted to the herbarium of University of Rajshahi, Bangladesh. |
| Preparation of the sample |
| After collection ripe fruits were dried into shadow. The dried fruit were cut into small pieces and ground into fine powder using a dry grinder. The grinded sample were sieved to get uniform particle size and kept it into air tight container. |
| Extraction |
| Powdered fruit (500 g) were placed into an amber coated bottle and soaked into 1500 mL of methanol. The contents were sealed into bottle for 7 days with occasionally stirred and shaken in every 30 minutes. After 7 days the whole mixture were filtered by Whitman No. 1 filter papers and filtrated were finally concentrated with rotary evaporator under reduced pressure below 50°C. About 16.298 gm of crude methanolic extract (CME) were found. |
| An aliquot of (12 gm) of concentrated CME were further fractionated by petroleum ether, Chloroform, Ethyl Acetate and pure double distilled water to get petroleum ether extract (PEF), Chloroform extract fraction (CEF), Ethyl Acetate fraction (EAF) and pure Aqueous fraction (AQF) respectively. The process were done on the basis of polarity [39,40]. Non-polar solvents were used first then less polar and finally polar solvents are added to the extract. The extraction methods are done by modified kupchan method. |
| Analysis of antioxidant properties |
| 1,2-diphenyl-2-picrylhydrazyl radical (DPPH) scavenging activity [41]: 0.1 mL of various concentrations of sample was mixed with 0.4 mL of 0.3 mM DPPH reagent prepared in methanol. Mixture were shaken and kept into dark at room temperature for 30 min. absorbance of the reaction mixture was taken at 517 nm. DPPH free radical scavenging activity was calculated by the following formula: |
| % of scavenging = |
| Determination of hydroxyl radical scavenging activity [42]: Hydroxyl radical scavenging activity of the samples was determined by using 2-deoxy-d-ribonucleic acid. To generate hydroxyl radical Ferric- Ascorbate-EDTA-H2O2 system were used. The reaction contained 2-deoxy-d-ribonucleic acid (2.8 nM), KH2PO4-KOH buffer (20 nM, pH 7.4). FeCl3 (100 μM), H2O2 (1.0 mM), Ascorbic Acid and various concentration of samples or standards (+) Catechin was used as a reference standard. Reaction mixture incubated for 1hour at 37°C after incubation 0.5 mL reaction mixture added to 1 mL 2.8% Thiobarbituric acid and 1 mL 1% ice cool Tricholoroacetic acid. The whole mixture incubated at 100°C for 30 minutes to develop colors. After cooling absorbances were taken at 532 nm against an appropriate blank solution. Following formula of calculating scavenging activity: |
| % of scavenging = |
| Determination of nitric oxide radical scavenging activity [43,44]: Nitric Oxide radical scavenging activity was determined by using sodium nitroprusside 5mM, prepared in phosphate buffer pH 7.4. 1 mL of various concentrations of fractions, sodium nitroprusside 0.3 mL was added. The test tubes were incubated for 5 hour at 25ºC. Afterward 0.5 mL of Griess reagent was added to the test tubes. Absorbance was taken at 546 nm. |
| Determination of reducing power [45,46]: Reducing power of the sample were determined by Oyaizu method [42]. Plant extract/ Standard (1 mL) were mixed with 2.5 mL Potassium buffer Potassium ferricyanide and incubated at 50°C for 20 min. 2.5 mL of CCl3COOH (10%) solution added to it. Then reaction mixture centrifuged at 3300 RPM for 10 min. After that 2.5 mL supernatant of the reaction mixture, 2.5 mL distilled water and 0.5 mL FeCl3 (1%) solution were taken into a test tube. Absorbance was taken at 700 nm. Ascorbic acid was used as reference standard. |
