Antiacid and Prodigestive Activity of a Novel Formulation
Received: 17-Dec-2021 / Manuscript No. JGDS-21-50109 / Editor assigned: 20-Dec-2021 / PreQC No. JGDS-21-50109(PQ) / Reviewed: 03-Jan-2022 / QC No. JGDS-21-50109 / Revised: 07-Jan-2022 / Manuscript No. JGDS-21-50109(R) / Accepted Date: 12-Jan-2022 / Published Date: 14-Jan-2022 DOI: 10.4172/2161-069X.1000666
Abstract
Background: The general increase of high calories and fat rich diets in developed and developing countries led to an increase in gastrointestinal related issues. The most diffuse gastrointestinal issues i.e., dyspepsia, gastroesophageal reflux, etc. are related to hyperacidity and/or leakage of gastric juice. While over the counter medication such as antacids is still considered the main defense line, their activity may hinder the digestive process by inactivating digestive enzymes, potentially worsening digestive difficulties. A solution to this undesirable effect comes from digestive enzyme supplementation (proteases, lipases and carbohydrates hydrolyzing enzyme). This supplementation can support the activity of digestive process released enzymes, reducing the “stomach heaviness” feeling as a consequence. In particular, considering the progressive enrichment in lactose containing foods of western diets, the supplementation of lactose hydrolyzing enzymes, such as β-galactosidase (lactase), is instrumental to enhance and support food digestion. The aim of the present work is to test the antacid and prodigestive efficacy of a new multifunctional formulation, Biochetasi Acidità e Digestione (BAD).
Methods: The antacid activity of BAD was determined with an in vitro approach, by following the progressive pH neutralization over time of a simulated gastric fluid. Neutralization tests were carried out starting from pH 2 (fasted condition) and pH 3 (fed condition). The prodigestive activity of BAD supplemented proteases, lipases and galactosidases was evaluated by performing in vitro digestion of a standard food, composed in equal percentage of proteins, lipids and carbohydrates, in the presence or absence of normal digestive enzymes (i.e., pepsin, trypsin, lipases, etc.). Protein hydrolysis was assessed by free amino acids quantification, while lipids and carbohydrates hydrolysis were investigated by triglycerides and released glucose quantification respectively. To investigate galactosidases specificity, in vitro digestion was performed either on a starch or lactose based standard food.
Results: Regardless physiological digestive situation (i.e., fed or fasted), BAD neutralizes gastric acidity to pH 4, guaranteeing the maintenance of pepsin activity at the gastric level. BAD supplemented proteases significantly improve protein digestion in the absence of normal digestive enzymes. Lipases supplemented by BAD induces an effective triglycerides hydrolysis both in the absence and presence of digestive lipases. BAD galactosidases significantly enhances lactose hydrolysis and their activity are not affected by normal digestive enzymes. Galactosidases specificity for lactose was proved by exposing starch based standard food without observing any effects.
Conclusion: BAD shows a multifunctional effect since it has an antiacid activity, while supporting proteins, lipids and lactose digestion with a prodigestive action. As such, it could be instrumental in limiting gastric acidity related side effects and the stomach heaviness sensation, due to slow digestion of an abundant or unbalanced meal.
