Animal Models of Tendinopathy Induced by Chemicals
Received: 01-Dec-2021 / Manuscript No. CMB-21-41284 / Editor assigned: 03-Dec-2021 / PreQC No. CMB-21-41284 (PQ) / Reviewed: 07-Jan-2022 / QC No. CMB-21-41284 / Revised: 13-Jan-2022 / Manuscript No. CMB-21-41284 (R) / Accepted Date: 13-Jan-2022 / Published Date: 20-Jan-2022 DOI: 10.4172/1165-158X.1000221
Abstract
Tendinopathy is a common disease that afflicts a wide range of people irrespective of age and gender. The underlying pathogenesis is still poorly understood. Since it is impossible to directly conduct experiments on humans, animal models of tendinopathy are essential not only to study its developmental mechanisms, but also to devise new treatment options for tendinopathy. Chemically-induced models are usually low-cost, reproducible, less labor-intensive and easy to perform. Chemicals that are currently being used to produce tendinopathy in animals include collagenase, cytokines, transforming growth factor-β1 (TGF-β1), fluoroquinolone, kartogenin, prostaglandin, statin, carrageenan and elastase. This paper discusses the development and use of animal models induced by chemicals.
Keywords
Tendon; Tendinopathy; Animal model; Chemicalinduced; Collagenase
Introduction
Tendinopathy, a disease of the musculoskeletal system which is prevalent in the general population and especially in athletes, is characterized by activity-related chronic pain, focal tendon tenderness, tendon swelling and intratendinous imaging changes. The etiology of the disease is not completely clear. Mechanical overloading of tendons is one of the commonly agreed factors. Other factors including age, gender, body weight, gene polymorphisms, and anatomical and biomechanical variations are thought to be involved in the etiology of tendinopathy [1]. Tendinopathy is becoming one of the most common non-fatal disease of the 21st century, and an important cause of work disability and loss of quality of life [2]. If not adequately treated, tendinopathy may lead to complete tendon rupture, which often requires surgical repair. Although some progress has been made and various treatments have been applied to treat tendinopathy in recent years, we still know little about the underlying pathogenesis of tendinopathy. One of the principal reasons is the limited availability of specimens. While tissue can be obtained surgically, the tissue obtained from patients undergoing surgical procedures is generally already well developed in terms of histopathology. Additionally it is rarely possible to obtain developing specimens from patients because their condition is usually not sufficiently severe to warrant surgical intervention. Hence, a validated animal model is essential to enable in-depth studies on the etiology and pathogenic mechanism of tendinopathy, to find out how the disease occurs and develops, and to seek new treatment for it.
Currently, there are many ways to establish animal models of tendinopathy, and most of them can be categorized into two groups. One is mechanical overloading which is considered to be the most common extrinsic factor causing tendinopathy [3-5]. The other model group, which relies on intrinsic factors, involves the introduction of chemicals into normal animal tendons [6-8]. This paper discusses the development and use of animal models induced by chemicals, highlights potential outcome measures that may be used in animal tendon research, and reviews current animal models of tendinopathy induced by chemicals.
Materials and Methods
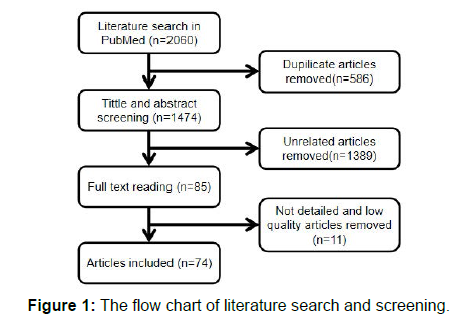
All literatures were retrieved from PubMed database. The keywords “tendinopathy,” “tendinosis” “tendinitis” and “animal model” were used for searching the literature published before September 2020. After screening the title, abstract and full text of each article, duplicate and irrelevant articles were removed, and 73 articles were finally included in this review. The flow chart of searched results presented in Figure 1.
Assessment criteria of tendinopathy animal models
To determine if an animal model is valid, there should be consensus criteria. Seeing that our knowledge about tendinopathy is limited, a standard criterion of tendinopathic animal models is not yet published. But there are some crucial features from human tendinopathy samples that animal models should consistently replicate in order to be valid. Histopathologically, the alteration in human samples include loss of matrix organization or collagen arrangement, excessive proliferation of tenocytes and changes in tenocyte morphology (hypercellularity), extensive neovascularization and vascular ingrowth (hypervascularity), increase in non-collagenous matrix such as glycosaminoglycans (GAG) [2,9-12]. In regard to mechanics, altered biomechanical properties should be apparent [13]. Also, changed molecular expression, such as matrix metalloproteinases (MMPs) and collagens, should be taken into account [10,14]. Animal models should replicate the above histopathological, mechanical and genetic features of tendinopathy in humans to be considered valid. If there are new findings in human tendinopathy pathology, animal model criteria should be updated accordingly.