| Determination of lipid peroxidation inhibition assay [47-49]: Lipid peroxidation inhibition assay were determined by Liu method with slight modification. About 150 g adult long Evan rats brain were dissected and homogenized with a homogenizer in ice-cold phosphate buffer (50 mM, pH 7.4) to produce a 1/10 homogenate (as lipid source). The homogenate was centrifuged for 15 min in 10000 rpm at 4°C. The final supernatant was used as liposome for an in vitro lipid peroxidation assay. Supernatant treated with hydrogen peroxide (10 μM) and different concentrations of plant extract where hydrogen peroxide induced lipid peroxidation. Lipid peroxides treated with thiobarbituric acid to form a pink color and absorbance taken at 532 nm. The difference between the control and plant extract treated sample was the measured decrease in thiobarbituric acid reacting substances formation, reflecting reduced hydroxyl radical-induced lipid peroxidation. |
| Determination of AChE inhibitory activity [50-52]: |
| Ellman’s colorimetric method was applied to run AChE inhibitory assay and acetylthiocholine iodide used as a substrate. For the enzyme source, the bovine brains were homogenized in a homogenizer with ten volumes of a homogenation buffer (10 Mm Tris-HCl, pH 7.2), which contained 1 M NaCl, 50 mM MgCl2 and1%Triton X-100 and centrifuged at 10000 rpm for 30 min. The resulting supernatant was treated with super saturated ammonium sulphate solution and floating precipitation was collected. Solution of this precipitation was used as an enzyme source. Temperatures were maintained (4°C) throughout the excretion procedure. A bicinchoninic acid kit was used to determine protein concentration and bovine serum albumin used as a protein standard. AChE hydrolysis rate were monitored spectrophotometrically. Each extract or standard (500 μL) was mixed with an enzyme solution (500 μL) and incubated at 37°C for 30 min. Absorbance at 405 nm was read immediately after adding Ellman’s reaction mixture (3.5 mL 0.5 mM acetylthiocholine, 1 mM 5, 5´-dithiobis (2-nitro benzoic acid)) in a 50 mM sodium phosphate buffer (pH 8.0) to the above reaction mixture. To verify the reaction reading were repeated for 10 min at 2 min intervals. The blank reaction was measured by substituting buffer saline for the enzyme (Figure 1). Donepezil was used as a positive control. The percentage inhibition of AChE activity was calculated using the following formula: |
| % of Inhibition of AChE = |
| Determination of BuChE inhibitory activity [50-52]: Modified Ellman’s colorimetric methods were used to perform BuChE inhibitory assay, where butyrylthiocholine iodide used as a substrate. Human blood plasma used as enzyme source. BuChE hydrolysis rate were monitored spectrophotometrically. Each extract or standard (500 μL) was mixed with an enzyme solution (500 μL) and incubated at 37°C for 30 min. Absorbance at 405 nm was read immediately after adding Ellman’s reaction mixture (3.5 mL 0.5 mM butyrylthiocholine, 1 mM 5, 5´-dithio-bis (2-nitro benzoic acid)) in a 50 mM sodium phosphate buffer (pH 8.0) to the above reaction mixture. To verify the reaction reading were repeated for 10 min at 2 min intervals. The blank reaction was measured by substituting saline for the enzyme. Galantamine was used as a reference standard (Figure 2). The percentage inhibition of AChE activity was calculated using the following formula: |
| % of Inhibition of BuChE = |
| Statistical analysis |
| All analyses tests were done three times to get most reliable results. Experimental data were presented as mean ± SD. For graphical analysis Microsoft Excel 2010 were used. For statistical evaluation free R-software version 2.15.1 were utilized. By using the t-test Significant differences (P-value<0.05) between the means were determined. |
| Results |
| AChE inhibitory activity |
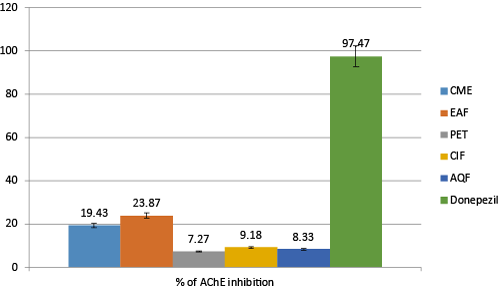
