Research Article Open Access
Antennal and Behavioral Response of Cydia pomonella and Lobesia botrana Moths to Allyl Cinnamate
| Marta Giner1*, Mercè Balcells2 and Jesús Avilla1 | ||
| 1Department of Forest Science and Crop Production, University of Lleida, Lleida (Spain) and Department of Crop Protection, IRTA Center, Lleida, Spain | ||
| 2Department of Chemistry, University of Lleida, Lleida, Spain | ||
| Corresponding Author : | Marta Giner Department of Forest Science and Crop Production, University of Lleida Lleida (Spain) and Department of Crop Protection IRTA Center, Lleida, Spain Tel: 876-566 3088 E-mail: mginergil@gmail.com |
|
| Received: August 27, 2014; Accepted: November 11, 2014; Published: November 13, 2014 | ||
| Citation: Giner M, Balcells M, Avilla J (2014) Antennal and Behavioral Response of Cydia pomonella and Lobesia botrana Moths to Allyl Cinnamate. Adv Crop Sci Tech 2:155. doi: 10.4172/2329-8863.1000155 | ||
| Copyright: © 2014 Giner M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Electroantenographical (EAG) response to allyl cinnamate were assessed on virgin and mated Cydia pomonella and Lobesia botrana adults to determine whether this compound could be used within integrated management programs (IMP). Adult behavioral reaction was later assessed in a wind tunnel, with and without the main compound of the corresponding female sex pheromone. Allyl cinnamate elicited antennae responses of C. pomonella and L. botrana, both males and females. Allyl cinnamate EAG response was as high as pheromone response, and it was not reduced after mating. In wind tunnel assays, allyl ester itself was not attractive to C. pomonella males, but its presence did not interfere with the pheromonal action when the number of contacts was compared. For females, a higher proportion of codling moths moved towards the source when allyl cinnamate was in the wind-tunnel plume. No differences were recorded depending on the mating status of codling moth adults. The same trend was observed in L. botrana males and females.
Results suggest that allyl cinnamate acts as a female behavioral modifier, but more assays are required to
determine its role in insect communication in field conditions before inclusion in integrated pest management.
| Keywords | |
| Allyl cinnamate; Behavioral response; Cydia pomonella; EAG; Lobesia botrana; Wind-tunnel | |
| Introduction | |
| Cydia pomonella (L.) (codling moth) and Lobesia botrana (Dennis and Schiffermüller) (grapevine moth) (Lepidoptera: Tortricidae) are key pests in pome, pear and walnut orchards, and in vineyards, respectively [1]. Both species are pests of high-value crops and have low tolerance thresholds, leading to repeated insecticide treatments (with or without other pest control interventions) during the season. To solve negative effects of insecticide use [2,3], research has developed alternative pest control methods. | |
| Mating disruption, based on the use of sex pheromones, is widely used in many countries to control codling and grapevine moths [4,5]. However, its application requires some specific field characteristics in order to succeed [6-8]. Other techniques based on the use of sex pheromones (mass-trapping and, attract and kill) are also available, but less used [9]. All these techniques focus in male moth control, but is also described an effect in female behavior by exposure to its own sex pheromone [10,11]. | |
| Tree fruit volatiles are usually added to pheromone traps to increase the number of moth captures [12-18]. Some of these plant volatiles synergize response to sex pheromone [19,20]. | |