Current chemicals to produce animal models of tendinopathy
Collagenase: Among the chemicals to produce animal models of tendinopathy, collagenase is the earliest and most widely used15. It was initially pioneered by Silver et al to study tendinitis by mimicing the intrinsic condition of tendon rupture. Briefly, it was found to induce a reproducible lesion consistent with spontaneous tendon injury which showed tendon degeneration accompanied by a classic inflammatory response which exist about one week [15,16]. Collagenase is currently being used by many research teams to establish tendinopathy models in animals including horse, sheep, rabbit and rat, in various anatomic locations such as the superficial digital flexor tendon (SDFT), deep digital flexor tendon(DDFT), Achilles tendon, patellar tendon and rotator cuff [17-23].
Collagenase is usually applied by intratendinous injection (Table 1). After injection, the tendon exhibits collagen matrix and fiber disorganization, increase in rounded cell density, and s marked increase in vascularity [6,22,24,25]. Altered biomechanical properties including larger cross-sectional area, decreased load to failure, lower stiffness, etc, have all been observed [25-28] (Table 1). These characteristics are similar to those observed in human samples. And the severity of collagenase induced injury seems to be dose-related [6].
In human, collagen type I is the major collagen type in healthy tendon, but when matrix degeneration resulting from tendinopathy occurs, a decrease in collagen type I and an increase in collagen type III occurs [29,30]. Findings from Liu et al in their collagenase model of rats displayed the same results as previously described [31]. In their research, they also found sustained or increased expression in decorin, biglycan, fibromodulin and aggrecan which are consistent with clinical samples [11,32,33] and increased expression in substance P(SP) and calcitonin gene-related peptide(CGRP) which positively correlates with activity-related tendon pain. Dahlgren and colleagues reported type III collagen expression is initially increased in endotenon and subsequently in the parenchyma of healing tendon [34].
Matrix metalloproteinases are thought to participate in the pathogenesis of tendinopathy. MMPs are members of a family of enzymes that can break down proteins such as collagen. It makes tendon more susceptible to microdamage, and further accelerate lesions. Numerous researchers reported a substantial increase in the expression of MMPs (MMP-1,MMP-3,MMP-9,MMP-13), and a decrease in the expression of its counterpart inhibitor, i.e., tissue inhibitor of metalloproteinases (TIMP), in the collagenase model (Table 2). Injury treatments including piperine, low level laser therapy, photobiomodulation therapy, and platelet-rich plasma were performed in these experiments and showed inhibitory effects on MMPs [19,35- 40].
In conclusion, tendinopathy induced by collagenase exhibit many major qualities seen in clinical cases. It can be considered as an efficient and valid model of tendinopathy. However, it should be noted that drawbacks also exist. There is an acute inflammatory reaction after injection which is not seen in human. Also, a chronic healing response caused after collagenase injection is incompatible with clinical cases; for humans, the healing process is usually impaired [16].
Cytokines: Stone and colleagues wished to produce a model that better emulate the reversible lesions that represent the majority of the painful tendons seen in clinical practice. They injected cytokine preparation into the rabbit patellar tendon and compared the results with the collagenase-injection model (Table 1). The cytokine preparation is a mixture of interleukin-lα (IL- lα), TGF-β, basic fibroblast growth factor, and other unidentificd growth factors. At 4 weeks, tendons injected with the cytokine had increased cellularity, and a normal-appearing collagen matrix. No inflammatory cells were seen. Slightly increased vascularity in the tendon was noted. While there was a significant decrease in the ultimate load at 16 weeks, there was no change in tendon cross-sectional area (Table 2). No significant change in the collagen content and crosslinking density were seen. No matrix damage or evidence of collagen degradation was produced. There were only mild injuries induced by cytokines compared to collagenase, Taken together, there is a lack of evidence that cytokines injections produce a valid model of tendinopathy [41].
TGF-β1: In recent years, TGF-β1 has been used to establish a tendinopathy model in mice [8,42,43]. The reason for the use of TGF-β1 is that TGF-β1 has been demonstrated to stimulate both chondrogenesis in numerous tissue and cell culture models, and is a critical biological factor translating mechanical overuse injury of tendon cells, into a biological response. Male mice were injected with 100ng TGF-β1 in the mid portion of the Achilles tendon (Table 1), and the mice were killed at 48H, 2W and 4W thereafter. The injected tendons showed a robust increase in collagen III and ADAMTS5 at 48h after injection. Accumulation of GAG, as well as an increase in chondrocyte-like cells, and collagen disorganization, was observed at 2 and 4 weeks. Significant reductions in stiffness, maximum stress, tensile modulus, and an increase in cross-sectional area were seen at 2 weeks8. These results are consistent with those seen in human. But another study reported that after injection, alterations included hypercellularity, collagen disorganization, and chondroid deposits at 14 days, which largely dissipated by 28 days, suggesting a feeble pathological continuity [43] (Table 2).
