Analytical Method Development and Validation of Carbimazole by Using RP-HPLC on Bulk Drug and Marketed Formulation in Addiction Treatment
Received: 31-Aug-2022 / Manuscript No. jart-22-77393 / Editor assigned: 02-Sep-2022 / PreQC No. jart-22-77393 (PQ) / Reviewed: 16-Sep-2022 / QC No. jart-22-77393 / Revised: 19-Sep-2022 / Manuscript No. jart-22-77393 (R) / Accepted Date: 20-Sep-2022 / Published Date: 23-Sep-2022
Abstract
The goal of the present study was to develop and validate of an analytical method for the evaluation of Clotrimazole in bulk form. UV-VIS spectroscopic technique was used for the estimation of Clotrimazole. Organic solvents and chemicals were used as trial diluents. The selection of the diluents was carried out on the basis of solubility and stability factors upon which drug was fully solubilised and stable for sufficient time. The drug was slightly soluble in water and acetonitrile, Soluble in Methanol and Alcohol. Clotrimazole showed absorption maxima (λ max) at 263 nm. The recovery studies were established the accuracy of the proposed method and the results were validated as per ICH guidelines. Linearity was obtained by concentration versus absorbance for Clotrimazole with correlation coefficient r꞊0.999. Robustness of the method was calculated as the % RSD for peak area was calculated which should be less than 2%.The LOD and LOQ were calculated as 0.000228(μg/mL), 0.00775 (μg/ mL) respectively. The result analysis was validated statistically and recovery studies confirmed the accuracy and precision of the proposed method. The developed method can effectively be applied for the quality control analysis and determination of Clotrimazole in tablet dosage form.
Keywords
Addiction research; Addiction therapy; Addiction; Clotrimazole; HPLC method; Validation; Stability indicating; ICH guidelines
Introduction
Analytical chemistry deals with method of determining the chemical composition of sample. It is primarily concerned about determining the qualitative and quantitative composition of material. A qualitative method yields information about the identity of atomic or molecular species or functional group in the sample. A quantitative method in contrast provides numerical information as to the relative amount of one or more of these components. It is a scientific discipline used to study the chemical composition, structure and behavior of matter. The term chemical analysis may be defined as the application of a process or series of process to identify or quantify a substance, the component of a solution or mixture or the structure of chemical compounds. The purpose of chemical analysis is to gather and interpret chemical information that will be of value to society in wide range of contexts. It is involved in all the stages related to a drug, from its discovery, development, action, safety, formulation, use, quality control, packaging, storage, marketing etc. Any drug or dosage form for human use must have excellent quality and purity, free from impurities. This dosage form directly affects the human life and behavior so their analysis is important which is carried out using analytical methods. Analytical method development is the heart of analytical chemistry. It involves development and validation of new analytical method for the purpose of testing samples. Sample testing is done by using UV, IR, HPLC, HPTLC, GC-MS, and LC-MS etc. Analytical chemistry has since long, occupied an important in the development of science and technology. It is very broad and embraces a wide range of natural, chemical and instrumental technique and procedure [1].
Chromatographic Technique
Chromatography was invented and named by Russian botanist MikhaiTswett. Chromatography according to USP can be defined as a procedure by which solutes are separated by a differential migration process in a system consisting of two or more phases, one of which moves continuously in a given direction and in which the individual substances exhibit different mobilities by reason of differences in adsorption, partition, solubility, vapor pressure, molecular size, or ionic charge density (Figure 1). One of the phases is a fixed bed of large surface area, whereas the other is a fluid that moves through or over the surface of the fixed phase [2].