Keywords: Antiacid; Dyspepisa; Enzyme supplementation; Digestion; lactose; Protein; Carbohydrate; lipids
Keywords
Antiacid; Dyspepisa; Enzyme supplementation; Digestion; lactose; Protein; Carbohydrate; lipids
Introduction
The progressive increase in pace, quality and duration of life, especially in developed and developing Countries, significantly affected population habits. In particular, eating habits are currently characterized by high calories and fat rich diets, irregular meal pattern and moderate to fast eating rates [1-3]. As a consequence, the incidence of gastrointestinal related issues, from mild (i.e. gastric hyperacidity, digestive difficulties and indigestion as dyspepsia) to severe (i.e. gastroesophageal reflux disease (GERD), gastritis, peptic ulcer), has drastically increased, affecting the various national health institutes from an economic point of view [4-6]. While gastrointestinal related issues could be controlled with specific drugs (i.e., histamine-2 blockers and proton pump inhibitors (PPIs)), antacids are still considered the mainstay of treatment for acid peptic disorders [7]. These over the counter medications, generally composed of a metallic ion (i.e. aluminum, magnesium or sodium) combined with a base (i.e. hydroxide, carbonate, etc.), and a gas absorbing, foaming agent like dimethicone reduce gastric acidity by neutralizing the hydrochloric acid secreted by the parietal cells of gastric mucosa [8,9]. On the other side, gastric acidity reduction could induce digestive difficulties [8,10]. Most of antacids completely abrogates pepsin activity by neutralizing gastric juices to neutral pH (i.e., pH 7), significantly slowing down protein digestion and gastric emptying as a result [11-13]. Supplementation of enzymes specialized in the hydrolysis of the main biological macromolecules (i.e. proteases, lipases and carbohydrate hydrolyzing enzymes) could represent a solution to improve digestive efficacy and to limit antacid pepsin inactivating activity [14-16]. Several studies have suggested that oral digestive enzyme supplementation to older adults may reduce symptoms associated with occasional indigestion, alleviating the effects of bacterial fermentation of under digested food or under hydrolyzed chyme (i.e. gas production and bloating) due to a physiological decrease in digestive enzyme activity [17-19]. One of the main responsible for these unwelcomed side effects is lactose, a carbohydrate presents in milk and its derivatives. Milk and milk derived products are consumed by about 6 billion of people, with a consumption doubling from the 1960, in particular in developed countries [20-22]. Despite their high nutritional value (proteins, fat, vitamins, etc.) milk derived and lactose rich products represent a challenge for human digestion [23]. Indeed, the brush border intestinal enzyme responsible for lactose hydrolysis, β-galactosidase (also known as lactase), is only available in a small amount, that further decreases with aging. As a result of the mismatch between lactase availability and lactose based foods consumption, an increasing fraction of lactose remains undigested. Consequently, lactase supplementation could support the lactase intestinal pool in digesting lactose, limiting digestive difficulties and side effects connected to undigested lactose fermentation by the intestinal microflora.[24-27].
Considering the aforementioned digestive problems, a multifunctional formulation able to deal with gastric juice hyperacidity related issues, while limiting and reducing the “stomach heaviness” feeling connected to a slow and difficult digestion could represent a breakthrough solution. In the present work, a novel formulation Biochetasi Acidità e Digestione (BAD) is described for its antacid and pro-digestive efficacy by using an in vitro approach.
Materials And Methods
Materials
D-Glucose Assay Kit (GOPOD Format) was purchased by Megazyme Ltd (Co. Wicklow, Ireland). Trinonanoylglycerol and tripentadecanoylglycerol standards, solvents (ethanol, hexane, chlorophorm, methanol), salts, digestive enzymes (α-amylase from porcine pancreas, pepsin from porcine gastric mucosa, porcine bile extract, lipase from porcine pancreas, and pancreatin from porcine pancreas), bovine serum albumin (BSA), starch, lactose, olive oil, amino acid standards of acidic and neutral amino acids, and bile were purchased from Merck KGaA (Darmstadt, Germany). Ninhydrin reagent comprising special ninhydrin buffer R2 and ninhydrin reagent R1 were purchased from Wako Pure Chemical Industries (Osaka, Japan). The reference standard mixture (GLC 461) for triglyceride quantification was purchased from Nuchek Prep (Elysian, MN, United States). Omegawax column was purchased from Supelco Inc. (Bellefonte, PA, United States). The basic amino acids type B standard and ion exchange column were purchased from Hitachi (Tokyo, Japan). Centrifuge concentrators Microcon YM, 3 kDa was purchased by Millipore (Burlington, MA, United States).
Methods
The determination of the antacid and prodigestive activity, with an in vitro digestive model, was performed on Biochetasi Acidità e Digestione (BAD) formulation, which composition in active principles is reported in (Table SM1).
