Case Report Open Access
Angiotensin Converting Enzyme (ACE) Inhibitor Associated Small Bowel Angioedema and Ascites Presenting as Recurrent Acute Abdomen: A Case Report
Pardeep Mittal*, Abhishek Agarwal, Frank Miller, William Small, Peter Harri and Courtney C Moreno
Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, 30322, USA
- *Corresponding Author:
- Pardeep Mittal, MD
Director MRI Body Imaging, Department of Radiology and Imaging Sciences
Emory University School of Medicine, Atlanta, GA, 30322, USA
Tel: 404 723 8531
Fax: 4047783800
E-mail: pmittal@emory.edu
Received date: July 9, 2016; Accepted date: August 12, 2016; Published date: August 19, 2016
Citation: Mittal P, Agarwal A, Miller F, Small W, Harri P, et al. (2016) Angiotensin Converting Enzyme (ACE) Inhibitor Associated Small Bowel Angioedema and Ascites Presenting as Recurrent Acute Abdomen: A Case Report. J Gastrointest Dig Syst 6:464. doi: 10.4172/2161-069X.1000464
Copyright: © 2016 Mittal P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License; which permits unrestricted use; distribution; and reproduction in any medium; provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
We describe a case of a patient who presented with recurrent abdominal pain due to small bowel angioedema believed to be caused by a recently started ACE inhibitor with classic imaging features. Very few cases of visceral angioedema due to ACE inhibitor use are described in the literature. This case demonstrates a unique direct causal association between the onset of drug administration and symptomatic bowel angioedema as well as ascites, spontaneous resolution on stopping ACE inhibitor, and recurrence of acute clinical symptoms and imaging features on resumption of the drug.
Keywords
Angiotensin converting enzyme inhibitor; Small bowel angioedema; Ascites
Introduction
Recent years have witnessed increasing use of angiotensin converting enzyme (ACE) inhibitors in hypertension and as drugs of choice in systolic dysfunction heart failure making it highly important to recognize all possible side effects and rectify them at the earliest time to reduce the morbidity. ACE inhibitors are known to cause acute GI symptoms due to various pathophysiological alterations including systemic hypotension leading to mesenteric hypoperfusion, pancreatitis, and angioedema. ACE inhibitor induced visceral angioedema is a rare and its incidence is not well documented [1,2]. Isolated involvement of the bowel presenting as acute abdomen is extremely uncommon and a direct temporal association between the drug intake and symptoms often gives the clue to the diagnosis. We present a rare case of recurrent ACE inhibitor related acute abdominal pain with classic imaging features of small bowel angioedema as well as ascites.
Case Report
A 37-year-old African-American woman presented to emergency department with acute onset of severe intermittent abdominal cramps, nausea and multiple episodes of vomiting of 12 h duration. She also had a few episodes of watery diarrhea. There was no history of fever, chills, stridor, facial swelling, dysuria or hematuria. She complained of no other constitutional symptoms. She gave no previous history of gastrointestinal illnesses or recent travel. The patient also gave a history of long standing hypertension secondary to polycystic renal disease and associated renal failure. Her past history also revealed allergies to drugs such as acetaminophen and oxycodone. Vital signs were normal except for slightly raised blood pressure (130/86 mmHg) and physical examination revealed diffuse tenderness over the entire abdomen.
