Research Article Open Access
Anti-Inflammatory Effects of Bullatine A on LPS-Induced RAW264.7 Cells by Endoplasmic Reticulum Stress
Ren W1, Zhou H1, Xian-Ju H1* and Gao Y2
1School of Pharmacy, South-central University for Nationalities, Wuhan, P.R. China
2Department of Pharmacology & Toxicology, Beijing Institute of Radiation Medicine, Beijing, P.R. China
- *Corresponding Author:
- Xian-Ju Huang
School of Pharmacy, South-central University for Nationalities
Wuhan, P.R. China
Tel: 0086-27-67841196
Fax: 0086-27-67841196
E-mail: huangxianju1972@aliyun.com
Received date: November 04, 2014; Accepted date: December 08, 2014; Published date: December 10, 2014
Citation: Ren W, Zhou H, Xian-Ju H, Gao Y (2014) Anti-Inflammatory Effects of Bullatine A on LPS-Induced RAW264.7 Cells by Endoplasmic Reticulum Stress. Interdiscip J Microinflammation 1:123. doi: 10.4172/2381-8727.1000123
Copyright: © 2014, Xian-Ju H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at International Journal of Inflammation, Cancer and Integrative Therapy
Abstract
Objective: Bullatine A (BLA), a diterpenoid alkaloid of the genus Aconitum, possesses anti-rheumatic, antiinflammatory and anti-nociceptive effects. The mechanism underlying the anti-inflammatory effect was further examined using murine RAW264.7 macrophage cell lines in vitro.
Methods: The effects of BLA on cell apoptosis/death and endoplasmic reticulum (ER) stress in RAW264.7 cells induced by lipopolysaccharide (LPS) were examined. The effects of BLA on pro-inflammatory cytokines interleukin-6 (IL-6) as well as nitric oxide (NO), intracellular reactive oxygen species (ROS) and Ca2+ in RAW264.7 cells induced by LPS were examined.
Results: The results showed that BLA protected RAW 264.7 cells against apoptotic death induced by LPS via attenuated the production of pro-inflammatory factor interleukin-6 (IL-6), nitric oxide (NO), intracellular reactive oxygen species (ROS) and Ca2+ in LPS-treated RAW 264.7 cells.
Conclusion: The results demonstrated a significant anti-inflammatory and ER stress inhibitory properties of BLA. It also indicated that it may have beneficial therapeutic effects in the treatment of conditions associated with an overproduction of inflammatory cytokines, including pain, inflammation, cancer, and neurodegenerative diseases.
Keywords
Bullatine A; anti-inflammation; RAW 264.7; LPS; endoplasmic reticulum stress
Introduction
The processed tubers of genus Aconitum were important materials in traditional Chinese medicine for the treatment of pain, inflammation, and some neuronal disorders [1]. Pharmacological researches have shown that the alkaloids of the genus Aconitum mainly possess therapeutic effects such as analgesia and anti-inflammatory [2]. The bullatine A (BLA) (Figure 1), a diterpenoid alkaloid of the genus Aconitum, is one of the major compounds isolated from Aconiti brachypodi Radix (Family Ranunculaceae) [3,4]. However, the effect of BLA on inflammation remains to be elucidated and needs further investigation.
Inflammation is a complex process mediated by the action of various immune cells, such as natural-killer cell, neutrophiles, and macrophages. Macrophages are key players of the immune system [5] and play a central role to mediate many different immunopathological phenomena during inflammation. When the body is stimulated by pathologic injury, activated macrophages release numerous pro-inflammatory cytokine and inflammatory mediators [6]. Hence, the macrophage cell line provides an excellent model for drug screening and evaluation of potential inhibitors of the inflammatory response.
The endoplasmic reticulum (ER) is multifunctional organelle which coordinates lipid biosynthesis, protein folding, and calcium storage and release. ER homeostasis is vital for cell function and survival. Disturbances in the redox status, calcium storage, or protein glycosylation in the ER compromise the ER protein folding capacity, resulting in the accumulation of unfolded proteins in the ER which is defined as ER stress. Moreover, the ER stress is also provoked by inflammatory situations and is involved in a wide range of pathophysiological conditions, including systemic inflammation caused by LPS [7]. Increasing Ca2+ and reactive oxygen species (ROS) were also reported under ER stress [8]. Exposure to LPS induces inflammatory induction of pro-inflammatory cytokines such as TNF-α, interleukin-6 (IL-6), and IL-1β, thus triggered a cascade of inflammatory reaction.