High performance liquid chromatography (HPLC)
High performance liquid chromatography (HPLC) is the most versatile and widely used analytical technique. It utilizes a liquid mobile phase to separate the components of mixture. These components (or analytes) are first dissolved in a solvent, and then forced to flow through a Chromatographic column under high pressure. In the column, the mixture is resolved into its components. The interaction of solute with mobile and stationary phases can be manipulated through different choices of both solvent and stationary phases. As a result, HPLC acquires a high degree of versatility not found in other chromatographic systems and it has the ability to separate a wide variety of chemical mixtures. HPLC uses high pressure to force solvent through closed columns containing very fine particles that gives high resolution separations. The technique is used to separate and determine species in a variety of organic, inorganic, and biological materials. HPLC is used either in the liquid-solid adsorption chromatography mode or the liquid-liquid partition chromatography mode, either normal or reversed phase. Both partition and adsorption chromatography operates on differences in solute polarity since polarity is important in determining both adsorption and solubility. As a general rule, highly polar materials are best separated using partition chromatography, while very non polar are separated using adsorption chromatography [2-4].
Types of HPLC
• Normal phase HPLC
• Reversed phase HPLC
Carbimazole is an aitithyroid agent that decreases the uptake and concentration of inorganic iodine by thyroid, it also reduces the formation of di-iodotyrosine and thyroxine. Once converted to its active form of methimazole, it prevents the thyroid peroxidase enzyme from coupling and iodinating the tyrosine residues on thyroglobulin, hence reducing the production of the thyroid hormones T3 and T4 [5,6].
Pharmaceutical grade carbimazole was supplied by Taj mahal chemicals ,India and used without further purification. All the chemicals used are of HPLC and AR grade. Chemicals used are as Methanol (SDFC Chem lab), 0.05% buffer TEAwith OPA water PH3 (Research chem lab) and the carbon dioxide free double distilled water for HPLC purpose.
Instruments
FTIR madefrom Bruker having software OPUS 7.5, U.V.-Visible double beam spectrophotometer Shimadzu (UV 1800) is used for the analysis. The HPLC system consisted of a pump is GRADIENT PUMP and the column used are (Cosmosoil) C18 Column (4.6mm × 250mm) for the validation studies. The ultrasonicator (model- ICO 900/2000) I used [7-9].
Chromatographic conditions
Accurately weigh and transfer 10mg Carbimazole working standard into 10 ml volumetric flask as about dilute Methanol prepared in completely and make volume up to the mark with the same solvent to get 1000μg/ml standard (stock solution) and 15 min sonicated to dissolve it and from the resulting solution 0.1ml was transferred to 10 ml volumetric flask and the volume was made up to the mark with mobile phase methanol: buffer (0.05% TEA with OPA pH adjusted 3 ) Water solvent. The resulting 10μg/ml of solution was subjected to chromatographic analyses using mobile phases of different strengths with chromatographic conditions mentioned below:
Standard stock solution
Accurately weight and transfer 10mg Carbimazole working standard into 10 ml volumetric flask as about diluent Methanol completely and make volume up to the mark with the same solvent to get 1000μg/ml standard (stock solution) and 15 min sonicated to dissolve it and the resulting stock solution 0.1ml was transferred to 10 ml volumetric flask and the volume was made up to the mark with mobile phase methanol : buffer(0.05% TEA(OPA pH 3)) prepared in (90ml MEOH :10ml BUFFER v/v) solvent [10].
Validation of method for analysis of Carbimazole
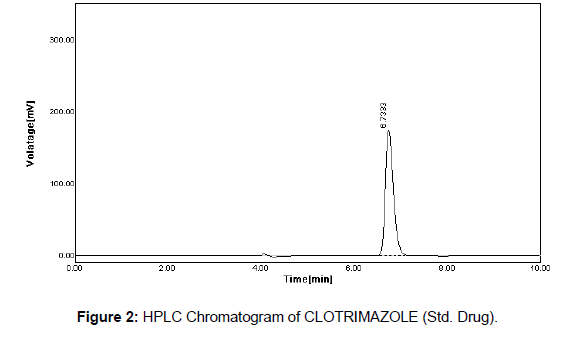
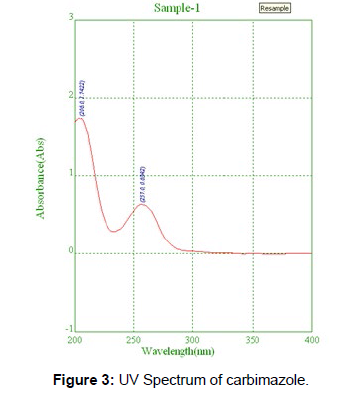
From Carbimazole standard stock solution, different working standard solution (5-25μg/ml) were prepared in mobile phase 20μl of sample solution was injected into the chromatographic system using mixed volume loop injector Chromatograms were recorded. The peak area was plotted against corresponding concentrations to obtain the calibration graph (Figures 2 & 3).