| Our research group has been working on allyl ester synthesis using glycerol as starting material [21,22], which is produced in large amounts as a by-product in biodiesel production (http://www.biodiesel.org/). The insecticidal properties of some allyl esters have been assessed previously in order to give an added value to the surplus of glycerol [21,23,24]. As several volatile compounds from codling and grapevine moth host-plants are used by adults for host and mate localization [25- 28], an effect in moth behavior due to allyl esters used as fruit aromas [29] was suspected. Moreover, some volatile compounds described as moth attractants [30-32] are chemically related to allyl cinnamate. This fact drives to hypothesize that the latter may influence codling moth and grapevine moth adult behavior. | |
| This paper describes for the first time the capacity of allyl cinnamate to elicit antennal response and to modify behavior from C. pomonella and L. botrana adults. Improved understanding of the role of this chemical compound may ultimately be incorporated into Tortricidae integrated pest management programmes (IPM). | |
| Materials and Methods | |
| Insects | |
| The experiments were conducted with a C. pomonella laboratory strain originated from a population collected in an unsprayed apple orchard in Lleida (north-east Spain), and with a L. botrana laboratory strain established in our laboratory from a mass-reared strain from INRA Bordeaux (France). Both strains were reared at the Crop Protection Laboratory of the UdL-IRTA Centre for Research and Development (Lleida, Spain) at 23 ± 2°C, with a photoperiod of 16:8 (L:D) and on agar-based semi-synthetic diet [33]. | |
| Cydia pomonella individuals were sexed as last instar larvae, whereas L. botrana individuals were sexed as pupae. The sexed individuals were kept separately by sex until adult emergence in the same conditions as the laboratory strains were. Next, four to six less than 24-h old adults of the same sex were transferred into plastic cages (diameter (d) = 15 cm, Height (H) = 5 cm), and maintained in the above mentioned conditions to obtain virgin individuals. To obtain mated adults, two or three couples were maintained together in the above mentioned plastic cages. Adults were used in electroantennogram (EAG) or wind-tunnel bioassays at second or third day after adult emergence. The mating status of females was ascertained by the presence of a spermatophore, by dissection of females after EAG recording or wind tunnel assay. Males were considered mated when females of their own group were ascertained to be mated. | |
| Chemicals | |
| Allyl cinnamate was purchased from Sigma-Aldrich (Madrid, Spain), and acetone for residue analysis was purchased from Panreac (Barcelona, Spain). | |
| E,E-8,10-dodecadienol (codlemone) (≥ 99.5% purity), the main compound of C. pomonella sex pheromone, and E,Z-7,9-dodecadienyl acetate (EZ79Ac), the main compound of L. botrana sex pheromone (≥ 99.5% purity), were purchased from PheroBank (Wageningen, the Netherlands). | |
| Electrophysiological assays | |
| An EAG apparatus from Synthech (Hilversum, the Netherlands) was used to record electroantennographical responses of adults. Signals after stimulus application (mV) were amplified (100×) and filtered (DC to KHz) with an ID-2 interface (Syntech), digitized on a PC and analyzed with the EAG2000 program. | |
| Each antenna was carefully cut from an insect that had previously been anesthetized with ice and immobilized using a fine needle. Another cut was done at the end of the antenna using a scalpel, and then the antenna was placed between EAG electrodes. Electrode gel (Parker, Orange, NJ) was used to facilitate connection between the antenna and electrodes. | |