The present study was thus conducted to investigate the effect of BLA on LPS-induced inflammation and ER stress response using murine RAW264.7 macrophage cell lines in vitro. The effects of BLA on pro-inflammatory cytokines IL-6, nitric oxide (NO) as well as the mRNA levels of IL-6 and inducible nitric oxide synthase (iNOS) were examined. Moreover, the intracellular ROS as well as Ca2+ in RAW264.7 cells induced by LPS were detected to further discuss the mechanism.
Materials and Methods
Materials, Chemicals and Reagents
The Bullatine A (BLA, FW 343.5, colorless crystal, purity 99.8%) was obtained from National Institutes for Food and Drug Control in China. It was dissolved in dimethyl sulfoxide (DMSO, Amresco, USA) and then freshly diluted in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, Logan, USA) at the desired concentration. The final DMSO concentration in the solution was not more than 0.5%. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Biotech (USA). Penicillin and streptomycin were purchased from Hyclone (Logan, Ut ah, USA). LPS, Griess reagent were obtained from Beyotime Institute of Biotechnology. Dichlorofluorescin diacetate (DCFH-DA) and hoechst 33258 were obtained from Sigma Chemicals Co. (USA). All other chemicals were of analytical grade unless otherwise stated.
The cell culture
Murine RAW 264.7 macrophage cell line was obtained from American Type Culture Collection (ATCC) and cultured in DMEM supplemented with 10% calf serum, 1% strepto-mycin/penicillin at 37°C in a humidified incubator under 5% CO2.
Analysis of cell viability
The cells were exposed to LPS or co-incubated with different concentration of BLA for 48 h before staining. Cell survival was observed with phase-contrast microscope (OLYMPUS, Japan). The cell viability was evaluated by MTT method using a microplate reader (TECAN A-5082, megllan, AUSTRIA) [9]. The results were expressed as a percentage of the control value.
Measurement of apoptosis by hoechst 33258 staining
The cells were exposed to LPS or co-incubated with different concentration of BLA for 48 h before staining. Cell apoptosis and nuclear morphology of the cells were detected using the method of hoechst 33258 staining [10]. Briefly, the cells were stained by Hoechst 33258 (1 µg/ml) at room temperature in dark for 15 min. The cells were then washed twice with D-hanks, examined and immediately photographed under a fluorescence microscope (Nikon Corporation, Chiyoda-ku, Tokyo, Japan). Apoptotic cells were defined on the basis of nuclear morphology changes, such as chromatin condensation and fragmentation.
Measurement of NO and IL-6 concentration
The supernatant of RAW 264.7 cells was collected for the detection of NO and IL-6. The production of NO was monitored using Griess Reagent System Kit (Beyotime Institute of Biotechnology, China). The IL-6 was measured by ELISA method using commercial kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
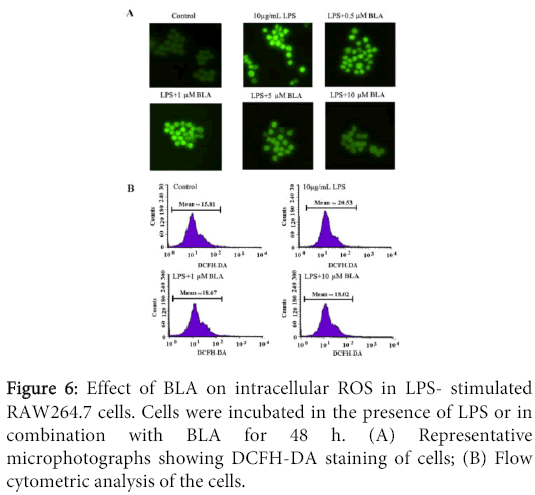
Measurement of intracellular ROS by DCFH-DA stainning
Intracellular ROS were monitored by using the DCFH-DA fluorescent probe as previously described [11]. Intracellular H2O2 or low-molecular-weight peroxides can oxidize DCFH-DA to the highly fluorescent compound dichlorofluorescein (DCF). Briefly, RAW264.7 cells were plated as 1×106 cells/well in 6-well culture plates and treated with 10 µg/mL LPS or in combination with different concentrations (0.5-10 µM) of BLA for 48 h. After treatment, the cells were washed twice in DMEM to remove excess nanoparticles. Cells were incubated with 10 mM DCFH-DA at 37 for 30 min, then washed twice with DMEM. The cells were then analyzed for ROS generation using fluorescence microscope (Nikon Corporation, Chiyoda-ku, Tokyo, Japan) and BD FACS Aria III flow cytometry (488 nm excitation, 530-540 nm emission), respectively. A minimum of 20,000 events were analyzed per sample and the results expressed as fold-change of fluorescence intensity over control.