Precision
The method was established by analyzing various replicates standards of Carbimazole all the solution was analyzed thrice in order to record any intra-day & inter-day variation in the result that concluded (Table 1).
| Conc. (μg/mL) | Mean | SD± | % RSD | |
|---|---|---|---|---|
| Morning | 10 | 97.92 | 0.20 | 0.21 |
| Evening | 10 | 97.88 | 0.087 | 0.089 |
Table 1: Intra-day Precision.
Accuracy
Recovery studies were performed to validate the accuracy of developed method. To pre analyzed tablet solution, a definite concentration of standard drug (50%, 100%, and 150%) was added. Robustness The mobile phase composition was changed in (±1 ml/ min-1) proportion in the mobile phase composition, and the flow rate was (±1 ml/ min-1) and the change in detection wavelength (±1 ml/ min-1) and the effect of the results were examined. it was performed using 10μg/ml solution of Carbimazole. Ruggedness It is the degree of reproducibility of the test result under the variety likes change in flow rate. Results and Discussion Optimization of chromatographic conditions Several columns were used for optimizing the chromatographic condition. The parameters being focused were improvisation of retention time, separation of degradation products and column life. The columns provided good peak shapes and no peak splitting was observed with any impurity [11]. UV Spectroscopy UV absorption of 10 μg/mL solution of Carbimazole in methanol was generated and absorbance was taken in the range of 200-400 nm. Λmax. Linearity From Carbimazole standard stock solution, different working standard solution (5-25 μg/ml) were prepared in mobile phase 20 μl of sample solution was injected into the chromatographic system using mixed volume loop injector Chromatograms were recorded. The calibration curve yielded correlation coefficient (r2) 0.999 and 0.999 for Carbimazole respectively. Precision The method was established by analyzing various replicates standards of Carbimazole all the solution was analyzed thrice in order to record any intra-day & inter-day variation in the result that concluded. Accuracy Recovery studies were performed to validate the accuracy of developed method. To pre analyzed tablet solution, a definite concentration of standard drug (50%, 100%, and 150%) was added. Robustness The Robustness of a method is its ability to remain unaffected by small deliberate changes in parameters. To evaluate the robustness of the proposed method, small but deliberate variations in the optimized method parameters were done. The effect of changes in mobile phase composition and flow rate, wavelength on retention time and tailing factor of drug peak was studied.
The mobile phase composition was changed in (±1 ml/min-1) proportion and the flow rate was varied by of optimized chromatographic condition.Robustness parameters were also found satisfactory; hence the analytical method would be concluded (Table 2-4).
| Conc. (μg/mL) | Mean* | SD± | %RSD | |
|---|---|---|---|---|
| Day 1 | 10 | 98.06 | 0.120 | 0.122 |
| Day 2 | 10 | 97.95 | 0.062 | 0.063 |
Table 2: Inter-day Precision.
Level of Recovery (%) |
Drug | Mean % Recovery | Standard Deviation | % RSD |
|---|---|---|---|---|
| 50% | Carbimazole | 98.99 | 0.053 | 0.053 |
| 100% | Carbimazole | 101.89 | 0.023 | 0.023 |
| 150% | Carbimazole | 99.42 | 0.050 | 0.050 |
Table 3: Statistical Validation of Recovery Studies of Carbimazole.
