Research Article Open Access
Analytical Considerations When Monitoring Pain Medications by LC-MS/MS
Amadeo Pesce1*, Cameron West1, Robert West1, Sergey Latyshev1, David Masters-Moore1, Patrick Friel2, John M. Hughes2, Perla Almazan1, Elizabeth Gonzales1 and Charles Mikel11Millennium Research Institute, San Diego, CA, USA
2Agilent Technologies, Santa Clara, CA, USA
- *Corresponding Author:
- Amadeo Pesce
Millennium Research Institute, San Diego, CA, USA
E-mail: pesceaj@ucmail.uc.edu
Received date: May 17, 2012; Accepted date: June 23, 2012; Published date: June 28, 2012
Citation: Pesce A, West C, West R, Latyshev S, Masters-Moore D, et al. (2012) Analytical Considerations When Monitoring Pain Medications by LC-MS/MS. J Anal Bioanal Techniques S5:003. doi: 10.4172/2155-9872.S5-003
Copyright: © 2012 Pesce A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Background: Laboratory urine drug testing of patients on chronic opioid therapy requires providing a large test menu of medications commonly prescribed for this population as well as metabolites and illicit substances. It has been shown that liquid chromatography-tandem mass spectrometry (LC-MS/MS) is the preferred method to analyze urine specimens for these substances.
Purpose of the study: To describe the challenges and some of the techniques to validate the analytical procedures used to identify and quantify these medications and substances.
Methods: Using data obtained from testing over one million specimens, the authors developed a proposed test menu. Potential isobaric interferences were established by using literature references. A list of potentially interfering medications was obtained by using the proposed test menu and the most commonly prescribed medications. Finally, criteria were designed to detect possible carryover.
Results: The LC-MS/MS instrumentation eliminated all potential interferences and provided quantitative data over the test range needed to monitor these patients. Carryover could be eliminated by setting the carryover thresholds for each analyte.
Conclusions: Reference laboratories utilizing LC-MS/MS technology to conduct urine drug testing for pain clinicians should employ specific techniques described in this study to develop an optimal test menu and validate procedures that include isolating retention times for isobaric compounds, identifying interfering substances including impurities in medicinal and illicit substance preparations, monitoring ion suppression, and avoiding carryover.
Keywords
LC-MS/MS; Mass spectrometry; Chronic pain; Drug testing; Interference testing; Validation; Isobaric compounds
Introduction
Urine drug testing of patients on chronic opioid therapy is a recognized component of the standard of care for this population [1-3]. Because a significant portion of this population experiences symptoms of other conditions including anxiety and depression, treatment often involves multiple medications. Additionally, a small percentage of these people take non-prescribed medications and/or illicit substances [4-10].
Treating physicians often utilize urine drug testing to monitor patients’ use of their prescribed medications to ensure that they are receiving optimal treatment. This establishes that patients are taking their medications, minimizes the potential for drug-drug interactions, and informs the physician if and when a patient has used a nonprescribed medication or illicit substance that could place their health at risk [11,12].
Laboratories performing analyses for pain physicians must offer a wide menu of tests to encompass commonly prescribed medications as well as illicit substances [13]. In addition to identifying parent compounds, laboratories should also be able to identify metabolites of these medications or illicit substances, such as 6-acetylmorphine, which may be present at very low concentrations in urine specimens [14,15]. To meet these needs in an efficient manner, laboratories must accurately quantify a wide range of concentrations for a large number of compounds, and accomplish this expediently.
Several testing methods utilizing mass spectrometry technology are capable of determining the presence of specific compounds. Gas chromatography-mass spectrometry (GC-MS) has been used for chemical analysis since the 1970’s [16-19] and is employed by many laboratories, particularly those involved with workplace drug testing. However, the newer liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods have become viable alternatives for urine substance analysis of clinical specimens [14,15,20]. Several advantages of LC-MS/MS as compared with GC/MS include: 1) less complex specimen preparation and consequently shorter preparation time, 2) fewer interferences with other substances, 3) potential to work with small specimen volume requirements, and 4) the ability to measure multiple analytes in a single method [21]. Additionally, when used with optimal concentration cutoffs and validated methods, LCMS/ MS analyses provide definitive results with no false positives or false negatives [20,22].
Although LC-MS/MS is an excellent technology, as with any method there are challenges that need to be met in order to provide optimal identification and quantification of test analytes [23]. In the interest of augmenting the existing body of literature on mass spectrometric techniques for monitoring the presence of multiple prescription medications, the present authors have endeavored to present the analytical considerations as well as the techniques used to validate LC-MS/MS procedures for urine drug testing.
Methods
Over one million urine specimens from patients being treated for pain with opioid therapy were submitted by treating clinicians for analysis at Millennium Laboratories, San Diego, CA, USA. The specimens were de-identified and the data aggregated. No human subjects were harmed and the retrospective data analyses were approved by Aspire® IRB, Santee, CA, USA. Each physician test requisition typically included a list of medications the patient was known to be taking as well as which specific tests were to be performed. No specimens were excluded.