| Each chemical (stimulus) was presented by applying 0.1 μg of it to a piece of filter paper (2×2 cm). The piece of paper was then inserted into a Pasteur pipette, which was placed so that the tip of the pipette was 5 cm from the antenna. A puff of air (300 mL min-1) through the pipette then carried the stimuli to the antenna. | |
| Allyl cinnamate intrinsic activity | |
| Twenty antennae per species (C. pomonella and L. botrana), sex (male and female) and mating status (virgin and mated) were used in the bioassay. Five consecutive puffs of allyl ester, pheromone and acetone (control puffs) were applied to each antenna in randomized order. Each puff was separated 30 s to minimize potential onset of antennae. No fatigue was observed in the antennae used in the bioassay. | |
| The response to each stimulus was calculated as the mean response to the five puffs, and was compared to mean response to control puffs (acetone) by t-test (P<0.05). If significant greater response was observed to stimuli compared to control, mean response to stimuli was corrected as follow: corrected EAG response = mean EAG response to stimulus – mean EAG response to acetone. Corrected EAG responses were transformed [log (x + 1)] and analyzed by t-test or one-way ANOVA followed by Tukey-Kramer HSD test (P<0.05) for each species, sex and mating status. Statistical analysis was carried out with the JMP 8.0.1 program (SAS Institute, Cary, NC). | |
| Synergism between Codlemone and Allyl cinnamate | |
| Five consecutive puffs of codlemone (0.1 μg), a mixture of codlemone and allyl cinnamate (0.05 + 0.05 μg), allyl cinnamate alone (0.1 μg) and control puffs (acetone) were applied to each antenna (N = 20) of C. pomonella virgin males and females. | |
| Mean corrected EAG response (calculated as described in the intrinsic activity assay) for each treatment (codlemone, allyl cinnamate and mixture) were compared for each sex by one-way ANOVA followed by Tukey-Kramer HSD test (P < 0.05) using the JMP 8.0.1 program (SAS Institute, Cary, NC). | |
| Wind-tunnel assay | |
| The assay was conducted in a glass wind tunnel (H=50 cm, Long (L)=200 cm and wide=50 cm) situated in a room maintained at 23 ± 2°C. Light was supplied by an incandescent light bulb situated on the ceiling of the room (2 lux for C. pomonella and 100 lux for L. botrana, following results from our research group). Two ventilators either side of the wind tunnel operated simultaneously, producing an air flow of 0.15 cm s-1 through the tunnel (measured using an anemometer). | |
| The assay was performed during the first two hours of scotophase, and at least 40 insects were used per treatment. Insects were tested individually and only once. Each individual was placed into a twoside open glass tube (d=2.5 cm, L=15 cm), which was oriented to the stimulus source and situated on a 20 cm high metal stand (the insect starting point). One 8 mm red rubber septum (Sigma-Aldrich, Spain) loaded with the main compound of the sex pheromone, allyl ester, or a mixture of pheromone and allyl ester in acetone (Table 1) was used as source of the stimulus. The source was situated on a 20 cm high metal stand located at 150 cm from the insect starting point. For solvent (acetone, code 0:0), 10 μL were added to the septum. | |