| Changes induced by chemicals | ||||
|---|---|---|---|---|
| Chemicals | Histopathological | Mechanical | Biochemical | Conclusion |
| Collagenase | 1.fiber disorganization 2.hypervascularity 3.hypercellularity |
1.increased cross-sectional area 2.decreased load to failure 3.lower stiffness |
1.decreased Coland increased Col content 2.increased ecpression in decorin, biglycan, fibromodulin, aggrecan, SP, CGRP, MMPs |
1.The most widely used modeling method. 2.Reproduced many major qualities seen in clinical cases. 3.Acute inflammation and chronic healing response caused by collagenase is not seen in clinical cases. |
| Cytokines | 1.hypercellularity 2.slightly increased vascularity |
1. significant decrease in the ultimate load at 16 weeks | absent | 1.Less valid ,because the model is lack of enough reproduced symptoms of human tendinopathy. |
| TGF-β1 | 1.Collagen disorganization 2. hypercellularity 3.chondroid deposits |
1.significant reduction in stiffness, maximum stress, tensile modulos 2.increased cross-sectional area |
1.accumulation of GAG 2.increase in Col and ADAMTS5 |
1.Reproduced many major qualities seen in clinical cases. 2.Unstable pathological changes and short of long-term status of the model. 3.A useful model for acute tendinopathy |
| Fluoroquinolone | 1.fiber disorganization | absent | 1.decrease in Col , elastin, fibronectin, β1-integrin | 1.Reproduced some features of tendinopathy. 2.Not valid for general tendinopathy research. |
| KGN | 1.Collagen disorganization 2.hypervascularity 3.hypercellularity |
absent | 1.proteoglycan accumulation 2.up-regulated aggrecan, Col , sox-9 in TSCs |
1.Reproduced many major qualities seen in clinical cases. 2.Short of long-term status of the model. 3. Lack of mechanical evidence. |
| PG | 1.hypercellularity 2.tendon disorganization and degeneration |
absent | absent | 1.Reproduced good histopathological features of tendinopathy but evidence in other aspects are Insufficient. |
| Statin | 1.fiber disorganization | 1.decreased maximum stress and load | 1.increase in GAG 2.increase in MMPs expression 3.decrease in Col |
1.Reproduced some features of tendinopathy. 2.Not valid for general tendinopathy research. |
| Carrageen | 1.matrix degeneration 2.cell infiltration 3.Collagen disorganization 4.hypercellularity 5.angiogenesis |
1.decreased ultimate failure load | 1.presence of MMP9 2.degradation of non-collagenous proteins and GAG |
1.Reproduced some major features of tendinopathy. 2.Distinct infiltration of inflammatory cells caused by carrageen is incompatible with clinical cases. |
| Elastin | 1.hypervascularity 2.hypercellularity 3.Collagen fiber disorganization and fragmentation |
1.increased tendon thickness 2.decreased weight-bearing |
1.decreased Col expression 2.increased Col expression |
1.Reproduced many major qualities seen in clinical cases. 2.Need more literature in future to verify its validity. |
| Abbreviations: Col , collagen type ; Col, collagen type ; Col , collagen type; GAG, glycosaminoglycan; SP, substance P; CGRP, calcitonin gene-related peptide; MMPs, matrix metalloproteinases; TSCs, tendon stem cells. | ||||
Table 2: Current chemicals to produce animal models of tendinopathy.
In conclusion, the injection of TGF-β1 can induce pathological changes in mice which similar to those seen in human tendinopathy. But deficiencies also exist. First, the long-term status of the model is not available because of the short duration of induced changes which only last for 4 weeks. Second, the progression of pathological changes is unstable. For the above reasons, this model may be useful for studies on acute tendinopathy, it does not provide an opportunity to conduct studies on chronic tendinopathy. Further efforts should be designed to prolong the observed changes to establish a more human injury simulating model.
Fluoroquinolone: Fluoroquinolone is an antibiotics which is widely used in the clinic. There have been reports that fluoroquinolones can cause tendon lesions including pain, swelling and even rupture [44- 46]. The clinical observations suggest that fluoroquinolone symptoms related to tendinopathy, and thus fluoroquinolone is used to produce tendinopathy in animals (Table 1). Kashida and Kaot [47] compared the toxic potentials of 10 fluoroquinolones on the Achilles tendon in juvenile rats. Ten fluoroquinolones were orally administered, and animals were sacrificed 24 hours after administration. Results showed edema and increased mononuclear cells in the tendon sheath. Among 10 fluoroquinolones, fleroxacin and pefloxacin were the most toxic, while norfloxacin, ciprofloxacin and tosufloxacin showed no toxicity. Shakibaei and colleagues further examined the effects of ciprofloxacin and fleroxacin. After orally administering ciprofloxacin to dogs for 5 days, there were decreases in collagen type I, elastin, fibronectin and β1 integrin content in the Achilles tendon [48]. Detachment of tenocytes from the extracellular matrix (ECM), a decrease in fibril diameter and an increase in fibril separation in Achilles tendon were reported 4 weeks after a single dose of fleroxacin in rats [49] (Table 2). Although fluoroquinolones reproduced some features of tendinopathy, this model lacks of universality. Fluoroquinolones are generally not involved in most cases of tendinopathy, so this model is quite fluoroquinolonesspecific. Meanwhile, not every patient taking fluoroquinolones shows the symptom of tendinopathy. The use of fluoroquinolones to establish animal model of tendinopathy is thereby questioned.