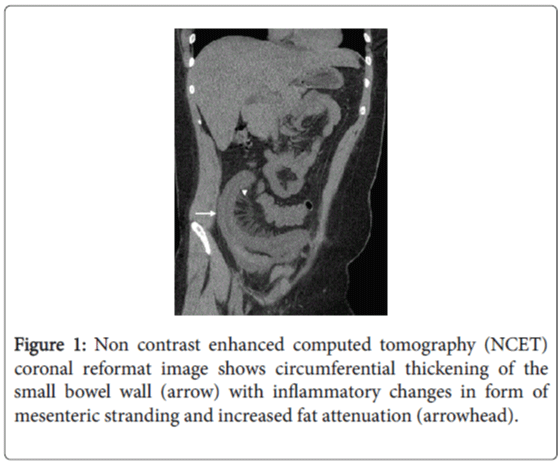
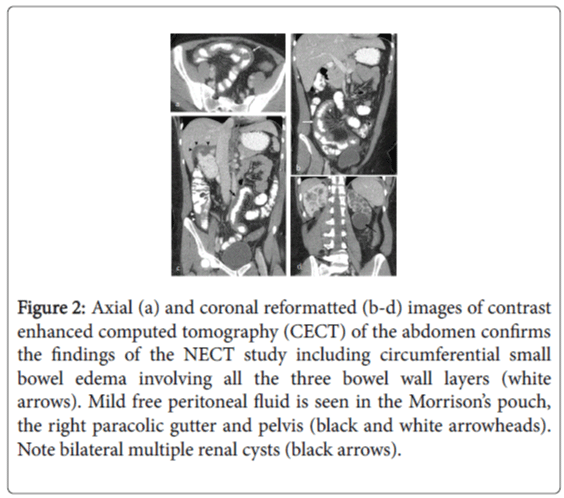
A non-enhanced CT (NECT) of the abdomen was first performed, which revealed severe localized ileal wall thickening in the right lower quadrant with surrounding inflammatory changes in the form of mesenteric stranding and increased fat attenuation without features of intestinal obstruction, pneumatosis or intraperitoneal free air (Figure 1). Mild free peritoneal fluid, moderate cardiomegaly with a small pericardial effusion and bilateral polycystic kidneys were the other positive findings. These NECT findings were confirmed with a contrast-enhanced CT (CECT) of the abdomen performed a few hours later using oral and 150 cc Omnipaque 300 (iohexol, GE Healthcare) intravenous contrast (Figure 2). Based on the imaging features a concern for ischemic bowel disease was raised at this point.
Figure 2: Axial (a) and coronal reformatted (b-d) images of contrast enhanced computed tomography (CECT) of the abdomen confirms the findings of the NECT study including circumferential small bowel edema involving all the three bowel wall layers (white arrows). Mild free peritoneal fluid is seen in the Morrison’s pouch, the right paracolic gutter and pelvis (black and white arrowheads). Note bilateral multiple renal cysts (black arrows).
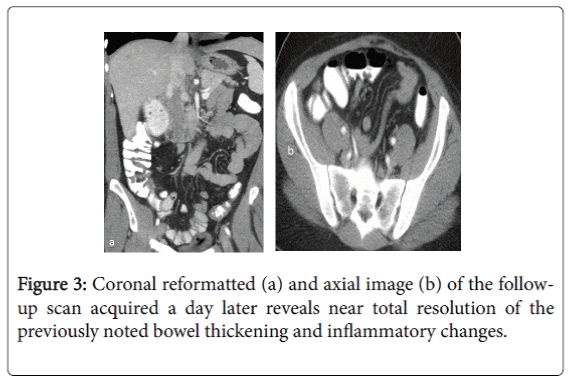
The patient was admitted, put on IV fluids and a NG tube inserted which showed 500 cc of bilious output throughout the night. Colonoscopy, performed the next morning, showed no signs of inflammation in the colon or terminal ileum. The patient improved symptomatically in the meantime and started having bowel movements. The NG tube was thus removed and a clear diet started. A CECT of the abdomen was repeated, which revealed near-complete interval resolution of the previously noted small bowel wall thickening, inflammatory changes in the right lower quadrant and the free peritoneal fluid (Figure 3). She was discharged to home in stable condition following complete resolution of her transient symptoms, but with no clear explanation of their etiology. She was advised to report to the emergency department if her abdominal symptoms recurred.