| The colorimetric method of Ellman, [50] which is based on determining the amount of Thiocholine releases when acetylcholine is hydrolyzed by acetylcholinesterase enzyme. The thiocholine released in the reaction mixture were determined by using DTNB. As reduction of DTNB in Hippocampus and cortex is most remarkable hallmark of AD, therefore, elevation of Ach in the synaptic cleft by inhibiting AChE (enzyme that causes breakdown of Ach) are widely accepted therapeutic strategy of AD. The inhibitory activity of AChE by CME other subfractions of A. augusta were determined by Ellman’s method. The inhibitory activities of various fractions are given in Table1 (Figure 3). |
| From the Table 1 it is found that AChE inhibitory activity of A. augusta is concentrated depended. With increase of concentration activity increases and after a certain period it saturates. Among all the fractions EAF is found most active which inhibits 23.83 ± 2.12% AChE at concentration 100 μg/mL, whereas CME, ClF, AQF and PEF inhibits 19.43 ± 1.429%, 9.18 ± 2.622%, 8.33 ± 1.871% and 7.23 ± 2.028% respectively at the same concentration. Donepezil used as a reference standard which inhibits 97.47 ± 2.375% at the same concentration. |
| BChE inhibitory activity [50-52] |
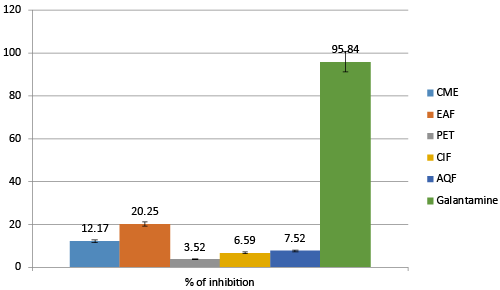
| BChE has been found active in the progression of AD as it is also reduces the availability of Ach by hydrolyzing it. BChE have synergistic effect with ACHE to hydrolyze ACH. Inhibiting this enzyme also found effective in treating AD patients. BChE inhibitory activity of different fraction of A. augusta are given in Table 2. |
| The result truly indicates that EAF was the most active fraction in inhibiting BuCH. At the concentration of 100μg/mL EAF inhibits 20.25 ± 1.522% of BuCH whereas CME, PET, ClF and AQF inhibits 12.17 ± 1.821%, 3.52 ± 0.938%, 6.59 ± 1.829% and 7.52 ± 1.128% of BCH respectively. For reference Galantamine was used and at the concentration of 100 μg/mL it inhibits about 95.84 ± 2. 575%. |
| DPPH radical scavenging activity |
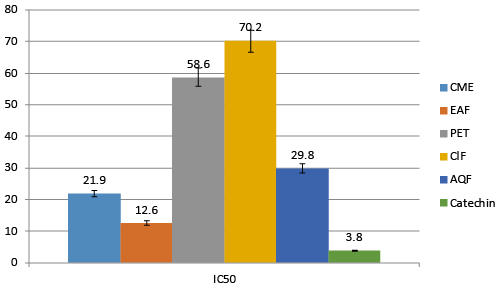
| Free radical causes huge damage to the brain and neurons. Scavenging of these radicals is an important strategy to treat AD. For free radical scavenging activity, DPPH radical scavenging model is well accepted. DPPH is a stable colored free radical which loses its color when free radicals are scavenged. The percentage of free radical scavenging activity was relatively high in EAF compare to others. IC50 value of EAF is 12.6 μg/mL whereas CME, AQF, ClF and PET have 21.9 μg/mL, 29.8 μg/mL, 70.2 μg/mL and 58.6 μg/mL respectively. Reference standard Catechin possesses IC50 of 3.8 μg/mL (Figure 4). |
| Nitric oxide (NO) scavenging activity |
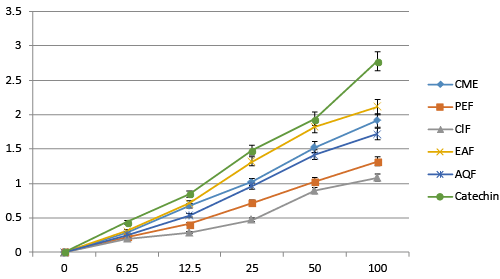
| NO free radical plays an important role in the pathogenesis of inflammation. This inflammatory process can lead to oxidative stress. By inhibiting these free radicals nerve cells can be safe from free radical related inflammations, which is beneficial for AD patients. Fractions of A. augusta shows NO free radical scavenging activities. Among all the fractions EAF shows highest scavenging activity compared to others with the IC50 of 55.63 μg/mL. CME, PEF, ClF and AQ have IC50 67.19, 82.92, 132.17 and 72.17 μg/mL respectively. Standard Ascorbic acid has IC50 of 47.29 μg/mL (Figure 5). |
| Hydroxyl radical scavenging activity |
| Hydroxyl radicals are most reactive oxygen species that causes lipid oxidation and fat oxidation and other biological damages. These may cause oxidative stress and ultimate cell death by apoptosis. For detecting hydroxyl scavenging activity Fe3+-Ascorbate-EDTA_ H2O2 system were applied to all extracts by evaluating the degree of deoxyribose degeneration. |
| All of the extract gives hydroxyl radical scavenging activity. Among all EAF found most prominent with the IC50 of 18.75 μg/mL compared to CME, PET, ClF and AQF with IC50 of 25.93, 45.92, 56.24 and 35.31 μg/mL respectively (Figure 6). Catechin was used as reference standard, which exhibits IC50 9.5 μg/mL. |
| Lipid Peroxidation Inhibition activity |