Evaluation of the antiacid activity: The formulation antiacid activity was determined with an in vitro assay, based on in vitro gastric digestion. Briefly, according to the formulation posology, 4.4 grams of the formulation, equivalent to two tablets, were resuspended in 100 mL of double distilled water (ddH2O) and mixed for 30 seconds. At the mixing end, 500 mL of simulated gastric fluid, which composition is detailed in compatible with the average volume of gastric fluid during in human in vivo digestion, were added and pH measured at different time points, from 0 to 120 min (Table SM2). The time points were selected accordingly to the gastric transition time (120 min) of the in vitro digestion model (see below), and the potential antacid activity of the formulation was investigated in both fasted (pH 2) and fed (pH 3) conditions [28,29].
In vitro digestive process: The prodigestive efficacy of the formulation was investigated with an in vitro approach, according to the harmonized protocol proposed by Minekus et al., 2014 [28]. Briefly, 5 grams of the standard food (SF) composed by an equiproportional blend of BSA (proteins), olive oil (triglycerides) and lactose or starch (carbohydrates), was mixed with 5 mL of simulated saliva (pH 7) without amylase for 2 min [30]. Next, the standard food was mixed with 10 mL of simulated gastric juice (pH 3) containing pepsin (2000 U/mL of digesta) for 2 hours. To properly simulate its posology and the overall formulation on digestive fluids volume ratio, 176 mg of the formulation were added 20 min after the start of the gastric phase. Then, 20 mL of simulated intestinal juice (pH 7) containing pancreatin (100 U trypsin activity/mL of digesta), lipases (2000 U/mL), bile (10 mmol/L in the total digesta) were added and incubated for 120 min. The oral, gastric and intestinal steps were performed at 37 °C under constant gentle mixing (100 rpm in a horizontal shaker). At the end of the digestive process, the digests were centrifuged at 2750 g to separate the undigested fraction (i.e., pellet) from the supernatant, containing the release products of macronutrient digestion (i.e., amino acids, triglycerides and glucose). For free amino acids, the supernatant was added with a protease inhibitor cocktail and stored at 4°C prior to analysis, while the supernatant was directly stored at -20 °C for triglyceride analysis. Finally, for glucose analysis, supernatant aliquots were boiled at 100°C for enzyme inactivation and stored at -20°C. To isolate the digestive contribution of the formulation, the in vitro digestion was performed both with and without digestive enzymes (α-amylase from porcine pancreas, pepsin from porcine gastric mucosa, lipase from porcine pancreas, and pancreatin from porcine pancreas). The composition of the simulated digestive fluids is reported in (Table SM2).
Triglyceride quantification: The triglyceride quantification was performed by gas chromatography with flame ionization detector (GC-FID). Briefly, lipids were extracted from 100 μL of sample using a chloroform methanol solution in accordance with the Folch method [31]. Lipids extract were resolved in classes by thin layer chromatography as previously reported [32].
The triacylglycerols fraction was hydrolyzed with HCl methanol and the resulting free fatty acids were trans esterified and extracted with hexane. Hexane containing fatty acid methyl esters (FAME) was directly taken for gas chromatographic analysis.
Gas chromatography analysis was performed with an Agilent 5890 gas chromatograph (GC) equipped with a flame ionization detector. FAMEs derived from triacylglycerols were resolved with an Omegawax column (30 m x 0.25 mm internal diameter × 0.25 μm film thickness) with 1 μl injection volume running in on column mode. Oven temperature was programmed as follow: 60 °C for tree minutes, increased 20 °C/minute to 205 °C, where remained constant for 15 minutes. Temperature then increased 0.4 °C/minute up to 213 °C, which was maintained for 10 minutes and finally increased to 240 °C at 5.0°C/min and held for 8 minutes. Peaks were identified in relation to a reference standard mixture. The internal standard method, based on internal standards trinonanoylglycerol and tripentadecanoylglycerol, was used for quantitative analysis. Obtained results were expressed as triglyceride percentage (%), compared to the amount of triglyceride in the SF.