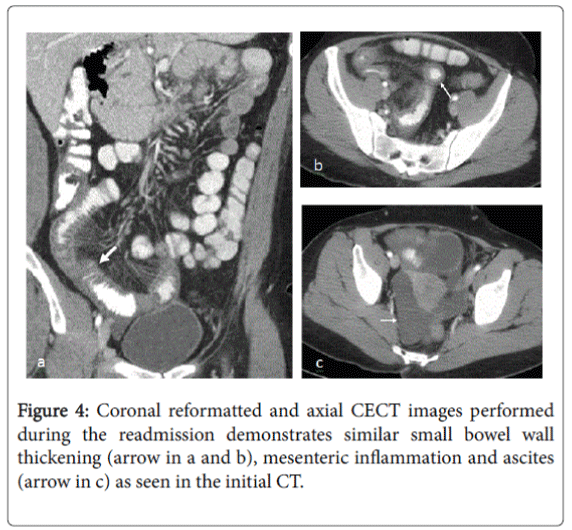
The patient was asymptomatic for a few days but returned to the emergency department five days later with similar complaints of severe abdominal pain, intractable nausea, vomiting, and diarrhea. She also complained of the development of new hives along her extremities and her trunk. Abdominal CECT was performed which revealed interval development of bowel wall thickening and inflammation in the distal ileum similar to the findings on her initial study that had resolved in the interim (Figure 4). Also, there was interval accumulation of significant pelvic free fluid. On detailed evaluation of her chart, an interesting association of patient’s symptoms was observed with her oral use of a newly started angiotensin-converting-enzyme (ACE) inhibitor, Lisinopril. She started her Lisinopril on the day that she initially presented to the emergency room with abdominal pain, nausea, vomiting, and diarrhea.
Lisinopril was discontinued during the duration of stay at the hospital and not resumed until the day prior to this current admission when she again developed similar symptoms as well as imaging features. Thus, her clinical presentation was considered consistent with ACE inhibitor-induced angioedema of the intestine based on strong association with the medication intake and symptoms. Lisinopril was removed from her treatment regimen and she showed considerable improvement in her symptoms during the second term of hospital stay. The patient is symptom free on follow-up period of one year and she is off ACE inhibitors during the last one year.
Discussion
Angioedema is a non-inflammatory disease characterized by increased capillary permeability leading to extravasation of intravascular fluid and resultant edema of the mucosa [3-5]. Angioedema is a known, potentially life-threatening side effect of treatment with ACE inhibitors, occurring in 0.1-0.5% of patients treated with these drugs [2,6]. African Americans are known to have a substantially increased risk of ACE inhibitor-associated angioedema compared with Caucasian subjects and that this increased risk has not been attributed to an effect of dose, specific ACE inhibitor, or concurrent medications [7].
Angioedema caused by ACE inhibitors very often is localized on the lips, the tongue, pharynx and larynx, in many cases necessitating emergency measures. Other sites that could potentially be involved include hands and feet, arms, legs and scrotum. In general, angioedema occurs within hours to days after initiation of therapy; however, delayed occurrence of angioedema up to 10 years of therapy has been reported [8,9]. Gastrointestinal tract symptoms of angioedema can mimic acute abdomen. The more commonly reported symptoms include abdominal pain lasting from hours to days before initial presentation [1]. According to one study 35% of patients diagnosed with small bowel angioedema had undiagnosed abdominal pain before their diagnosis [10]. Nausea, vomiting and watery diarrhea are other frequently reported symptoms [11-13]. The underlying pathophysiology of bowel angioedema due to ACE inhibitors in not clear and various pathophysiologic mechanisms have been proposed. ACE inhibitors decrease the inactivation of bradykinin and thus increase the circulating levels of bradykinin. One of the proposed pathophysiological mechanisms of edema is the vasodilation and altered vascular permeability due to increased circulating bradykinin. Tissue specific and antinuclear antibodies induced by ACE might lead to immunologic reaction resulting in angioedema. Another hypothesis suggests that induction of C1 esterase deficiency by ACE inhibitors may also contribute to visceral edema [6,14,15].