Kartogenin (KGN): Chondrocyte-like cells have been observed in the degenerated tendon regions in tendinopathy patients [50]. Kartogenin is a small heterocyclic compound that can stimulate endogenous stem/progenitor cells such as mesenchymal stem cells to differentiate into chondrocytes in mice [51]. In order to create an animal model with a more relevant injury location, Yuan et al implanted fine beads of a bio-compound called kartogenin into rat tendons [52] (Table 1). After 5 weeks of normal cage feeding, rats were sacrificed. Gross appearance showed thickening of the paratenon tissues. Hypercellularity, hypervascularity, collagen disorganization, anti-CD31 antibody and extensive amounts of proteoglycans were revealed in stained tendon sections. These findings indicate that KGN induces localized chondrogenesis with neo-vascularization in rat Achilles tendons. They also performed in vitro experiments to understand the cellular mechanism by which KGN induces the formation of cartilage-like tissues in vivo. Results showed that KGN up-regulated three chondrocyte specific genes, aggrecan, collagen type II and Sox-9 in tendon stem cells(TSCs) treated with KGN in vitro [52] (Table 2). Briefly, certain features of degenerative tendinopathy frequently observed in clinics have been captured by KGN-induced in an animal tendinopathy model. These findings suggest KGN has the potential to be a useful model of tendinopathy. Disadvantage is that the implantation procedure is a little more complex than injection and gavage. And further studies should focus on the long-term alterations of KGN model.
Prostaglandin (PG): Prostaglandin has been used to induce tendinopathy, it is based on up-regulated production of prostaglandin-E2 (PGE2) by human tendon fibroblasts under mechanical stimulation in vitro [53] and increased PGE2 expression after exercise in vivo [54]. PGE2 was injected into the rabbit patellar tendon once a week for 4 weeks (Table 1). Following treatment, hypercellularity, abnormal tissue architecture, tendon disorganization and degeneration were evident, coupled with decreased fibril diameter (Table 2). Interestingly, despite PGE2 being a known inflammatory mediator, there were no inflammatory cells in the tendon 1 week after repeated PGE2 injection. This suggests that degeneration may be the principal effect of PGE2 instead of inflammation [55]. PEG1 was also applied to the peritendinous Achilles tendon of rats. Acute inflammation was manifested 1 week after injection, followed by weeks of degeneration characterized by increased vascularity, cellularity and fiber disorganization [56]. The limitation of PGEs is the lack of longterm effects of prostaglandin injections. Evidence in biomechanics and molecular level processes has not been reported.
Statin: Statins are widely prescribed medications used for the treatment of hyperlipidemia. Tendinitis and tendon ruptures have been observed in frequent user of stains. Effected tendons include the distal biceps tendon, the quadriceps tendon, the patellar tendon and the Achilles tendons, the latter of which is the most commonly affected one [57-60]. Statins, therefore, may be involved in the etiology of tendinopathy and may be used as agents to produce tendinopathy in animals (Table 1). Statins may change the ECM components in the tendon, with increases in GAGs and decrease in collagen I accompanied by active expression of MMPs [61]. The use of atorvastatin and simvastatin in a rat model demonstrated reduced epitenon thickness, fiber disorganization, increased amount of ED1+ macrophages and impaired biomechanical strength in the Achilles tendon [62] (Table 2). Other research also reported foci of dystrophic calcification in Achilles tendon with improved biomechanica properties of the tibias simultaneously [63]. Although statins may induce tendinopathy in human, they are generally not involved in most cases of tendinopathy and neither do they induce tendinopathy in every patient. It may be useful to explore the side-effect of statins on tendons, but the valid of statin-indued tendinopathy animal model is doubtable.
| Chemicals | Operate Method | Species | Anatomical Location |
|---|---|---|---|
| Collagenase | Ultrasound guided local injection or direct local injection | Rat, mouse, rabbit, horse, sheep, | Achilles tendon, patella tendon, deep or superficial digital flexor tendon |
| Cytokine | Direct local injection | Rabbit | Patellar tendon |
| TGF-β1 | Direct local injection | Mouse, | Achilles tendon |
| Fluoroquinolones | Gavage administration | Rat, dog | Achilles tendon |
| KGN | Surgical implantation | Rat | Achilles tendon |
| PG | Direct local injection | Rat, rabbit | Achilles tendon, Patellar tendon |
| Statin | Gavage administration | Rat | Achilles tendon |
| Carrageen | Direct local injection | Rat | Achilles tendon, patellar tendon, footpad |
| Elastase | Ultrasound guided local injection | Rat | Achilles tendon |
| Abbreviations: TGF-β1, transforming growth factor-β1; KGN, kartogenin; PG, prostaglandin | |||
Table 1: Operate method of chemicals to make animal model.