Glucose quantification: The amount of glucose resulting from starch or lactose digestion was determined with a commercial colorimetric assay. Briefly, digestion supernatant samples stored at -20 °C were left to thaw on ice, diluted in ethanol and centrifuged at 14000 g for 5 min. 7 μL aliquot of the resulting supernatants was placed in a microplate well together with 300 μL GOPOD (a mixture of glucose oxidase, peroxidase, and 4-aminoantipyrine in a potassium phosphate and ρ-hydroxybenzoic acid buffer). The microplate was then incubated at 50°C for 20 min, protected from light, and absorbance was read at 505 nm with a Synergy 4 multiwell plate reader (BioTek Instruments, VT, USA). A fresh glucose standard curve, based on highly purified glucose (purity ≥ 99.5%) was used with each set of extracts, ranging from 0 to 2 mg/mL. All standards and extracts were analyzed in triplicate. Glucose results were expressed as mg. For lactose digestion, obtained results were also expressed as percentage (%) of the expected amount of glucose derived from complete lactose digestion.
Free amino acids quantification: Free amino acid analysis of digests supernatant was conducted on an L8800 amino acid analyzer (Hitachi) with two single channel colorimeters. Detection was carried out at two wavelengths (570 and 440 nm) according to the manufacturer’s instructions. Separation was performed on an ion exchange column packed with sulfonated styrene divinylbenzene copolymer (4.6 × 60 mm) using a stepwise gradient of Li citrate buffers at elution rate 0.4 ml/min and temperature gradient during chromatographic separation (the column was thermostated in the temperature interval 30°C-70°C). A mixture of amino acid standards was used for calibration of the instrument. Aliquots of standard mixtures containing 2 nmol of each introduced amino acid were used. Online post column amino acid derivatization was used for quantitative assessment of amino acids in the eluate, which occurred on mixing of the eluate with ninhydrin reagent solution supplied by a separate pump (+136°C, rate 0.35 ml/min). The ninhydrin reagent comprised special ninhydrin buffer R2 and ninhydrin reagent R1. The stained products of derivatization were detected at 570 nm for all amino acids except proline and hydroxy proline, which were recorded colorimetrically at 440 nm. MultiChrom for Windows software (Ampersand Ltd., Russia) was used for processing the chromatographic data.
The samples were centrifuged through membrane ultrafilters and filtrate aliquots (100 μl-120 μl) were taken for amino acid analysis.
Statistical analysis: All data are presented as mean ± standard deviation (SD). To determine statistically significant differences between treatments were present, a t-test analysis was performed. T-test is a statistical method used to test differences between two means. The differences between groups were considered significant at p<0.05. All statistical analyses were performed with Origin Lab software.
Results
Antiacid activity
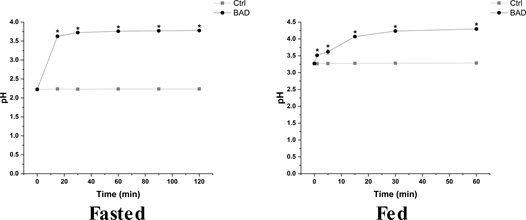
The main focus of antacid formulations is the reduction of gastric acidity by neutralizing the acidic gastric juice to a neutral pH. To investigate the antiacid activity, BAD formulation was added to simulated gastric fluids at pH 2 and 3 respectively to mimic fasted and fed conditions according to Walczak and Minekus protocols and pH was measured over time (Figure 1 and Table SM3) [28,29]. BAD formulation significantly increases the pH in both fasted (from pH 2.22 ± 0.00 to pH 3.77 ± 0.02) and fed (from pH 3.27 ± 0.03 to pH 4.29 ± 0.00) conditions. Moreover, BAD antiacid activity is rapidly exerted in both physiological conditions (i.e., fed and fasted), with the maximum pH reached in about 15 min. As such, BAD shows a significant, quick and effective antiacid activity.