| In AD patients ROS production by Aβ protein causes various pathophysiological effects including lipid peroxidation and cellular degeneration. Lipid peroxidation causes the formation of low molecular weights lipids by oxidation of poly unsaturated fatty acids. In lipid peroxidation inhibition test oxidized polyunsaturated fatty acid reacts with two molecules of thiobarbituric acid and produce pink chromogen. |
| Extracts exhibits lipid peroxidation inhibition activity in various degree. Among all the fractionsEAF found most prominent. IC50 of EA fraction was 37.31 μg/mL while CME, PEF, ClF and AQF had IC50 of 47.22, 91.33, 112.34 and 66.71 μg/mL (Figure 7) Catechin was used as reference standard, which exhibits IC50 16.54 μg/mL. |
| Discussion |
| Reduction of Birth rate and increased life expectancy resulted increase of average age which also rings age related problem in forward [53]. AD one of the most common age related problem having no cure at all. But several factors can be effective to suppressive severity of this disease. A number of factors have been identified, oxidative stress, and cholinergic dysfunctions are the major contributor factor of AD [54,55]. Earlier studies have been reported that, there some plant derivative product that have direct use on this disease. So, as a source of new medicine plants are always good participants. |
| A. augusta was used from the very past for various disease condition. It has been renowned for its anti-diabetic, anti-rheumatic and anti-gonorrheal use [56]. in this study we evaluate its antialzheimer’s activity. Our finding suggested that A. augusta also possess anti-oxidative activity but al ACHE and BuCHE inhibitory activity. It is well recognized that inhibition of ACHE is one of the most effective strategy. In this study we found that inhibition of ACHE by CME and its subtraction were occurred by dose dependent manner and the highest activity was found in EAF (23.87%) compared to other fractions. Under similar condition Donepezil an anti-AD drug with potential ACHE inhibitory activity, inhibit at the enzyme almost 97%.Our results show moderate inhibitory activity of A. augusta. It resembles that Donepezil have much more activity than this fraction. But EAF is just a fraction not a single isolated compound. |
| BuCHE another crucial enzyme involved in AD [57]. It hydrolyzes both BuCHE and ACHE and also influence the activity of ACHE in breakdown of ACHE. So, breakdown of BuCHE is also an effective way in the treatment of AD. Our study revealed the A. augusta have anti-BuCHE activity. Several fractions show various type of ability to hydrolyze BuCHE. Among the entire fraction EAF was most prominent in case of BuCHE inhibition. EAF found most active fraction with inhibition capability of 12.17%. It is found active compared to others which indicates A. augusta possess both ACHE and BuCHE inhibitory activity. |
| It is found that antioxidant have a promising effect in the treatment of AD by effecting A-β protein formation and oxidative stress [58-61]. Different fraction of A. augusta gives different potentialantioxidant activities. Antioxidants provide their activity mainly scavenging the free radicals that formed into the cells. In our in-vitro study we found that, these fractions isolated from the plant part shows various degrees of antioxidant activities. In DPPH free radical scavenging activity EAF found most active than others with IC50 of 12.6 μg/mL. The order of antioxidant activity was like this CME>AQF>PEF>CIF (Table 3). |
| For hydroxyl and Nitric Oxide free radical scavenging activity, EAF founds bet. Studies indicate that, ROS which formed during oxidative stress induce cellular and molecular abnormalities in AD. HO and NO are the main ROS that cause damage to neuron. Among all extracts EAF found most active with IC50 of the other fractions [62-66]. |