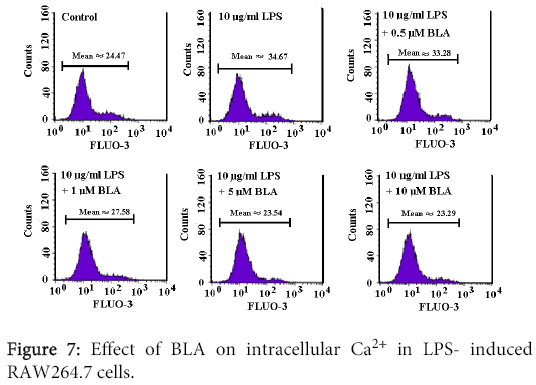
Measurement of intracellular Ca2+
Intracellular Ca2+ measurements were performed as described previously [12]. After treatment with 10 µg/mL LPS or in combination with different concentrations (0.5-10 µM) of BLA for 48 h, RAW 264.7 cells were washed in DMEM and then loaded with Fluo3/AM (Calbiochem; Bad Soden, Germany) in DMEM (10 μM). The cells were incubated at 37 for 20 min and washed twice with DMEM. The Fluo3/AM-loaded cells were re-suspended in 700 μL DMEM. Then, Ca2+ dependent fluorescence intensity was measured using flow cytometry (488 nm excitation, 530-540 nm emission).
Realtime reverse transcriptional polymerase chain reaction (Realtime RT-PCR)
Briefly, Total RNA was isolated from cells using Trizol Reagent according to the manufacture’s instructions. One microgram of RNA was reverse transcribed to cDNA with the use of ReverTra AceTM First Strand cDNA Synthesis Kit (Promega, USA). cDNA was amplified by quantitative real-time PCR (Bio-Rad, USA) using SYBR® Premix Ex TaqTM RT-PCR kit (Takara, Code QPK-201, Japan). Each 25 μL reaction mixture consisted of 12.5 μL SYBR® Premix Ex TaqTM, 0.5 μL of each primer (10 μm), 2 μL of cDNA, and 9.5 μL Rnase Free dH2O. Cycling conditions were as follows: step1, 30 s at 95; step 2.40 cycles at 95 for 5 s, 60 for 30 s; step 3, dissociation stage. Data from the reaction were collected and analyzed by the complementary computer software. Relative quantification of gene expression was calculated using 2ΔΔCt data analysis method as previously described and normalized to β-actin in each sample. The primers used in this study were:
IL-6: fw TGATGATAACCTGCTGGTGTG and rw TCGTTGCTTGGTTCTCCTTG;
iNOS: fw ACATCGACCAGAAGCTGTCC and rw TGAGCCTATATTGCTGTGGC.
Statistical analysis
Statistical analysis was performed using SPSS 11.5 for windows. All results were presented as mean ± S.E.M. Group differences were analyzed using one-way analysis of variance (ANOVA) followed by LSD's post hoc tests. A probability of P<0.05 was considered significant.
Results
Effect of BLA on LPS- stimulated cell viability
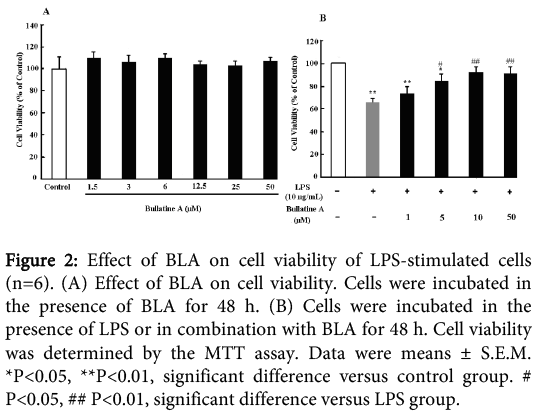
As shown in Figure 2A, B LA has no obvious cytotoxicity on RAW 264.7 cells under dosage of 50 µM for 48 h. No significant difference was observed in MTT assay at A570 values between the groups (P>0.05). However, incubation of 10 μg/mL LPS for 48 h could induce significant cytotoxicity on the cells. BLA (1-50 μM) could dose-dependently prevent the decreased cell viability, which indicated the protective effect of BLA on LPS-induced injury.