Prodigestive activity
The prodigestive activity of BAD was evaluated with an in vitro digestive process, by digesting a standard food composed of olive oil (triglycerides), bovine serum albumin (proteins) and lactose or starch (carbohydrates) in the presence or absence of digestive enzymes. While in the absence of digestive enzymes it was possible to directly assess tha activity of BAD supplemented enzymes, the formulation contribution to the digestive process was analyzed in the presence of the normal pool of digestive enzymes (i.e. pepsin, trypsin, lipases, amylases, etc.).
Protein digestion: During the digestive process, proteins are degraded into amino acids by well-known gastric and intestinal proteases, such as pepsin, trypsin and chymotrypsin. The quantification of free amino acids from protein hydrolysis in digestive fluids represents a reliable indication of both physiological and supplemented proteases efficacy. In the absence of normal set of digestive proteases, proteases supplemented in BAD significantly increase the free amino acids concentration in the digestive fluids (Table 1). In the presence of digestive proteases containing fluids, no significant improvements in pro-digestive effects were observed by adding BAD (Table 2 and Figure SM1). With digestive enzymes protein hydrolysis is significantly higher, flattening BAD protease contribution (Table 1 and Table 2).
Table 1: Prodigestive effect on proteins of BAD proteases in the absence of digestive enzymes. To assess the prodigestive activity of BAD towards proteins, a standard food, containing BSA as a protein source, was digested with an in vitro digestive process model in the absence of potentially interfering digestive enzymes. The proteolytic activity of BAD supplemented proteases was determined by measuring released free amino acids. The results are reported a mean ± standard deviation. Ctrl: control; SF: Standard Food; BAD: Biochetasi Acidità e Digestione; LOQ: Limit of Quantification.
| Treatment | Total Free Amino Acids (mg/kg) |
|---|---|
| Ctrl | <LOQ |
| SF | <LOQ |
| BAD + Standard Food | 55.5 ± 15.2 |
Table 2: Prodigestive effect on proteins of BAD proteases in the presence of digestive enzymes. To assess the prodigestive activity of BAD towards proteins, a standard food, containing BSA as a protein source, was digested with an in vitro digestive process model, in presence of digestive enzymes. The proteolytic activity of BAD supplemented proteases was determined by measuring released free amino acids. The results are reported a mean ± standard deviation. Ctrl: control; SF: Standard Food; BAD: Biochetasi Acidità e Digestione.
| Treatment | Total Free Amino Acids (mg/kg) |
|---|---|
| Ctrl | 2676.7 ± 41.9 |
| BAD | 2627.7 ± 43.9 |
| Standard Food | 10886.3 ± 67.1 |
| BAD + Standard Food | 10743.0 ± 168.6 |
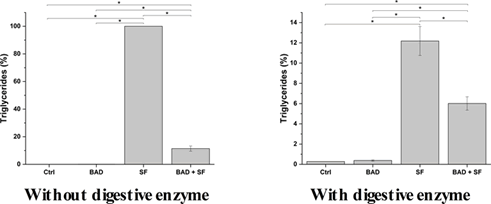
Lipid digestion: Lipids are processed during digestion by specialized enzymes, lipases, in particular at the intestinal level. Lipases hydrolyze triglycerides, leading to the release of glycerol and fatty acids. The impact of BAD lipases on lipid digestion was evaluated by exposing a standard food containing olive oil, rich in triglycerides, to in vitro digestion either in the presence or absence of digestive enzymes. In the absence of digestive enzymes, BAD formulation significantly decreases the amount of triglycerides of about 90% the initial value (Figure 2A and Table SM4). In the presence of lipases comprising digestive enzyme, BAD formulation further enhances triglyceride hydrolysis, as shown in (Figure 2B and Table SM4). Besides antacid activity, BAD improves lipid hydrolysis potentially reducing dyspepsia frequency while facilitating food digestion.