| When lipids are attacked by ROS lipid peroxidation occurs which cause formation of carbon radical that react with O2 resulting the formation of peroxyl radical and caused lipid peroxides generation [67-70]. Brain cells contain high percentages of fat. Thus, lipid peroxidation in AD patient is more dangerous than others. A. augusta found active in lipid peroxidation inhibition.The inhibition rate varies extract to extract but EAF found most active among all. |
| The ACHE and BuCHE inhibitory property with antioxidant activity of A. augesta were supported by the presence of endogenous alkaloids, tannin, steroids, polyphenol and flavonoids in it, as these are involved in absorbing or neutralizing free radicals. |
| It was formed to reduce oxidative stress and inhibition of ACHE, BuCHE. Our overall study indicates that A. augusta can contribute to inhibition of both ACHE and BuCHE which also possess antioxidant activity. |
| Conclusion |
| The result suggested that EAF extended from A. augusta effectively inhibits both ACHE and BuCHE. This fraction also possesses antioxidant stress. As a result, EAF has found a potential source of cholinesterase inhibitor and antioxidant which can be an effective and safe alternative treatment for AD. |
References
- FratiglioniL,Winblad B, Strauss EV (2007) Prevention of Alzheimer’s disease and dementia. Major findings from the Kungsholmen project. Physiology and Behavior 92: 98-104.
- Terry Jr, Buccafusco JJ (2003) The cholinergic hypothesis of age and Alzheimer’s disease- related cognitive deficits: recent challenges and their implications for novel drug development. J PharmacolExpTher 306: 821-827
- Kudo T, Tanii H, Takeda M (2007) Neurodegerative dementias involving aberrant protein aggregation. Psychogeriatrics 7:114-117.
- Masters CL, Selkoe DJ (2012) Biochemistry of amyloid ß-protein and amyloid deposits in alzheimer disease. Cold Spring Harbor Perspectives in Medicine 2: 1-24.
- Iqbal K, Wang X, Blanchard J, Liu F, Gong CX, et al. (2010) Alzheimer’s disease neurofibrillary degeneration: pivotal and multifactorial. Biochemical Society Transactions 38: 962-966.
- Tanaka T, Kazui H, Morihara T, Sadik G, Kudo T, et al. (2008) Post marketing survey of donepezil Hydrochloride in Japanese patients with Alzheimer’s disease with behavioral and psychological symptoms of dementia (BPSD). Psychogeriatrics 8: 114-123.
- Mehta M, Adem A, Sabbagh M (2012) New acetylcholinesterase inhibitors for Alzheimer’s disease. International Journal of Alzheimer’s disease.
- Kadowaki H, Nishitoh H, Urano F (2005) Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activationCell DeathandDifferentiation12: 19-24.
- Valencia A, Morán J (2004) Reactive oxygen species induce different cell death mechanisms in cultured neurons.Free Radical Biology and Medicine36: 1112-1125.
- Reed TT, Owen J, Pierce WM, Sebastian A, Sullivan PG et al (2009) Proteomic identification of nitrated brain proteins in traumatic brain-injured rats treated post injury with gammaglutamylcysteine ethyl ester: insights into the role of elevation of glutathione as a potential therapeutic strategy for traumatic brain injury. Journal of Neuroscience Research 87: 408-417.
- Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, et al, (2000) Intake of flavonoids and risk of dementia. Eur J Epidemiol 16: 357-363.
- Morris MC, Beckett LA, Scherr PA, Hebert LE, Bennett DA (1998) Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease.Alzheimer Dis AssocDisord: 121-126.
- Youdim KA, Joseph JA (2001) A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects.Free RadicBiol Med. 30: 583-594.
- Atmani D, Chaher N, Atmani D (2009) Flavonoids in human health: from structure to biological activityCurrent NutritionandFood Science 5: 225-237.
- Darvesh AS, Carroll RT, Bishayee A (2010) Oxidative stress and Alzheimer’s disease. Dietary polyphenols as potential therapeutic agents. Expert Rev Neurother 10: 729-745.