| The effect of the allyl cinnamate alone or mixed to the pheromone at different proportions (Table 1) was tested on virgin males and females of C. pomonella and L. botrana. Moth behavior (activation, nonoriented flight, oriented flight and contact with source) in response to the stimulus was recorded for three minutes. The wind tunnel was cleaned with acetone after each experimental day and used material (glass tubes and metal stands) was washed with acetone and oven-dried at 200°C overnight. The percentage of insects that showed a specific behavior (activation, non-oriented flight, oriented flight and contact) for each species, sex and stimulus were compared by Χ2 test (P<0.05) using the GraphPad program (Graph Pad Software Inc., La Jolla, CA). Comparison between results obtained per sex on C. pomonella for the combination codlemone+allyl cinnamate and the sum of results for codlemone and allyl cinnamate separately were compared by t- test (P<0.05) using the GraphPad program to ascertain synergism. | |
| Results | |
| Electrophysiological assays | |
| Allyl ester intrinsic activity: Allyl cinnamate elicited virgin C. pomonella female antenna and EAG responses as great as the one produced by codlemone. The EAG response was maintained after mating (Table 2). | |
| For C. pomonella males, allyl cinnamate also caused a significant EAG response (1.070 ± 0.460 mV), not significantly different from the response to codlemone (0.753 ± 0.068 mV). The EAG response to allyl cinnamate did not reduced after mating (0.756 ± 0.207 mV) (P<0.05). | |
| For L. botrana virgin males, EAG values regarding allyl cinnamate were not significantly different than the pheromone ones (0.635 ± 0.141 and 0.892 ± 0.403, respectively). A significant reduction of response was recorded to pheromone after mating (0.323 ± 0.086; P>0.05), but not to allyl cinnamate (0.237 ± 0.059; P<0.05), as observed in C. pomonella. In the case of females, only virgins elicited a response followed by allyl cinnamate stimulation (0.305 ± 0.019; P<0.05). | |
| Synergism among Codlemone and Allyl cinnamate: No synergism was observed among codlemone and allyl cinnamate in C. pomonella antennae. No differences in EAG response were observed between codlemone - allyl cinnamate mixture and codlemone alone (Pmales = 0.91; Pfemales = 0.57) or allyl cinnamate alone (Pmales= 0.76; P females = 0.55). | |
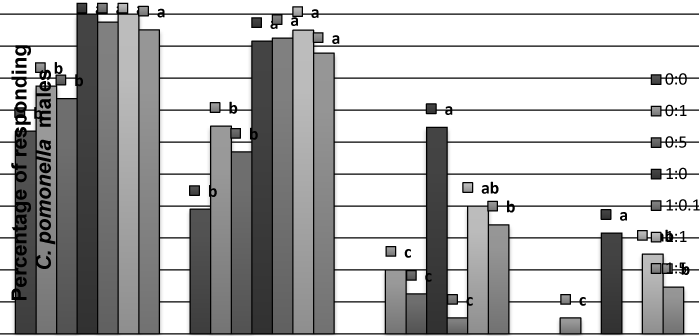
| Wind tunnel: In the wind-tunnel, the codlemone caused the complete range of behaviors from C. pomonella males (Figure 1). The addition of allyl cinnamate to codlemone blend at different doses did not have a significant effect on activation and non-oriented flight behavioral steps when compared to the ones produced by codlemone alone. The addition of allyl cinnamate to codlemone at 1: 0.1 and 1: 5 caused some reduction on oriented flight and contact behavior, but not at 1:1 (Figure 1). | |
| When allyl cinnamate was presented alone a significant reduction in percent of males that reach each behavioral step was recorded. If these values were compared to the solvent alone, a greater percent of males were activated and started the flight in the presence of the allyl ester, but few contacts were scored (Figure 1). | |
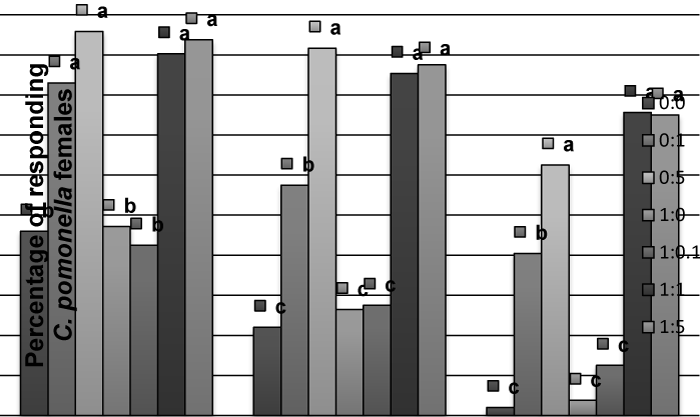
| In the case of C. pomonella females, codlemone did not cause significant behavioral effect but the presence of allyl cinnamate (alone or mixed with codlemone) did. A higher percent of virgin females were activated and flew to the source when allyl cinnamate was present (Figure 2). The same was observed in the case of mated females (data not shown). No differences were recorded in the response to allyl cinnamate between virgin or mated C. pomonella females (P>0.05). No increase of attraction was observed in the case of mated males (data not shown), compared to virgins. | |
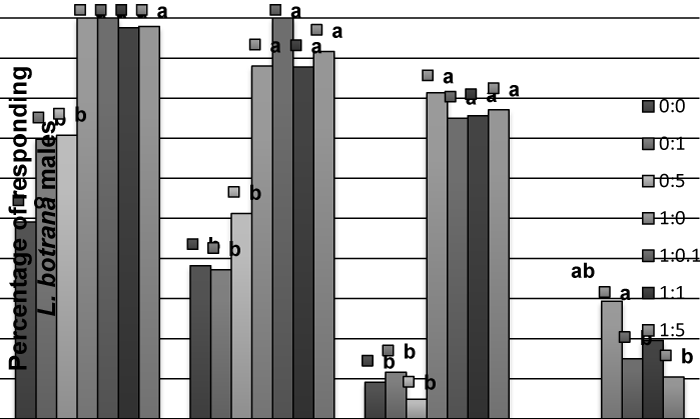
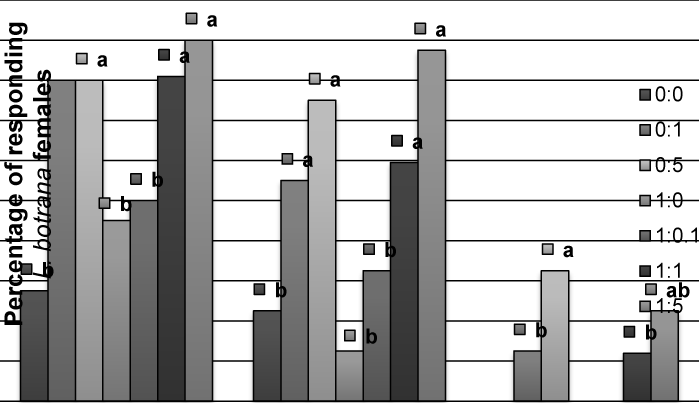