Figure 2: Effect of BLA on cell viability of LPS-stimulated cells (n=6). (A) Effect of BLA on cell viability. Cells were incubated in the presence of BLA for 48 h. (B) Cells were incubated in the presence of LPS or in combination with BLA for 48 h. Cell viability was determined by the MTT assay. Data were means ± S.E.M. *P<0.05, **P<0.01, significant difference versus control group. # P<0.05, ## P<0.01, significant difference versus LPS group.
Effect of BLA on LPS- induced cell apoptosis
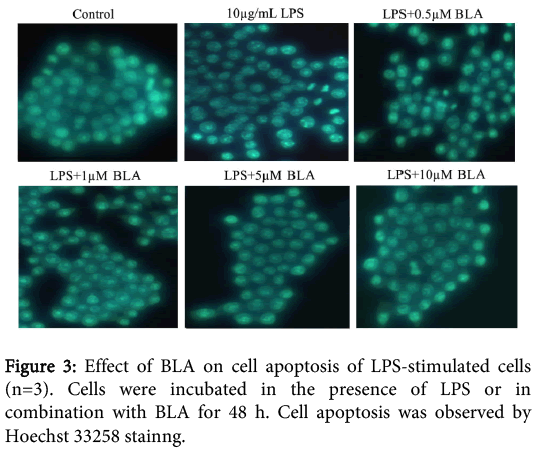
Nuclear morphology was assessed with membrane-permeable reagent Hoechst 33258. The nuclei of normal cells exhibited homogeneous and diffuse staining with regular contours and a round shape. After exposure to LPS for 48 h, the majority of cells exhibited an asymmetric and bright blue fluorescence. The number of condensed nuclei, one of the typical hallmarks for apoptosis, increased. However, co-incubation of BLA (0.5-10 μM) could significantly prevent LPS-induced cell apoptosis, which was consistent with the protective effect of BLA on LPS-induced cell viability Figure 3.
Effects of BLA on iNOS and IL-6 mRNA levels in RAW 264.7 cells induced by LPS
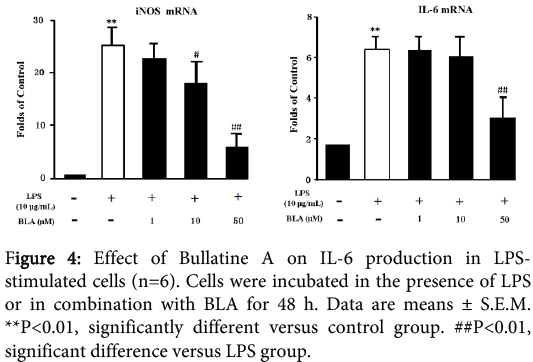
The treatment of RAW264.7 cells with LPS (10 μg/ml) for 48 h could increase the mRNA levels of IL-6 and iNOS to some degree as shown in Figure 4. The BLA (1-50 µM) could significantly down-regulate the mRNA levels in a dose-dependentmanner.
Effect of BLA on NO and IL-6 production of RAW264.7 cells induced by LPS
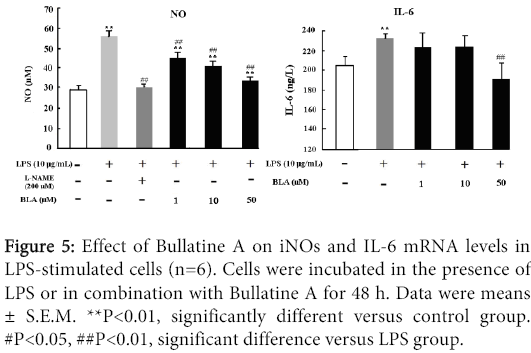
The effects of BLA on NO and IL-6 production in LPS-stimulated RAW 264.7 cells were shown in Figure 5. Exposure of LPS (10 μg/ml) could lead to redundant release of NO and IL-6, which could be dose-dependently inhibited by BLA (1-50 µM) as depicted in Figure 5. As a positive control drug, L-NAME which is widely used to inhibit endothelial synthesis of NO, also displayed evident inhibitory property on NO production. This reduction in NO and IL-6 accumulation correlates with decreased iNOS and IL-6 expression, respectively.