- Medicinal Plants of Bangladesh. (Accessed on 12 December, 2014)
- Rajagopal K, Sasikala K(2008) Antidiabetic activity of hydro-ethanolic extracts of Nymphaeastellataflowers in normal and alloxan induced diabetic rats. African journal of pharmacy and pharmacology 2: 173-178.
- Huang Yn, Zhao XL, Gao XL, Zhao ZF, Jing Z, et al. (2010)Intestinal a-glucosidase inhibitory activity and toxicological evaluation of Nymphaeastellata flowers extract. J Ethnopharmacol131: 306-312.
- Ishrat J, Mamun MAA, Hossen MA, Sakir JAMS, Shamimuzzaman M, et al. (2012) Antioxidant, Analgesic and Anti-Inflammatory Activities of Nymphaeanouchali Flowers. Research Journal of Pharmacology 6: 62-70.
- Pandurangan SB, Paul AS, Savarimuthu I, Ali AA (2013) Antinociceptive, Immunomodulatory and Antipyretic Activity of Nymphayol Isolated from Nymphaeastellata (Willd.) Flowers. Biomolecules and Therapeutics 21: 391-397
- Dash BK, Sen MK. Ala K, Hossain K, Islam R, et al. (2013) Antibacterial activity of Nymphaeanouchali (Burm. f) flower. Annals of Clinical Microbiology and Antimicrobials 12: 27.
- Hossain MA, Park JY, Kim JY, Suh JW, Park SC (2014) Synergistic Effect and Antiquorum Sensing Activity of Nymphaeatetragona (Water Lily) Extract. BioMed Research International ID 562173.
- Lakshmi G, Smitha N, Ammu S, Priya C, Bhaskara RKB (2014) Phytochemical profile, in vitro antioxidant and hemolytic activities of various leaf extract of Nymphaeanouchalilinn: an in vitro study. International Journal of Pharmacy and Pharmaceutical Sciences 6: 548.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology 299: 152-178.
- Asaduzzaman,Uddin MJ, Kader MA, Alam AHMK, Rahman AA, et al. (2014) In vitro acetylcholinesterase inhibitory activity and the antioxidant properties of Aeglemarmelos leaf extract: implications for the treatment of Alzheimer's disease.Psychogeriatrics 14: 1-10.
- Oyaizu M (1986) Studies on products of browning reactions: antioxidant activities of products of browning reaction prepared from glucose amine. Indian J Pharm Sci44: 307-315.
- Choi HY, Jhun EJ, Lim BO (2000) Application of flow injection-chemilumineacence to the study of radical scavenging activity in plants. Phytotherapy Research 14: 250-253.
- Halliwell B, Gutteridge JMC (1981) Formation of thiobarbituric acid reactive substances from deoxyribose in the presence of iron salts: The role of superoxide and hydroxyl radicals FEBS Letters128: 347-352.
- Liu F, Ng TB (2000) Antioxidative and free radical scavenging activities of selected medicinal herbs.Life Sciences 66: 725-735.
- Ellman GL, Courtney KD, Andres V, Feather-stone RM (1961) A new and rapid colorimetric determination ofacetylcholinesterase activity Biochemical Pharmacology7: 88-95.
- Bonda DJ, Wang X, Perry G (2010) Oxidative stress in Alzheimer disease: Apossibility for prevention. Neuropharmacology 59: 290-294.
- Xiao J, Tundis R (2013) Natural products for Alzheimer's disease therapy: basic and application. Journal of PharmacologyandPharmacotherapeutics65: 1679-1680.
- Singhal AK, Naithani V, Bangar OP (2012) Medicinal plants with a potential to treat Alzheimer and associated symptoms. International Journal of Nutrition, Pharmacology, Neurological Diseases2: 84-91.
- Massoud F, Gauthier S (2010) Update on the pharmacological treatment of Alzheimer’s disease. Current Neuropharmacology 8: 69-80.
- Neugroschl J, Sano M (2010) Current treatment and recent clinical research in Alzheimer's disease. Mt Sinai J Med. 77: 3-16.