| In L. botrana, allyl cinnamate did not produce an effect in males, and the addition to pheromone causes a significant reduction in percent of contacts with the source at 1:0.1 or 1:5 proportions, but not at 1:1 (Figure 3), as observed in C. pomonella. For females, allyl cinnamate caused a significant activation, non-oriented flight and oriented flight – compared to solvent – (Figure 4), but no contacts were observed. | |
| Discussion | |
| To our knowledge, this is the first report of allyl cinnamate eliciting antennal response of C. pomonella and L. botrana moths and (or) causing a behavioral reaction in a wind tunnel. | |
| Allyl cinnamate elicited an antenna response in both males and females of C. pomonella and L. botrana, independently of the state of mating. This indicates that its action would not be strictly related with the mating process but has to be involved in general activity (feeding, host-localization). This observation contrasts with the one produced by several plant volatiles, which are more attractive after mating [34,35]. | |
| It is interesting to note that the same EAG recordings were scored by both codlemone and allyl cinnamate alone, or when blended, suggesting that the same receptors could be involved in the perception process. This possibility was previously described for plant volatiles [25,36-38]. In the case of allyl cinnamate this fact should be more deeply studied to be confirmed. | |
| Although an effect of allyl esters of fatty acids could be suspected from similarities in chemical structure to butyl hexanoate (moth attractant) [15], no effect of alkyl allyl esters were recorded in C. pomonella or L. botrana (unpublished data). | |
| It is known that the size of EAG response does not always indicate a behavioral response [34]. Even though male and female antenna elicitation was recorded, only females were attracted to sources baited with allyl cinnamate in the wind-tunnel. An increase of fluttering was observed in males and allyl cinnamate alone does not clearly produce a behavior of source contact in any case. This lack of contact could be related with the fact that the behavior was observed for three minutes. More assays should be done with increased observation time or semifield assays to reaffirm the properties of allyl cinnamate as Tortricidae moth attractant. | |
| No increase of attraction was observed in the wind-tunnel assay after mating, reassuring the results from the EAG recordings and indicating the lack of allyl cinnamate effect on the mating process. | |
| Only on C. pomonella females a synergism between pheromone and allyl cinnamate was suggested in the wind-tunnel. Other authors have described a similar synergism to plant volatiles [21,25]. This could be extremely interesting if confirmed in a field situation. | |
| Allyl cinnamate is suggested as a candidate to bait pheromonal traps with the aim of increasing moth captures in pest monitoring, or in attract & kill and mass-trapping strategies in Tortricidae pest control. | |
| The fact that allyl cinnamate is authorized for use as food aroma (http://eur-lex.europa.eu/) indicates its availability to use in ethologic control. Moreover, allyl esters can be synthesized from glycerol or fat wastes [21,22], what fosters the re-use of industrial sub products. However, more assays need to be done to fix the proportion of allyl cinnamate into the blend to improve the attractiveness of moths [39,40] and asses it in field conditions, as well as the fate of allyl cinnamate in field conditions. | |
| Acknowledgements | |
| M.G. was financed by fellowship n° BES-2008-004779, and M.G., M.B. and J.A. were supported by Spanish Ministry of Economy and Competitiveness research grant AGL 2010-17486. | |
References
- value="1" id="Reference_Titile_Link">International Society of Pest Information (ISPI) (2009) Pest directory (CD-ROM for Windows 98 & up).