Parameter |
Conc. (µg/ml) |
Area (mean ±SD) |
%RSD |
|---|---|---|---|
| MP composition(89ml+11ml)Methanol + 0.05% TEA water with OPA ph3 | 5 | 526.41±4.28 | 0.81 |
| MP composition(91ml+09ml)Methanol + 0.05% TEA with (OPA)water ph3 | 5 | 329.03±6.48 | 1.65 |
| Wavelength change 256nm | 5 | 496.4±8.14 | 1.64 |
| Wavelength Change 258nm | 5 | 447.52±3.48 | 0.78 |
| Flow rate change(0.6 ml) | 5 | 474.72±6.33 | 1.33 |
| Flow rate change(0.8 ml) | 5 | 486.7±8.91 | 1.83 |
Table 4: Result of Robustness Study of Carbimazole.
The changes were did flow rate (±1 ml/ min-1), pH of mobile phase composition, and Wavelength. % RSD for peak area was calculated which should be less than 2%.the result shown in analytical method that concluded.
Conclusion
Simple, rapid, accurate and precise RP-HPLC have been developed and validated for the routine analysis of carbimazole in API and tablet dosage forms. Both methods are suitable for the simultaneous determination of carbimazole in Single-component formulations without interference of each other. The developed methods are recommended for routine and quality control analysis of the investigated drugs in two component pharmaceutical preparations. The amount found from the proposed methods was in good agreement with the label claim of the formulation. Also the value of standard deviation and coefficient of variation calculated were satisfactorily low, indicating the suitability of the proposed methods for the routine estimation of tablet dosage forms .
Acknowledgement
The author is thankful to the Principal of KCT’s R. G. Sapkal College of Pharmacy, Anjaneri,Nashik for providing the facilities at college.
Conflict of Interest
Authors declare that they don’t have any conflict of interest.
References
- Brissette I, Scheier MF, Carver CS (2002) The role of optimism in social network development,coping, and psychological adjustment during a life transition. J Pers Soc Psychol 82: 102-120.
- Chen KJ, Yang CC, Chiang HH (2018) Model of coping strategies, resilience, psychological well-being, and perceived health among military personnel. J Med Sci 38: 73.
- Dergisi A (2006) Examining of the Relationships between Professional Burnout, Work Engagement and Job Satisfaction. 3: 49-80.
- Gautam A (2015) Life Satisfaction and Life Orientation as predictors of Psychological Well Being. Inter J Indian Psychol 3: 20-27.
- Haleem M, Masood S, Aziz M, Jami H (2017) Psychological Capital and Mental Health of Rescue Workers.Pakistan J Psychol Res32: 1-15.
- Ahmad S, Arshad T, Kausar R (2015) Psychological correlates of distress in rescue 1122 workers in Pakistan.Int J Emerg Ment Health17: 486-494.
- Ahmed MA (2015) The role of self-esteem and optimism in job satisfaction among teachers of private universities in Bangladesh. Asian Business Rev 1: 114-120.
- Baptiste NR (2008) Tightening the link between employee wellbeing at work and Performence: A new dimension for HRM.Manag Dec46: 284-309.
- Bayani AA, Mohammad KA, Bayani A (2008) Reliability and validity of Ryff’s psychological well-being scales.Iran j psychiat clin psychol14: 146-151.
- Behson SJ (2012) The Importance of the Critical Psychological States in the Job Characteristics Model: A Meta-Analytic and Structural Equations Modeling Examination.Cur Res Soc Psychol1: 170-175.
- Behson SJ (2010) Using relative weights to reanalyze ‘settled’ areas of organizational behaviour research: The job characteristics model and organizational justice. Inter J Manag Infor Sys 1: 15-43.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Kanchan R, Phadtare DG (2022) Analytical Method Development and Validation of Carbimazole by Using RP-HPLC on Bulk Drug and Marketed Formulation in Addiction Treatment. J Addict Res Ther 13: 488.
Copyright: © 2022 Kanchan R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3695
- [From(publication date): 0-2022 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 2759
- PDF downloads: 936