Figure 5: Effect of Bullatine A on iNOs and IL-6 mRNA levels in LPS-stimulated cells (n=6). Cells were incubated in the presence of LPS or in combination with Bullatine A for 48 h. Data were means ± S.E.M. **P<0.01, significantly different versus control group. #P<0.05, ##P<0.01, significant difference versus LPS group.
Effects of BLA on intracellular ROS production of RAW 264.7 induced by LPS
The effect of BLA on intracellular ROS production of RAW 264.7 induced by LPS was shown in Figure 6. As shown in Figure 6A, cells induced by LPS produced stronger DCF signals than normal cells, LPS in combination with different concentrations of BLA (0.5-10 µM) resulted in a marked reduction in DCF fluorescence (Figure 6B), indicating a inhibitory effect of BLA on intracellular ROS production.
Effect of BLA on intracellular Ca2+ concentration of LPS-induced RAW264.7 cells
As shown in Figure 7, 10 μg/ml LPS significantly increased Fluo-3 fluorescence, demonstrating an increase of intracellular Ca2+ concentration of the cells. BLA (1-10 µM) could remarkably reduced the elevation of intracellular Ca2+ (Figure 7).
Discussion
Chronic inflammation has been linked to a wide variety of diseases such as atherosclerosis [13], Alzheimer’s disease [14], diabetes [15], and carcinogenesis [16]. Macrophages play a critical role in regulating inflammation and can be activated by external stimuli to produce various inflammatory mediators such as NO and ROS. Our present results showed that BLA treatment prevents LPS-induced RAW 264.7 cell death/apoptosis via attenuated the production of pro-inflammatory factors and inhibiting ER stress response through intracellular ROS and Ca2+, demonstrating a significant anti-inflammatory properties of BLA.
NO, which is mainly generated by iNOS under the inflammatory conditions [17], plays a key role in each step of the pathological processes during inflammation [18]. In fact, nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin [19] and tolfenamic acid [20], are currently used for both cancer prevention and treatment [21]. Moreover, NO is also a major source of Ca2+ elevation and can leads to an increase in intracellular ROS production via intracellular Ca2+ influx [22]. We have shown here that RAW264.7 cells could be damaged after incubation with 10μg/mL of LPS, leading to IL-6 and NO release as well as transcriptional activation of iNOS and IL-6.
ER stress has been confirmed to be involved in multiple pathological processes, including inflammation, impaired autophagy, mitochondrial dysfunction and hypoxic responses. Studies suggest that altered redox homeostasis in the ER is sufficient to cause ER stress, which could in turn induces the production of ROS in the ER and mitochondria. Furthermore, as the major site of intracellular Ca2+ storage, the ER has the capacity to regulate Ca2+ homeostasis and Ca2+ related biological processes, and it has been shown that ER stress-associated Ca2+ depletion mediates apoptosis and disease development [23]. We demonstrated that LPS stimulation causes ROS generation induced by disruption of intracellular Ca2+ homeostasis, and that ROS stimulate the overproduction of pro-inflammatory cytokines, which leads to death/apoptosis in macrophages. The BLA (1–50 μM) potently inhibits RAW264.7 cell death/apoptosis induced by ATP, and thus suppressed ER stress -mediated inflammatory responses.
In conclusion, this study demonstrates the anti-inflammatory effect of BLA are mediated, at least in part, through attenuation of ER stress. Our results should provide valuable insight into the pathogenesis of BLA on ER-associated cell death as a key mediator in LPS-induced inflammation. Since BLA has little toxicity and is much safer than other alkaloids isolated from the genus Aconitum [24], the results also demonstrated that it may have beneficial therapeutic effects in the treatment of conditions associated with an overproduction of inflammatory cytokines, including pain, inflammation, cancer, and neurodegenerative diseases.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81102897 and 81374064) and Chinese National Project of “Twelfth Five-Year” Plan for Science & Technology Support (2012BAI27B06-2).
References
- Wang BX (1996) Pharmacology of Modern Traditional Chinese Medicines. Tianjin: China Tianjin Science and Technology Press425-430.
- Ameri A (1998)The effects of Aconitum alkaloids on the central nervous system. Prog Neurobiol56: 211-235.
- Ren W, Yuan L, Li J, Huang XJ, Chen S, Zou DJ, et al. (1012) Ethanolic extract of Aconiti Brachypodi Radix attenuates nociceptive pain probably via inhibition of voltage-dependent Na+ channel. Afr J Tradit Complement Altern Med9: 574-583.