- value="2" id="Reference_Titile_Link">Devine GJ, Furlong MJ (2007) Insecticidal use: context and ecological disturbances. Agric Human Values 24: 281-306.
- value="3" id="Reference_Titile_Link">Rodríguez MA, Marques T, Bosch D, Avilla J (2011) Assessment of insecticide resistance in eggs and neonate larvae of Cydiapomonella (Lepidoptera: Tortricidae). Pesticide Biochemistry and Physiology 100: 151-159.
- value="4" id="Reference_Titile_Link">Angeli G, Anfora G, Baldessari M, Germinara GS, Rama F, et al. (2007) Mating disruption of codling moth Cydiapomonella with high densities of Ecodian sex pheromone dispensers. J ApplEntomol 131: 311-318.
- value="5" id="Reference_Titile_Link">Dunkelblum E (2007) Pest management programs in vineyards using male mating disruption. Pest ManagSci 63: 769-775.
- value="6" id="Reference_Titile_Link">Sauer AE, Karg G (1999) Variables affecting pheromone concentration in vineyards treated for mating disruption of grapes vine moth Lobesiabotrana. J ChemEcol 24: 289-302.
- value="7" id="Reference_Titile_Link">Witzgall P, Bäckman AC, Svensson M, Koch U, Rama F, et al. (1999) Behavioural observations of codling moth, Cydiapomonella, in orchards permeated with synthetic pheromone. Biocontrol 44: 211- 237.
- value="8" id="Reference_Titile_Link">Louis F, Schirra K-J (2001) Mating disruption of Lobesiabotrana (Lepidotpera: Tortricidae) in vineyards with very high population densities. Pheromones for Insect Control in Orchards and Vineyards. IOBC wprs Bull 24: 75-79.
- value="9" id="Reference_Titile_Link">Mansour M (2010) Attract and kill for codling moth Cydiapomonella (Linneeus) (Lepidoptera: Tortricidae) control in Syria. J ApplEntomol 134: 234-242.
- value="10" id="Reference_Titile_Link">Stelinki LL, Il’ichev AL, Gut LJ (2006) Antennal and behavioral responses of virgin and mated oriental fruit moth (Lepidoptera: Tortricidae) females to their sex pheromone. Ann EntomolSoc Am 99: 898-904.
- value="11" id="Reference_Titile_Link">Kuhns EH, Pelz-Stelinki K, Stelinski LL (2012) Reduced mating success of female tortricid moths following intense pheromone auto-exposure varies with sophistication of mating system. J ChemEcol 38:168-175.
- value="12" id="Reference_Titile_Link">Knight AL, Light DM (2004) Use of (E,Z)-2,4-decadienoic acid in codling moth management: improved monitoring in Barlett pear with high dose lures. J EntomolSoc British Columbia 101: 59-66.