Figure 2: Prodigestive effect on lipids of BAD lipases in the absence and presence of digestive enzymes. To assess the prodigestive activity of BAD towards lipids, a standard food, containing olive oil as a lipid (triglycerides) source, was digested with an in vitro digestive process model, in absence (without digestive enzymes) or presence (with digested enzymes) of digestive enzymes. The lipolytic activity of BAD supplemented lipases was determined by measuring triglycerides concentration at the end of the digestive process. The results are reported as percentage (%) compared to the amount of triglycerides detected in the standard food digestion without enzymes. Ctrl: Control; SF: Standard Food; BAD: Biochetasi Acidità e Digestione. *p<0.05.
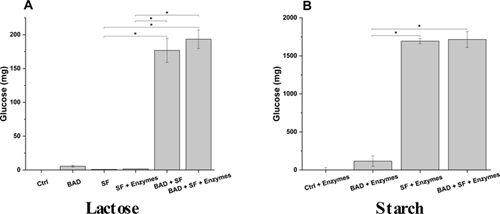
Carbohydrate digestion: BAD mediated lactose digestion was evaluated by applying the in vitro human digestive process to standard food containing lactose, and measuring the amount of released glucose. In the presence of BAD, lactose hydrolysis is higher than in the absence of formulation, 176.8 ± 17.7 mg versus 5.6 ± 1.2 mg of glucose respectively (Figure 3A and Table SM5). In particular, BAD induces a 31-fold increase of lactose digestion, releasing about 33% of expected glucose (Figure SM2).
Figure 3: Prodigestive effect on carbohydrates of BAD galactosidases in absence and presence of digestive enzymes. To assess the prodigestive activity of BAD towards carbohydrates, a standard food, containing lactose or starch as a carbohydrates source, was digested with an in vitro digestive process model, in absence or presence of digestive enzymes. The carbohydrate hydrolytic activity of BAD supplemented lactase was determined by measuring the amount (mg) of released glucose at the end of the digestive process. Ctrl: Control; SF: Standard Food; BAD: Biochetasi Acidità e Digestione. *p<0.05.
As expected, no significant contribution to lactose digestion is observed for the digestive enzymes secreted during physiological digestion, among which α-amylase is the most importat carbohydrate-hydrolyzing one (Figures 3A and 3B and Table SM5). The specificity of BAD supplemented β-galactosidase was further demonstrated by substituting lactose with starch as a carbohydrate source. In this condition, BAD does not contribute to starch hydrolysis since the amount of released glucose is similar in the presence or absence of the formulation (Figure 3B and Table SM5).
Considering the presented results, BAD formulation is effective in promoting lactose hydrolysis during digestion, potentially reducing the side effect linked to lactose digestion resistance.
Discussion
In this work, the efficacy of BAD formulation as antacid and prodigestive formulation was investigated. Antiacid formulations need to rapidly neutralize an adequate volume of gastric acid, maintaining an ideal pH of 5 to 6 for a sufficient time, compatible with gastric digestion [8]. Based on the results, BAD is an effective antiacid formulation in both fasted and fed conditions. Indeed, the formulation neutralizes gastric juices within 15 minutes from administration, and it is able to maintain its neutralizing activity for up to 2 hours, corresponding to the average transition time of food in the stomach. Independently from the considered physiological digestive condition (i.e., fasted or fed), BAD formulation neutralizes gastric juice to a pH of about 4, lower than other commercially marketed products that usually reach value of 7 or above, highlighting its efficacy before (fasted condition) and after starting digestion (fed condition). BAD provides an optimal equilibrium between antacid action and maintenance of gastric digestive function. While pepsin activity is retained of about 70% at pH 4 it is almost completely abrogated at pH 7 [33,34]. As such, BAD is effective in countering gastric juice acidity related issues while avoiding shutting down pepsin activity with the consequent reduction of digestive process. On the other hand, hyperacidity causes a further lowering of pH for which pepsin is less active [33]. So BAD mediated pH increase might still permit the return of pepsin activity to values for which it can activally act as a protease.