Diagnosing ACE inhibitor induced angioedema as the cause of acute abdomen is not straightforward. ACE inhibitors can cause GI symptoms due to various other pathophysiological alterations that result in systemic hypotension leading to mesenteric hypoperfusion, pancreatitis and lastly, angioedema [16-18]. On the other hand, bowel angioedema produced by other etiologies can usually present with overlapping symptoms; however, a strong association with history of medication and associated symptoms is often the diagnostic clue to suggest ACE inhibitor associated angioedema. There is no definite diagnostic test for ACE inhibitor induced visceral angioedema [9]. Occasionally diagnosis is made after resumption of the ACE inhibitors and returning of the symptoms as described in our case [1].
In bowel angioedema, CT classically demonstrates segmental involvement of small bowel with circumferential edema and wall thickening [3]. Prominent mucosal enhancement is seen with prominence of the mesenteric vessels and mesenteric fat stranding. There is usually a striking contrast between the low-attenuation edematous submucosa, which separates the outer muscular layers and serosa from brightly enhanced thickened mucosa. It is less commonly seen to involve the large bowel and rarely the rectum [13]. Noncomplicated ascites is a consistent associated finding. The CT findings invariably resolve on removal of the implicating agent, an ACE inhibitor in this case. This sort of reversible and segmental nature of the small bowel edema has a limited radiological differential diagnosis including ischemia, Henoch Schonlein purpura, and intramural bleeding from trauma, anticoagulation, or hemophilia [5].
As there are no specific diagnostic tests to confirm ACE inhibitor induced bowel angioedema, the diagnosis is based on the temporal relationship between the symptoms and the classic radiological findings with the drug administration, exclusion of other causes of angioedema. The resolution of the bowel edema after discontinuation of the ACE inhibitor is the key in diagnosis [5,13]. The fact that our patient started her Lisinopril the same morning that she had the episode, discontinued it during her stay in the hospital leading to spontaneous resolution of symptoms, and recurrence of symptoms after resuming Lisinopril strongly implicates ACE inhibitor as the cause for her small bowel edema and associated symptoms. Absence of similar symptoms during the period of follow-up since her ACE inhibitor was replaced with another class of anti-hypertensives also points to the ACE inhibitor as causative agent.
Although her complaints in the initial episode were purely gastrointestinal in nature, the second episode of acute abdomen was associated with development of hives along her extremities and trunk. GI manifestations may be the first and only presenting symptoms of this problem [19,20]. Angioedema has no pathognomonic feature that distinguishes it from a true surgical emergency, however, the absence of peritoneal signs, fever, or leukocytosis and the presence of bowel sounds may justify a more conservative management. The results of surgical exploration in these patients are not diagnostic, as the usual findings are ascites and bowel edema. A high index of suspicion is important because if not recognized early, patients with ACE inhibitorinduced visceral angioedema may be subjected to multiple radiologic and invasive procedures [21,22].
Isolated bowel angioedema can also be caused by acquired and hereditary C1 esterase deficiencies. Although hereditary and acquired angioedema have overlapping signs and symptoms, the age of the patient at onset differs [23,24]. Almost half of patients with the hereditary form are young and have episodes before they are ten years old [23]. Patients with the acquired form of angioedema generally are found to have episodes after the fourth decade of life and, similarly, diagnosis is difficult [23,24].
Conclusion
ACE inhibitor induced visceral angioedema is a rare but documented side effect of the medication. Isolated involvement of the bowel presenting as acute abdomen is extremely uncommon and a direct temporal association between the drug intake and symptoms often leads to the diagnosis. It is also important to rule out other causes of angioedema. This patient was unusual as her symptoms resolved when stopping the medication and recurred after restarting Lisinopril. CT findings, although not specific for this pathology, help to exclude more urgent surgical etiologies. ACE inhibitors are not only the antihypertensive of choice in many cases but also are drug of choice in systolic dysfunction heart failure making it highly important to recognize all possible side effects and rectify them at earliest to decrease the morbidity.
References
- Bloom AS, Schranz C (2015) Angiotensin-Converting Enzyme Inhibitor-Induced Angioedema of the Small Bowel-A Surgical Abdomen Mimic. The Journal of emergency medicine 48: e127-129.