- Decker M, Kraus P, Heilmann J (2008) Design, synthesis and pharmacological evaluation of hybrid molecules out of quinazolinimines and lipoic acid lead to highly potent and selective butyrylcholinesterase inhibitors with antioxidant properties.Bioorg Med Chem 16: 4252-4261.
- Galasko D, Montine TJ (2010) Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomarkers in Medicine 4: 27-36.
- Lyras L, Cairns NJ, Jenner A (1997) An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. Journal of Neurochemistry 8: 2061-2069.
- Sadik G, Isalm R, Rahman MM, Khondkar P, Rashid MA, et al. (2003) Antimicrobial and cytotoxic constituents of Loranthusglobosus. Fitoterapia74: 308-311.
- Van Wagenen BC, Larsen R, Cardellina JH II, Randazzo D, Lidert ZC (1993) Ulosantoin, a potent insecticide from the sponge Ulosaruetzleri. J Org Chem58:335-337.
- Kadowaki H, Nishitoh H, Urano F, Sadamitsu C, Matsuzawa A, et al. (2005) Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation.Cell Death Differ 12: 19-24.
- Sponne L, Fifre A, Drouet B, Klein C, Koziel V, et al. (2002) Apoptotic neuronal cell death induced by the non-fibrillar amyloid-beta peptide proceeds through an early reactive oxygen species-dependent cytoskeleton perturbation.J BiolChem278: 3437-3445.
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, et al. (2001) Oxidative damage is the earliest event in Alzheimer disease. Journal of Neuropathology and Experimental Neurology 60: 759-767.
- Murakami K, Irie H, Ohigashi H, Hara M, Nagao T, et al. (2005) Formation and stabilization model of the 42- merAbeta radical: implications for the long-lasting oxidative stress in Alzheimer’s disease. J Am ChemSoc 127: 15168-15174.
- Tabner BJ, El-Agnaf OM, Turnbull S, German MJ, Paleologou KE, Hayashi Y, et al. (2005) “Hydrogen peroxide is generated during the very early stages of aggregation of the amyloid peptides implicated in Alzheimer disease and familial British dementia”Journal of Biological Chemistry 280: 35789-35792.
- Tabner BJ, Turnbull S, El-Agnaf OMA, Allsop D (2002) Formation of hydrogen peroxide and hydroxyl radicals from Aß and a-synuclein as a possible mechanism of cell death in Alzheimer’s disease and Parkinson’s disease. Free Radical Bio Med 32: 1076-1083.
- Arlt S, Beisiegel U, Kontush A (2002) Lipid peroxidation in neurodegeneration: new insights into Alzheimer’s disease. CurrOpinLipidol 13: 289-294.
- Hardas SS, Sultana R, Clark AM, Beckett TL, Szweda LI, et al. (2013) Oxidative modification of lipoic acid by HNE in Alzheimer disease brain. RedoxBiol 1: 80-85.
- Okhawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animals tissue by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Ellman GL, Courtney KD, Andres V Jr, Feath-erstone RM (1961) A new and rapid colorimetric determination of acetylcholinesteraseactivity. Bio- chem. Pharmacol 7:88-95.
- Wilson BW, Philip W (2005)Encyclopaedia of Toxicology. New York: Elsevier. Cholinesterase inhibition; pp. 588–599.
- Silver A (1974) The biology of cholinesterase. In: Neuberger A, Tatum EL, editors. Frontiers of Biology. Amsterdam: North Holland 1-596.
- Michael R, Sebastian S (2011) Extending life span by increasing oxidative stress. Free Radical Biology and Medicine51: 327-336.
- Ashe KH (2001) Learning and memory in transgenic mice modeling Alzheimer's disease. Learn. Mem., 8: 301-308
- Auld DS, Kar S, Quirion R (1998) ß-amyloid peptides as direct cholinergic neuromodulators: a missing link? Trends Neurosci 21: 43-49.
- Kirtikar KR, Basu BD (1918) Indian Medicinal Plants, SudhindraNathBasu, BahadurGanj, Allahabad 210-211.
- Karlsson D, Fallarero A, Brunhofer G, Mayer C, Prakash O, et al. (2012) The exploration of thienothiazines as selective butyrylcholinesterase inhibitors. Eur. J. Pharm. Sci47: 190-205.