- Huang XJ, Ren W, Li J, Chen LY, Mei ZN (2013)Anti-inflammatory and Anticancer Activities of Ethanol Extract of Pendulous Monkshood Root in vitro. Asian Pac J Cancer Prev14: 3569-3573.
- CarralotJP, Kim TK, Lenseigne B, Boese AS, Sommer P, Genovesio A, et al.(2009) Automated high-throughput siRNA transfection in Raw264.7macrophages: a case study for optimization procedure. J Biomol Screen 14: 151–160.
- Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, et al. (2006) Aldose reductase mediates the lipopolysaccharide-induced release ofinflammatory mediators in RAW264.7 murine macrophages. JBiol Chem281: 33019–33029.
- HoHJ, Huang DY, Ho FM, Leed LT, Lin WW (2014) Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase expression by endoplasmic reticulum stress. Cell Signal24: 2166–2178.
- Pahl HL, Baeuerle PA (1996)Activation of NF- cB by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett392: 129–136.
- Li J, Ren W, Huang XJ, Zou DJ, Hu X, et al. (1013) Bullatine A, a diterpenoid alkaloid of the genus Aconitum, could attenuate ATP-induced BV-2 microglia death/apoptosis via P2X receptor pathways. Brain Res Bull 97: 81-85.
- Yao G, Yang L, Hu Y, Liang J, Liang J, et al. (2006) Nonylphenol-induced thymocyte apoptosis involved caspase-3 activation and mitochondrial depolarization. Mol Immunol43: 915-26.
- Huang X, Li Q, Li H, Guo L (2009)Neuroprotective and Antioxidative Effect of CactusPolysaccharides In Vivo and In Vitro. Cell Mol Neurobiol 29:1211–1221.
- LangPA, KempeDS,MyssinaS,TanneurV,BirkaC,et al. (2005) PGE(2)inthe regula-tion of programmed erythrocyte death. Cell Death Differ12: 415-428.
- Ross R (1999) Atherosclerosis—an inflammatory disease. New Engl J Med 340: 115–126.
- Weiner HL and Frenkel D (2006)Immunology and immunotherapy of Alzheimer's disease. Nat Rev Immunol6: 404–416.
- 15 Garcia C, Feve B, Ferré P, HalimiS, Baizri H, et al.(2010) Diabetes and inflammation: fundamental aspects and clinical implications. Diabetes and Metab 36: 327–338.
- Mantovani A, Allavena P, Sica A, Balkwill F (2008)Cancer-related inflammation. Nature454: 436–444.
- Brown GC (2007) Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc T35: 1119–1121.
- Kanwar JR, Kanwar RK, Burrow H, Baratchi S, et al. (2009) Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr Med Chem16: 2373–2394.
- Jacobs EJ (2011) Will an aspirin a day help keep fatal cancer away? Lancet377: 3–4.
- Basha R, Baker CH, Sankpal UT,Ahmad S,et al.(2011) Therapeutic applications of NSAIDS in cancer: special emphasis on tolfenamic acid. Front Biosci3: 797–805.
- Aggarwal BB, Vijayalekshmi RV, Sung B (2009) Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res15: 425–430.
- Weber TJ, Smallwood HS, Kathmann LE, Markillie LM, et al. (2006) Functional link between TNF biosynthesis and CaM-dependent activation of inducible nitric oxide synthase in RAW 264.7 macrophages. Am J of Physiol-Cell Ph 290:C1512-1520.
- Mei Y, Thompson MD,Cohen RA, TongXY (2013) Endoplasmic Reticulum Stress and Related Pathological Processes. J Pharmacol Biomed Anal1: 1000107.
- Singhuber J, Zhu M, Prinz S, Kopp B, (2009) Aconitum in Traditional Chinese Medicine—A valuable drug or an unpredictable risk? J Ethnopharmacol 126: 18-30.
Relevant Topics
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery
- Immunology: Current Research
- Current Trend in Gynecologic Oncology
- Journal of Cancer Diagnosis
- Journal of Gastrointestinal Cancer and Stromal Tumors
- Cervical Cancer: Open Access
- Journal of Mucosal Immunology Research
- Journal of Oncology Research and Treatment
- Journal of Orthopedic Oncology
- Journal of Prostate Cancer
- Research and Reviews on Pathogens
Article Tools
Article Usage
- Total views: 17469
- [From(publication date):
December-2014 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 12746
- PDF downloads : 4723