- Scheirey CD, Scholz FJ, Shortsleeve MJ, Katz DS (2011) Angiotensin-converting enzyme inhibitor-induced small-bowel angioedema: clinical and imaging findings in 20 patients. AJR American journal of roentgenology 197:393-398.
- Inman WH, Rawson NS, Wilton LV, Pearce GL, Speirs CJ (1988) Postmarketing surveillance of enalapril. I: Results of prescription-event monitoring. Bmj 297:826-829.
- Israili ZH, Hall WD (1992) Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Annals of internal medicine 117:234-242.
- Marmery H, Mirvis SE (2006) Angiotensin-converting enzyme inhibitor-induced visceral angioedema. Clinical radiology 61:979-982.
- Karagiannis A, Pyrpasopoulou A, Tziomalos K, Florentin M, Athyros V (2006) Angioedema may not be a class side-effect of the angiotensin-converting-enzyme inhibitors. QJM : monthly journal of the Association of Physicians 99:197-198.
- Eck SL, Morse JH, Janssen DA, Emerson SG, Markovitz DM (1993) Angioedema presenting as chronic gastrointestinal symptoms. The American journal of gastroenterology 88:436-439.
- Orr KK, Myers JR (2004) Intermittent visceral edema induced by long-term enalapril administration. The Annals of pharmacotherapy 38:825-827.
- Vallurupalli K, Coakley KJ (2011) MDCT features of angiotensin-converting enzyme inhibitor-induced visceral angioedema. AJR American journal of roentgenology 196:W405-411.
- Campbell T, Peckler B, Hackstadt RD, Payor A (2010) ACE Inhibitor-Induced Angioedema of the Bowel. Case reports in medicine 2010:690695.
- Aggarwal A, Mehta N, Shah SN (2011) Small bowel angioedema associated with angiotensin converting enzyme inhibitor use. Journal of general internal medicine 26:446-447.
- Hachem CY, Balci NC, Desai D (2010) Recurrent nausea, vomiting, and abdominal pain. Gastroenterology 138:e1-2.
- Shahzad G, Korsten MA, Blatt C, Motwani P (2006) Angiotensin-converting enzyme (ACE) inhibitor-associated angioedema of the stomach and small intestine: a case report. The Mount Sinai journal of medicine, New York 73:1123-1125
- Kallenberg CG (1985) Autoantibodies during captopril treatment. Arthritis and rheumatism 28:597-598.
- Khan MU, Baig MA, Javed RA, Ali S, Qamar UR, et al. (2007) Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. International journal of cardiology 118:e68-69.
- Chase MP, Fiarman GS, Scholz FJ, MacDermott RP (2000) Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. Journal of clinical gastroenterology 31:254-257.
- Jeandidier N, Klewansky M, Pinget M (1995) Captopril-induced acute pancreatitis. Diabetes care 18:410-411.
- Standridge JB (1994) Fulminant pancreatitis associated with lisinopril therapy. Southern medical journal 87:179-181.
- Malde B, Regalado J, Greenberger PA (2007) Investigation of angioedema associated with the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Annals of allergy, asthma and immunology : official publication of the American College of Allergy, Asthma, and Immunology 98:57-63.
- Schmidt TD, McGrath KM (2002) Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. The American journal of the medical sciences 324:106-108.
- Bork K, Staubach P, Eckardt AJ, Hardt J (2006) Symptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiency. The American journal of gastroenterology 101:619-627.
- Voore N, Stravino V (2015) Isolated small bowel angio-oedema due to ACE inhibitor therapy. BMJ case reports 2015.
- Ciaccia D, Brazer SR, Baker ME (1993) Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. AJR American journal of roentgenology 161:1215-1216.
- Agostoni A, Cicardi M (1992) Hereditary and acquired C1-inhibitor deficiency: biological and clinical characteristics in 235 patients. Medicine 71:206-215.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 13425
- [From(publication date):
August-2016 - Apr 14, 2025] - Breakdown by view type
- HTML page views : 12444
- PDF downloads : 981