- Ahmed M, Rocha JB, Mazzanti CM, Morsch AL, Cargnelutti D, et al. (2007) Malathion, carbofuran and paraquat inhibit bungarussindanus (krait) venom acetylcholinesterase and human serum butyrylcholinesterasein vitro. Ecotoxicology16: 363-369.
- Tian Y, Zhou SF, Gao Y, Zhou YJ, Shi R, et al. (2011) Effects of repeated maternal oral exposure to low levels of trichlorfon on development and cytogenetic toxicity in 3-day mouse embryos. Food Chem. Toxicol49: 2655-2659.
- Becker RE, Unni LK, Greig NH (2009) Resurrecting clinical pharmacology as a context for alzheimer disease drug development. Curr. Alzheimer Res. 2009, 6, 79-81.
- López-Arrieta JM, Schneider L (2006)Metrifonate for Alzheimer’s disease. Cochrane Database Syst. Rev., CD003155.
- Gutierrez-Merino C, Lopez-Sanchez C, Lagoa R, Samhan-Arias AK, Bueno Cet al. (2011)Neuroprotective actions of flavonoids. Curr Med Chem18: 1195-1212.
- Jung HA, Min B-S, Yokozawa T, Lee J-H, Kim YS, Choi JS (2009) Anti-Alzheimer and antioxidant activities of CoptidisRhizoma alkaloids. Biol Pharm Bull32: 1433-1438.
- Fang Z, Jeong SY, Jung HA, Choi JS, Min BS, et al. (2010) Anticholinesterase and Antioxidant Constituents from Gloiopeltisfurcata. Chem Pharm Bull(Tokyo)58: 1236-1239.
- Cho SO, Ban JY, Kim JY Jeong HY, Lee IS, et al. (2009) Aralia cordataprotects against amyloid ß protein 25–35)–induced neurotoxicity in cultured neurons and has antidementia activities in mice. J PharmacolSci111: 22–32.
- Mishra S, Palanivelu K (2008)The effect of curucumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian AcadNeurol11: 13-19.
- Sayre LM, Smith MA, Perry G (2001) Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr Med Chem 8:721-738
- Lovell MA, Xie C, Markesbery WR (2001)Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging 22:187-194.
- Smith MA, Rottkamp CA, Nunomura A (2000) Oxidative stress in Alzheimer's disease. BiochimBiophysActa 1502:139-144.
- Keller JN, Hanni KB, Markesbery WR (1999) Oxidized low-density lipoprotein induces neuronal death: implications for calcium, reactive oxygen species, and caspases. J Neurochem 72:2601-2609.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
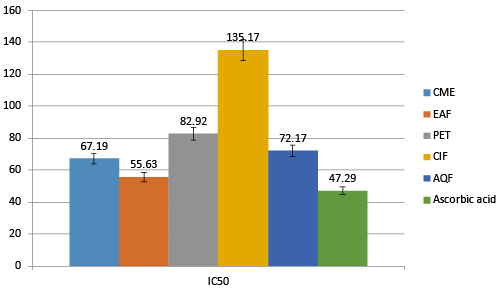 |
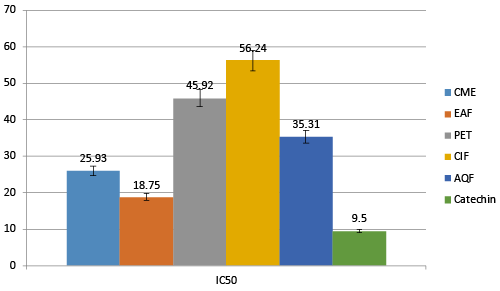 |
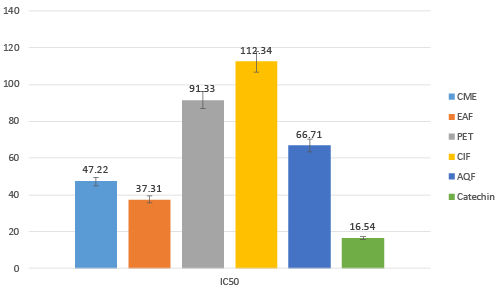 |
| Figure 5 | Figure 6 | Figure 7 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 14592
- [From(publication date):
November-2015 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 10156
- PDF downloads : 4436