- value="13" id="Reference_Titile_Link">Bengtsson M, Bäckman A-C, Liblikas I, Ramirez MI, Borg-Karlsson AK, et al. (2001) Plant odor analysis of apple: Antennal response of codling moth females to apple volatiles during phenological development. J Agric Food Chem 49: 3736-3741.
- value="14" id="Reference_Titile_Link">Reed HC, Landolt PJ (2002) Attraction of mated females codling moths (Lepidoptera: Tortricidae) to apples and apple odor in a flight tunnel. FlaEntomol 85: 324-329.
- value="15" id="Reference_Titile_Link">Hern A, Dorn S (2004) A female-specific attractant for the codling moth, Cydiapomonella, from apple fruit volatiles. Naturwissenschaften 91: 77-80.
- value="16" id="Reference_Titile_Link">Fernández DE, Cichón L, Garrido S, Ribes-Dasi M, Avilla J (2010) Comparison of lures loaded with codlemone and pear ester for capturing codling moths, Cydiapomonella, in apple and pear orchards using mating disruption. J Insect Sci 10: 139.
- value="17" id="Reference_Titile_Link">Knight AL (2010) Improved monitoring of female codling moth (Lepidoptera: Tortricidae) with pear ester plus acetic acid in sex pheromone-treated orchards. Environ Entomol 39: 1293-1290.
- value="18" id="Reference_Titile_Link">Knight AL, Light DM (2014) Combinal approaches using sex pheromone and pear ester for behavioural disruption of codling moth (Lepidoptera: Tortricidae). J ApplEntomol 138: 96-108.
- value="19" id="Reference_Titile_Link">Yang Z, Bengtsson M, Witzgall P (2004) Host plant volatiles synergize response to sex pheromone in codling moth, Cydiapomonella. J ChemEcol 30: 619-629.
- value="20" id="Reference_Titile_Link">Varela N, Avilla J, Anton S, Gemeno C (2011) Synergism of pheromone and host-plant volatile blends in the attraction of Grapholitamolesta males. EntomolExpAppl 141: 114-122.
- value="21" id="Reference_Titile_Link">Escribà M, Barbut M, Eras J, Balcells M, Avilla J (2009) Synthesis of allyl esters of fatty acids and their ovicidal effect on C. pomonella (L.). J Agric Food Chem 12: 345-358.
- value="22" id="Reference_Titile_Link">Escribà M, Eras J, Villorbina G, Balcells M, Blanch C, et al. (2011) Use of crude glycerol from biodiesel producers and fatty materials to prepare allyl esters. Waste Biomass Valorization 2: 285-290.
- value="23" id="Reference_Titile_Link">Giner M, Balcells M, Avilla J (2012) Insecticidal action of five allyl esters on eggs and larvae of three tortricid pests: laboratory tests. Bull Insectol 65: 63-70.
- value="24" id="Reference_Titile_Link">Giner M, Avilla J, Balcells M, Caccia S, Smagghe G (2012) Toxicity of allyl esters in insect cell lines and in Spodopteralittoralis larvae. Arch Insect PhysiolBiochem 79: 18-30.
- value="25" id="Reference_Titile_Link">Yan F, Bengtsson M, Witzgall P (1999) Behavioural response of female codling moths, Cydiapomonella, to apple volatiles. J ChemEcol 25: 1343-1351.
- value="26" id="Reference_Titile_Link">Reddy GVP, Guerrero A (2004) Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci 9: 253-261.
- value="27" id="Reference_Titile_Link">Tasin M, Anfora G, Ioriatti C, Carlin S, de Cristofaro A, et al. (2005) Antennal and behavioural response of grapevine moth Lobesiabotrana females to volatiles from grapevine. J ChemEcol 31: 77-87.
- value="28" id="Reference_Titile_Link">Trona F, Anfora G, Bengtsson M, Witzgall P, Ignell R (2010) Coding and interaction of sex pheromone and plant volatile signals in the antennal lobe of the codling moth Cydiapomonella. J ExpBiol 213: 4291-4303.