Besides antiacid activity, BAD has been also thought to exert a prodigestive function to support digestive process. Proteases in BAD are effective in enhancing protein hydrolysis, supporting digestive proteases such as the partially inactivated pepsin after gastric juice neutralization to hydrolyze proteins. As a consequence, BAD could be more effective in promoting and accelerating gastric protein digestion compared to market product, favoring a faster solution to stomach heaviness. Lipases supplemented with BAD are also effective in supporting the digestive process. BAD lipases reduce triglyceride content of the standard food up to 90% in the absence of digestive enzymes, while in their presence BAD lipases supports digestive lipases activity by reducing triglyceride content up to 94%. As such, BAD formulation shows a significant prodigestive activity towards lipids, and it could be instrumental for improving lipid digestion so reducing dyspepsia and other digestion related problems.
Lactase supplemented with BAD demonstrated to be effective in enhancing lactose digestion. BAD lactases significantly increase lactose digestion up to 31-fold, either in the presence or absence of digestive enzymes. Consequently, lactase supplemented by BAD could effectively support the brush border localized intestinal lactase pool in digesting the growing amount of lactose typically present in diets of developed and developing countries. By improving lactose digestion, DAB may help in reducing the side effects due to intestinal microflora fermentation of undigested lactose, such as microbiota dysbiosis, abdominal pain, diarrhea, bloating, flatulence, and abdominal cramping [35-38]. The supplementation of this enzyme is particularly relevant since the available pool of lactase decreases with ageing.
Conclusion
BAD showed to be a multifunctional formulation able to effectively neutralizes gastric acidity, while supporting proteins, lipids and lactose digestion. Due to its digestive enzyme supplementation, BAD exerts prodigestive action, limiting stomach heaviness related to digestion difficulties.
References
- Hu FB, Willett WC (2018) Current and future landscape of nutritional epidemiologic research. J American Med Asso 320:2073-2074.
- Venn D, Banwell C, Dixon J (2017) Australia’s evolving food practices: A risky mix of continuity and change. Public Health Nutr 20:2549-58.
- Pike KM, Dunne PE (2015) The rise of eating disorders in Asia: A review. J Eat Disord 3:1-14.
- Drossman DA (2016) Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 150:1262-1279.
- Oshima T, Miwa H (2015) Epidemiology of functional gastrointestinal disorders in Japan and in the world. J Neuro gastroenterology Motil 21:320-9.
- Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, et al. (2021) Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology 160:99-114.
- Kaye AD, Kaye AM, Urman RD (2015) Essentials of pharmacology for anesthesia, pain medicine, and critical care. Springer, New York.
- Jakaria M, Zaman R, Parvez M, Islam M, Haque M, et al. (2015) Comparative study among the different formulation of antacid tablets by using acid-base neutralization reaction. Global J Pharmacology 9:278-81.
- Udeze A, Jayeoba T, Innocent-Adiele H, Okerentugba O, Nwanze J, et al. (2012) Bacteriological Assessment of some selected antacid suspension products In Nigeria. New York Sci J 5:28-32.
- Maton P, Burton M (1999) Antacids revisited: A review of their clinical pharmacology and recommended therapeutic use. Drugs 57:855-70.
- Samloff IM, O’Dell C (1985) Inhibition of peptic activity by sucralfate. Am J Med 79:15-8.
- Piper DW, Fenton BH (1965) pH stability and activity curves of pepsin with special reference to their clinical importance. Gut 6:506-8.
- Pegu KD (2020) Pharmacology of antacids. SAJAA 26:133-6.
- Ianiro G, Pecere S, Giorgio V, Gasbarrini A, Cammarota G (2016) Digestive enzyme supplementation in gastrointestinal diseases. Curr Drug Meta 17(2):187-93.
- Layer P, Keller J (2003) Lipase supplementation therapy: Standards, alternatives, and perspectives. Pancreas 26(1):1-7.
- Keller J, Layer P (2003) Pancreatic enzyme supplementation therapy pancreat ic enzyme supplement at ion therapy. Curr Treat Options Gastroenterol 6:369-74.
- Laugier R, Bernard JP, Berthezene P, Dupuy P (1991) Changes in pancreatic exocrine secretion with age: Pancreatic exocrine secretion does decrease in the elderly. Digestion 50:202-11.