Carrageenan: Carrageenan is a polysaccharide extracted from the cell wall of Rhodophyta algae. It is widely used to induce inflammation in vivo [64,65]. It has long been debated whether inflammation takes part in the underlying pathogenesis of tendinopathy and carrageenan has been used to study the effects of inflammation on tendons (Table 1). Tillander et al. revealed that repeated subacromial injection of carrageenan resulted in bursitis featured by degenerative matrix, macrophage infiltration and bone and fibrocartilaginous metaplasia in the rat supraspinatus tendon [66]. Vieira et al. injected carrageenan into the paratenon of the deep digital flexor tendon to study the effect of inflammatory tissue adjacent to tendon. Although the injury was not directly introduced into tendon, remarkable results include presence of MMP-9, degradation of non-collagenous proteins and GAG, cellular infiltrate and less organized collagen bundles were detected in the tendon [67,68]. Experiments conducted by Vieira et al. were finished within 24 hours, which means their findings were instant consequences of carrageenan. Hypercellularity, fiber disorganization, angiogenesis, cell Infiltration, nuclear rounding and decreased ultimate failure load were illustrated in rat tendon after carrageenan injection for weeks [7,69,70] (Table 2). It should note that both instant and sustained use of carrageenan gives rise to a distinct infiltration of inflammatory cells which is usually non-existent in clinically diseased specimens. This makes carrageenan-induced tendinopathy less credible. However, because it was difficult to obtain early clinical samples, the presence of inflammation at the early stages or before the onset of symptoms in human tendinopathy cannot therefore be excluded. This model provides a possible way to study the intrinsic role of inflammatory cells in tendinopathy.
Elastase: Elastase is an elastin degarding enzyme. The degradation products of elastin stimulate the release of proteolytic enzymes-MMPs, which would further lead to the destruction of matrix and structural components of tendon [71,72]. Because elastase may affect tendon components, Wu et al. investigated the role of elastase on tendinopathy by peritendinous injections of elastase into rats [73] (Table 1). On the one hand, they examined the expression of elastase in tendons of patients with tendinopathy, and concluded that elastase expressions may be related to the severity of a tendon injury. On the other hand, experiment on rat model displayed positive results. Increased tendon thickness and decreased weight-bearing of tendon were observed after elastase injection on the Achilles tendons of rats. Histological changes included hypervascularity, hypercellularity, collagen fiber disorganization and fragmentation were found by staining. Decreased collagen type I expression and increased collagen type III expression were examined by western blot (Table 2). These studies suggested that elastase plays a critical role in the pathogenesis of chronic tendinopathy. Limitations included dosage of elastase, grading methods of tendinopathy and the lack of end-stage tendinopathy syndrome were noted by the author. Based on the findings, we can conclude that elastase may provide a potential animal model for tendinopathy, but additional verification is needed.
Conclusion
Tendinopathy is a common disease that torments a large percentage of the population. As experimentation on humans to investigate the etiology, pathogenesis and evaluate new treatments is impossible, the establishment of a valid animal model is therefore indispensable. In this paper, several methods for chemically inducing tendinopathy in animals are reviewed. Chemically-induced models are usually lowcost, reproducible, less labor-intensive and easy to perform compared to mechanically-induced models. Chemicals mimic the pathological features, not the known first cause of tendinopathy which is believed to be the over-loading of the tendon. However, we cannot always identify what is the first cause of a disease, and tendinopathy is clearly a multifactorial one, and using one single model to represent all aspect of tendinopathy is unrealistic. Given the limited circumstances presently, we can only try our best to simulate the natural process of disease occurrence. Combining various factors, for example applying chemical and mechanical factors at the same time, to create animal model may provide us with a model closer to natural in future. Parameters been selected are also essential to model assessment, future study should add better relevant parameters, such as pain assessment, in assessment criteria.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- Warden SJ (2007) Animal models for the study of tendinopathy. British J Sports Med 41: 232-240.
- Khan KM, Bonar F, Desmond PM, Cook JL, Young DA, et al. (1996) Patellar tendinosis (jumper's knee): findings at histopathologic examination,US and MR imaging: Victorian Institute of Sport Tendon Study Group. Radiology 200(3): 821-827.
- Glazebrook MA, Wright JR, Langman M, Stanish WD, Lee JM (2008) Histological analysis of achilles tendons in an overuse rat model. J Orthop Res 26(6): 840-846.
- Ng GY-F, Chung PY-M, Wang JS, Cheung RT-H (2011) Enforced bipedal downhill running induces Achilles tendinosis in rats. Connect Tissue Res 52(6): 466-471.
- Yoshida M, Funasaki H, Kubota M, Marumo K (2015) Therapeutic effects of high molecular weight hyaluronan injections for tendinopathy in a rat model. J Orthop Sci 20(1): 186-195.
- Orfei CP, Lovati AB, Vigano M, Stanco D, Bottagisio M, et al. (2016) Dose-Related and Time-Dependent Development of Collagenase-Induced Tendinopathy in Rats. PloS one 11(8).