- value="29" id="Reference_Titile_Link">Burdock GA (2010) Fenaroli’s handbook of flavour ingredients. 10th edition. Taylor and Francis group. CC Press. Orlando, Florida, USA. 2159.
- value="30" id="Reference_Titile_Link">Casado D, Gemeno C, Avilla J, Riba M (2006) Day-night and phenological variation of apple tree volatiles and electroantennogram responses in Cydiapomonella (Lepidoptera: Tortricidae). Environ Entomol 35: 258-267.
- value="31" id="Reference_Titile_Link">Massa MJ, Robacker DC, Patt J (2008) Identification of grape juice aroma volatiles and attractiveness to the Mexican fruit fly (Diptera: Tephritidae). FlaEntomol 91: 266-276.
- value="32" id="Reference_Titile_Link">Ortiz A, Echeverría G, Graell J, Lara I (2010) The emission of flavour-containing volatile esters by “Golden Reinders” apples is improved after mid-term storage by postharvest calcium treatment. Postharvest BiolTechnol 57: 114-123.
- value="33" id="Reference_Titile_Link">Pons S, Eizaguirre M, Sarasúa MJ, Avilla J (1994) Influencia del fotoperiodosobre la inducción de diapausa de Cydiapomonella (Lepidoptera: Tortricidae) en laboratorio y campo. InvestigacionesAgrariasProducciónProtección Vegetal 9: 477-491.
- value="34" id="Reference_Titile_Link">Ansebo L, Coracini MDA, Bengtsson M, Liblikas I, Ramirez M, et al. (2004) Antennal and behavioural response of codling moth Cydiapomonella to plant volatiles. J Econ Entomol 128: 488-493.
- value="35" id="Reference_Titile_Link">Landolt PJ, Guédot C (2008) Field attraction of codling moth (Lepidoptera: Tortricidae) to apple and pear fruit, and quantification of kairomones from attractive fruits. Ann EntomolSoc Am 101: 675- 681.
- value="36" id="Reference_Titile_Link">Shields VAC, Hildebrand JG (2001) Responses of a population of antennal olfactory receptor cells in the female moth Manducasexta to plant-associated volatile organic compounds. J ComprPhysiol 186: 1135-1151.
- value="37" id="Reference_Titile_Link">De Cristofaro A, Ioriatti C, Molinari F, Pasqualini E, Anfora G, et al. (2004) Electrophysiological responses of Cydiapomonella to codlemone and pear ester ethyl (E,Z)-2,4-dodecadienote: peripheral interactions in their perception and evidences for cells responding to both components. B Insectol 57: 137-144.
- value="38" id="Reference_Titile_Link">Ansebo L, Ignell R, Löfqvist J, Hansson BS (2005) Responses to sex pheromone and plant odours by olfactory receptor neurons housed in sensillaauricillica of the codling moth, Cydiapomonella (Lepidoptera: Tortricidae). J Insect Physiol 51: 1066-1074.
- value="39" id="Reference_Titile_Link">Tasin F, Casado D, Coracini M, Bengtsson M, Ioriatti C, et al. (2010) Flight tunnel response of codling moth Cydiapomonella to blends of codlemone, codlemone antagonists and pear ester. PhysiolEntomol 35: 249-254.
- value="40" id="Reference_Titile_Link">Solé J, Sans A, Riba M, Guerrero A (2010) Behavioural and electrophysiological responses of the European corn borer Ostrinianubilalis to host-plant volatiles and related chemicals. PhysiolEntomol 35: 354-363.
- value="41" id="Reference_Titile_Link">Giner M, Sans A, Riba M, Bosch D, Gago R, et al. (2009) Development and biological activity of a new antagonist of the pheromone of codling moth Cydiapomonella. J Agric Food Chem 57: 8514-8519.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
|||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 18337
- [From(publication date):
December-2014 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 13711
- PDF downloads : 4626