- Rémond D, Shahar DR, Gille D, Pinto P, Kachal J, et al. (2015) Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 6(16):13858-98.
- Vellas B, Balas D, Moreau J, Bouisson M, Guidet M (1988) Exocrine pancreatic secretion in the elderly. Int J Pancreato 3(6):497-502.
- Scrimshaw NS, Murray EB (1988) The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am J Clin Nutr 48:1079-159.
- Ugidos-Rodríguez S, Matallana-González MC, Sánchez-Mata MC (2018) Lactose malabsorption and intolerance: a review. Food and Funct 9(8):4056-68.
- Misselwitz B, Butter M, Verbeke K, Fox MR (2019) Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 68(11):2080-91.
- Pereira PC (2014) Milk nutritional composition and its role in human health. Nutrition 30(6):619-27.
- Kies AK (2014) Authorised EU health claims related to the management of lactose intolerance: Reduced lactose content, dietary lactase supplements and live yoghurt cultures. Wodhd Publis Ser Sci Techn Nutr 3:177-211.
- Felicilda-reynaldo RFD, Kenneally M (2016) Digestive enzyme replacement therapy: Pancreatic enzyme and lactase. Medsurg Nurs 25(3):182-5.
- Fassio F, Facioni MS, Guagnini F (2018) Lactose maldigestion, malabsorption, and intolerance: A comprehensive review with a focus on current management and future perspectives. Nutrients 10(11):1-12.
- Ibba I, Gilli A, Boi MF, Usai P (2014) Effects of exogenous lactase administration on hydrogen breath excretion and intestinal symptoms in patients presenting lactose malabsorption and intolerance. Biomed Res Int 2014:680196.
- Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, et al. (2014) A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct 5(6):1113-24.
- Walczak AP, Fokkink R, Peters R, Tromp P, Herrera Rivera ZE, et al. (2013) Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model. Nanotoxicology 7(7):1198-210.
- Diez-Sánchez E, Quiles A, Hernando I (2021) Interactions between blackcurrant polyphenols and food macronutrients in model systems: In vitro digestion studies. Foods 10(4):847.
- Folch J, Lees M, Sloane Stanley G (1957) A simple method for the isolation and purification of total lipides from animal tissue. J Biol Chem 226:497-509.
- Correani A, Visentin S, Cosmi E, Ponchia E, D’Aronco S, et al. (2018) The maternal-fetal gradient of free and esterified phytosterols at the time of delivery in humans. Clin Nutr 37(6):2107-12.
- Gray VA, Cole E, Riva Toma JMD, Ghidorsi L, Guo JH, et al. (2014) Use of enzymes in the dissolution testing of gelatin capsules and gelatin-coated tablets- revisions to dissolution <711> and disintegration and dissolution of dietary supplements <2040>. Dissolution Technologies 21(4):6-19.
- Corrente A (1954) A method for appraisal of antacid capacity. J Am Pharm Assoc 43(4):242-5.
- Forsgård RA (2019) Lactose digestion in humans: Intestinal lactase appears to be constitutive whereas the colonic microbiome is adaptable. Am J Clin Nutr 110:273-9.
- Deng Y, Misselwitz B, Dai N, Fox M (2015) Lactose intolerance in adults: Biological mechanism and dietary management. Nutrients 7(9):8020-35.
- Savaiano D (2013) Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): A randomized, double-blind clinical trial. Nutrition Journal 12(1):1-9.
- Garipoglu G, Ersoy N, Gülsen M, Özgürtas T (2021) Effect of lactose intolerance severity on food intake and quality of life in adults with lactose intolerance in Turkey. J Health Res 12:0617.
Citation: Gaio E, Tedesco E, Benetti F, Ciampanelli F, Benatti P, et al. (2021) Antiacid and Prodigestive Activity of a Novel Formulation. J Gastrointest Dig Syst.11:666 DOI: 10.4172/2161-069X.1000666
Copyright: © 2021 Gaio E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2677
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 2127
- PDF downloads: 550