- Eskildsen SM, Berkoff DJ, Kallianos SA, Weinhold PS (2019) The use of an IL1-Receptor Antagonist to Reverse the Changes Associated with Established Tendinopathy in a Rat Model. Scand J Med Sci Sports 29(1): 82-88.
- Bell R, Li J, Gorski DJ, Bartels AK, Shewman EF, et al. (2013) Controlled treadmill exercise eliminates chondroid deposits and restores tensile properties in a new murine tendinopathy model. J Biomech 46(3): 498-505.
- Maffulli N, Longo UG, Franceschi F, Rabitti C, Denaro V (2008) Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res 466(7): 1605-1611.
- Klatte-Schulz F, Minkwitz S, Schmock A, Bormann N, Kurtoglu A, et al. (2018) Different Achilles Tendon Pathologies Show Distinct Histological and Molecular Characteristics. Int J Mol Sci 19(2): 404.
- Movin T, Gad A, Reinholt FP, Rolf C (1997) Tendon pathology in long-standing achillodynia: Biopsy findings in 40 patients. Acta Orthop Scand 68(2): 170-175.
- Pingel J, Lu Y, Starborg T, Fredberg U, Langberg H, et al. (2014) 3-D ultrastructure and collagen composition of healthy and overloaded human tendon: evidence of tenocyte and matrix buckling. J Anat 224(5): 548-555.
- Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, et al. (1997) Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone-tendon complex. J Orthop Res 15(5): 719-726.
- Riley G (2005) Gene expression and matrix turnover in overused and damaged tendons. Scand J Med Sci Sports 15(4): 241-251.
- Silver IA, Brown PN, Goodship AE, Lanyon LE, McCullagh KG, et al. (1983) A clinical and experimental study of tendon injury, healing and treatment in the horse. Equine Vet J Suppl, pp: 1-43.
- Williams I, McCullagh K, Goodship A, Silver I (1984) Studies on the pathogenesis of equine tendonitis following collagenase injury. Res Vet Sci 36(3): 326-338.
- Durgam SS, Stewart AA, Sivaguru M, Johnson AJW, Stewart MC (2016) Tendon-derived progenitor cells improve healing of collagenase-induced flexor tendinitis. J Orthop Res 34(12): 2162-2171.
- Facon-Poroszewska M, Kielbowicz Z, Przadka P (2019) Influence of Radial Pressure Wave Therapy (RPWT) on collagenase-induced Achilles tendinopathy treated with Platelet Rich Plasma and Autologous Adipose Derived Stem Cells. Pol J Vet Sci 22(4): 743-751.
- Yan R, Gu Y, Ran J, Hu Y, Zheng Z, et al. (2017) Intratendon Delivery of Leukocyte-Poor Platelet-Rich Plasma Improves Healing Compared With Leukocyte-Rich Platelet-Rich Plasma in a Rabbit Achilles Tendinopathy Model. Am J Sports Med 45(8): 1909-1920.
- Oshita T, Tobita M, Tajima S, Mizuno H (2016) Adipose-Derived Stem Cells Improve Collagenase-Induced Tendinopathy in a Rat Model. Am J Sports Med 44(8): 1983-1989.
- Lui PP-Y, Chan L-S, Fu S-C, Chan K-M (2010) Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am J Sports Med 38(4): 757-764.
- Iacopetti I, Perazzi A, Maniero V, Martinello T, Patruno M, et al. (2015) Effect of MLS® laser therapy with different dose regimes for the treatment of experimentally induced tendinopathy in sheep: pilot study. Photomed Laser Surg 33(3): 154-163.
- Chen H-S, Su Y-T, Chan T-M, Su Y-J, Syu W-S, et al. (2015) Human adipose-derived stem cells accelerate the restoration of tensile strength of tendon and alleviate the progression of rotator cuff injury in a rat model. Cell Transplant 24(3): 509-520.
- Kamineni S, Butterfield T, Sinai A (2015) Percutaneous ultrasonic debridement of tendinopathy-a pilot Achilles rabbit model. J Orthop Surg Res 10: 70.
- Chen J, Yu Q, Wu B, Lin Z, Pavlos NJ, et al. (2011) Autologous tenocyte therapy for experimental Achilles tendinopathy in a rabbit model. Tissue Eng Part A 17: 2037-2048.
- Imai K, Ikoma K, Chen Q, Zhao C, An K, et al. (2015) Biomechanical and histological effects of augmented soft tissue mobilization therapy on achilles tendinopathy in a rabbit model. J Manipulative Physiol Ther 38: 112-118.
- de Cesar NC, Godoy-Santos AL, Pontin PA, Natalino RJM, Pereira CAM, et al. Novel animal model for Achilles tendinopathy: Controlled experimental study of serial injections of collagenase in rabbits. PloS one. 2018;13(2).
- Lee S-Y, Chieh H-F, Lin C-J, Jou I-M, Sun Y-N, et al. (2017) Characteristics of Sonography in a Rat Achilles Tendinopathy Model: Possible Non-invasive Predictors of Biomechanics. Sci Rep 7: 5100.
- Goncalves-Neto J, Witzel S, Teodoro W, Carvalho-Junior A, Fernandes T, Yoshinari H. (2002) Changes in collagen matrix composition in human posterior tibial tendon dysfunction. Joint Bone Spine 69(2): 189-194.
- Riley G, Goddard M, Hazleman B (2001) Histopathological assessment and pathological significance of matrix degeneration in supraspinatus tendons. Rheumatology (Oxford) 40: 229-230.
- Lui PP-Y, Chan L-S, Lee Y-W, Fu SC, Chan K-M (2010) Sustained expression of proteoglycans and collagen type III/type I ratio in a calcified tendinopathy model. Rheumatology (Oxford) 49(2): 231-239.
- Fu S, Chan K, Rolf C (2007) Increased deposition of sulfated glycosaminoglycans in human patellar tendinopathy. Clin J Sport Med 17(2): 129-134.
- Corps A, Robinson A, Movin T, Costa M, Hazleman B, et al. (2006) Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology (Oxford) 45(3): 291-294.
- Dahlgren LA, Brower-Toland BD, Nixon AJ (2005) Cloning and expression of type III collagenin normal and injured tendons of horses. Am J Vet Res 66(2): 266-270.
- Ueda Y, Inui A, Mifune Y, Takase F, Kataoka T, et al. (2019) Molecular changes to tendons after collagenase-induced acute tendon injury in a senescence-accelerated mouse model. BMC Musculoskelet Disord 20(1) :120.
- Marcos RL, Leal-Junior ECP, Arnold G, Magnenet V, Rahouadj R, et al. (2012) Low-level laser therapy in collagenase-induced Achilles tendinitis in rats: analyses of biochemical and biomechanical aspects. J Orthop Res 30(12): 1945-1951.
- Dirks RC, Galley MR, Childress PJ, Fearon AM, Scott A, et al. (2013) Uphill running does not exacerbate collagenase-induced pathological changes in the Achilles tendon of rats selectively bred for high-capacity running. Connect Tissue Res 54(6): 386-393.
- Marques AC, Albertini R, Serra AJ, da Silva EAP, de Oliveira VLC, et al. (2016) Photobiomodulation therapy on collagen type I and III, vascular endothelial growth factor, and metalloproteinase in experimentally induced tendinopathy in aged rats. Lasers Med Sci 31(9): 1915-1923.
- Marcos R, Arnold G, Magnenet V, Rahouadj R, Magdalou J, et al. (2014) Biomechanical and biochemical protective effect of low-level laser therapy for Achilles tendinitis. J Mech Behav Biomed Mater 29: 272-285.
- Gong F, Cui L, Zhang X, Zhan X, Gong X, et al. (2018) Piperine ameliorates collagenase-induced Achilles tendon injury in the rat. Connect Tissue Res 59: 21-29.
- Stone D, Green C, Rao U, Aizawa H, Yamaji T, et al. (1999) Cytokine-Induced Tendinitis: A Preliminary Study in Rabbits. J Orthop Res 17(2): 168-177.
- Bell R, Li J, Shewman EF, Galante JO, Cole BJ, et al. (2013) ADAMTS5 is required for biomechanically-stimulated healing of murine tendinopathy. J Orthop Res 31(10): 1540-1548.
- Trella KJ, Li J, Stylianou E, Wang VM, Frank JM, et al. (2017) Genome-wide analysis identifies differential promoter methylation of Leprel2, Foxf1, Mmp25, Igfbp6 and Peg12 in murine tendinopathy. J Orthop Res 35(5): 947-955.
- Zabraniecki L, Negrier I, Vergne P, Arnaud M, Bonnet C, et al. (1996) Fluoroquinolone induced tendinopathy: report of 6 cases. J Rheumatol 23(3): 516-520.
- Dekens-Konter JA, Knol A, Olsson S, Meyboom RH, de Koning GH (1994) Tendinitis of the Achilles tendon caused by pefloxacin and other fluoroquinolone derivatives. Ned Tijdschr Geneeskd 138(10): 528-531.
- Royer R, Pierfitte C, Netter P (1994) Features of tendon disorders with fluoroquinolones. Therapie 49(1): 75-76.
- Kashida Y, Kato M (1997) Characterization of Fluoroquinolone-Induced Achilles Tendon Toxicity in Rats: Comparison of Toxicities of 10 Fluoroquinolones and Effects of Anti-Inflammatory Compounds. Antimicrob Agents Chemother 41(11): 2389-2393.
- Shakibaei M, de Souza P, van Sickle D, Stahlmann R (2001) Biochemical changes in Achilles tendon from juvenile dogs after treatment with ciprofloxacin or feeding a magnesium-deficient diet. Arch Toxicol 75(6): 369-374.
- Shakibaei M, Stahlmann R (2001) Ultrastructure of Achilles tendon from rats after treatment with fleroxacin. Arch Toxicol 75(2): 97-102.
- Samiric T, Parkinson J, Ilic M, Cook J, Feller J, et al. (2009) Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol 28(4): 230-236.
- Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, et al. (2012) A stem cell-based approach to cartilage repair. Science 336: 717-721.
- Yuan T, Zhang J, Zhao G, Zhou Y, Zhang C-Q, et al. (2016) Creating an Animal Model of Tendinopathy by Inducing Chondrogenic Differentiation with Kartogenin. PloS one 11(2): e0148557.
- Wang J, Jia F, Yang G, Yang S, Campbell BH, et al. (2003) Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res 44: 128-133.
- Langberg H, Skovgaard D, Karamouzis M, Bülow J, Kjaer M (1999) Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. J Physiol 515: 919-927.
- Khan MH, Li Z, Wang JH-C (2005) Repeated Exposure of Tendon to Prostaglandin-E2 Leads to Localized Tendon Degeneration. Clin J Sport Med 15: 27-33.
- Sullo A, Maffulli N, Capasso G, Testa V (2001) The effects of prolonged peritendinous administration of PGE1 to the rat Achilles tendon: a possible animal model of chronic Achilles tendinopathy. J Orthop Sci 6: 349-357.
- Savvidou C, Moreno R (2012) Spontaneous distal biceps tendon ruptures: are they related to statin administration? Hand Surg 17(2): 167-171.
- Beri A, Dwamena F, Dwamena B (2009) Association between statin therapy and tendon rupture: a case-control study. J Cardiovasc Pharmacol 53(5): 401-404.
- Rubin G, Haddad E, Ben-Haim T, Elmalach I, Rozen N (2011) Bilateral, simultaneous rupture of the quadriceps tendon associated with simvastatin. Isr Med Assoc J 13(3): 185-186.
- Carmont MR, Highland AM, Blundell CM, Davies MB (2009) Simultaneous bilateral Achilles tendon ruptures associated with statin medication despite regular rock climbing exercise. Phys Ther Sport 10(4): 150-152.
- de Oliveira LP, Vieira CP, Da Re Guerra F, de Almeida Mdos S, Pimentel ER (2013) Statins induce biochemical changes in the Achilles tendon after chronic treatment. Toxicology 311: 162-168.
- de Oliveira LP, Vieira CP, Guerra FD, Almeida MS, Pimentel ER (2015) Structural and biomechanical changes in the Achilles tendon after chronic treatment with statins. Food Chem Toxicol 77: 50-57.
- Kaleagasıoglu F, Olcay E, Olgaç V (2017) Statin-induced calcific Achilles tendinopathy in rats: comparison of biomechanical and histopathological effects of simvastatin, atorvastatin and rosuvastatin. Knee Surg Sports Traumatol Arthrosc 25: 1884-1891.
- Morris C (2003) Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol 225: 115-121.
- Cicala C, Morello S, Alfieri A, Vellecco V, Marzocco S, et al. (2007) Haemostatic imbalance following carrageenan-induced rat paw oedema. Eur J Pharmacol 577: 156-161.
- Tillander B, Franzén L, Nilsson E, Norlin R (2001) Carrageenan-induced subacromial bursitis caused changes in the rat's rotator cuff. J Orthop Res 19: 441-447.
- Vieira CP, Aro AAD, Guerra FDR, Oliveira LPD, Almeida MDSD, et al. (2013) Inflammatory process induced by carrageenan in adjacent tissue triggers the acute inflammation in deep digital flexor tendon of rats. Anat Rec (Hoboken) 296: 1187-1195.
- Vieira CP, Aro AAd, Almeida MdSd, deMello GC, Antunes E, Pimentel ER. Effects of acute inflammation induced in the rat paw on the deep digital flexor tendon. Connective tissue research. 2012;53(2):160–168.
- Berkoff DJ, Kallianos SA, Eskildsen SM, Weinhold PS (2016) Use of an IL1-Receptor Antagonist to Prevent the Progression of Tendinopathy in a Rat Model. J Orthop Res 34(4): 616-622.
- Cinar BM, Circi E, Balcik C, Guven G, Akpinar S, et al. (2013) The effects of extracorporeal shock waves on carrageenan-induced Achilles tendinitis in rats: a biomechanical and histological analysis. Acta Orthop Traumatol Turc 47(4): 266-272.
- Yamaguchi T, Yokokawa M, Suzuki M, Higashide S, Katoh Y, et al. (2000) The time course of elastin fiber degeneration in a rat aneurysm model. Surg Today 30(8): 727-731.
- Molácek J, Treska V, Kobr J, Certík B, Skalický T, et al. (2009) Optimization of the model of abdominal aortic aneurysm--experiment in an animal model. J Vasc Res 46(1): 1-5.
- Wu Y-T, Wu P-T, Jou I-M (2016) Peritendinous Elastase Treatment Induces Tendon Degeneration in Rats: A Potential Model of Tendinopathy In Vivo. J Orthop Res 34(3): 471-477.
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Citation: Zhu B, Peng Y, Li Y, Liu X, Liu X (2022) Animal Models of Tendinopathy Induced by Chemicals. Cell Mol Biol, 68: 221. DOI: 10.4172/1165-158X.1000221
Copyright: © 2021 Zhu B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2393
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1868
- PDF downloads: 525