Test methods
Quantitative, multi-analyte liquid chromatographic tandem mass spectrometric assays were used to identify and confirm the presence of the analytes listed in Table 1. These assays include many of the medications (as well as some metabolites) requested by physicians who treat pain. The assays also include some illicit substances and metabolites.
| Compound Name | Precursor Ion | Product Ion 1 | Product Ion 2 |
|---|---|---|---|
| Buprenorphine-D4 | 472.4 | 59.2 | N/A |
| Buprenorphine | 468.3 | 396.1 | 55.2 |
| Norbuprenorphine-D3 | 417.3 | 55 | N/A |
| Norbuprenorphine | 414.3 | 83 | 55 |
| Naltrexone-D3 | 345.2 | N/A | N/A |
| Propoxyphene-D5 | 345.2 | 58.1 | N/A |
| Naltrexol | 344.2 | N/A | N/A |
| Fentanyl-D5 | 342.2 | 105.1 | N/A |
| Naltrexone | 342.2 | N/A | N/A |
| Propoxyphene | 340.2 | 266.1 | 58.1 |
| Zolpidem-COOH | 338.2 | N/A | N/A |
| Fentanyl | 337.2 | 188.2 | 105.1 |
| Paroxetine-D6 | 336.2 | N/A | N/A |
| 6-Acetylmorphine-D3 | 334.2 | 165.1 | N/A |
| Paroxetine | 330.2 | N/A | N/A |
| α-OH-alprazolam-D5 | 330.2 | 302 | N/A |
| 6-Acetylmorphine | 328.2 | 211.1 | 165.1 |
| Norpropoxyphene | 326 | 252 | 44 |
| α-OH-alprazolam | 325.1 | 297 | 216 |
| Oxycodone-D6 | 322.2 | 247.1 | N/A |
| Lorazepam | 321 | 275 | 229 |
| Oxycodone | 316.2 | 256.1 | 241.1 |
| Fluoxetine-D6 | 316.1 | N/A | N/A |
| Zolpidem-D6 | 314.3 | N/A | N/A |
| Methadone-D3 | 313.2 | 105 | N/A |
| Methadone | 310.2 | 265.1 | 105 |
| Fluoxetine | 310.1 | N/A | N/A |
| Zolpidem | 308 | N/A | N/A |
| Codeine-D6 | 306.2 | 115 | N/A |
| Hydrocodone-D6 | 306.2 | 202.2 | N/A |
| Temazepam-D5 | 306 | 260 | N/A |
| Oxymorphone-D3 | 305.3 | 201.1 | N/A |
| Oxymorphone | 302.3 | 227 | 198 |
| Norfluoxetine-D6 | 302.2 | N/A | N/A |
| Noroxycodone | 302.2 | 284 | 187 |
| Duloxetine-D3 | 301.2 | N/A | N/A |
| Temazepam | 301 | 255 | 177 |
| Codeine | 300.2 | 152 | 115 |
| Hydrocodone | 300.2 | 199? | 128 |
| Duloxetine | 298.1 | N/A | N/A |
| Norfluoxetine | 296.1 | N/A | N/A |
| Benzoylecgonine-D3 | 293.3 | 171.4 | N/A |
| Hydromorphone-D6 | 292.2 | 128.1 | N/A |
| Morphine-D6 | 292.2 | 152.1 | N/A |
| Oxazepam-D5 | 292.1 | 246.1 | N/A |
| Benzoylecgonine | 290.3 | 168.3 | 105.3 |
| Oxazepam | 287 | 241 | 104 |
| Hydromorphone | 286.2 | 185 | 128.1 |
| Morphine | 286.2 | 165.1 | 152.1 |
| Norhydrocodone | 286.1 | 199.1 | 115.1 |
| 7-NH2-clonazepam | 286 | 222 | 121.1 |
| Venlafaxine-D6 | 284.3 | N/A | N/A |
| Doxepin-D3 | 283.1 | 87 | N/A |
| Amitriptyline-D3 | 281.2 | 91 | N/A |
| EDDP-D3 | 281.2 | 234.1 | N/A |
| Imipramine | 281.1 | 86 | 58 |
| Doxepin | 280.1 | 107 | 84 |
| Venlafaxine | 278.4 | N/A | N/A |
| Amitriptyline | 278.2 | 105 | 91 |
| EDDP | 278.2 | 234.1 | 186 |
| Cyclobenzaprine | 276.2 | 231 | 191 |
| MDPV | 276.2 | N/A | N/A |
| Nordiazepam | 271 | 165 | 140 |
| Carisoprodol-D7 | 268.2 | 183.3 | N/A |
| Tramadol-13CD3 | 268.2 | 58.1 | N/A |
| Desipramine | 267.1 | 72 | 44 |
| O-desmethylvenlafaxine | 264.3 | N/A | N/A |
| Nortriptyline | 264.2 | 233 | 155 |
| Tramadol | 264.2 | 58.1 | N/A |
| Carisoprodol | 261.2 | 176.1 | 97? |
| Meperidine-D4 | 252.3 | 224.2 | N/A |
| Phencyclidine-D5 | 249.2 | 86.1 | N/A |
| Meperidine | 248.3 | 220.3 | 174.2 |
| Phencyclidine | 244.2 | 91.1 | 86.1 |
| Secobarbital-D5 | 244 | N/A | N/A |
| Methylphenidate-D9 | 243.2 | 93 | N/A |
| Ketamine-D4 | 242.1 | 224.1 | N/A |
| Secobarbital | 239 | N/A | N/A |
| Normeperidine-D4 | 238.3 | 164.2 | N/A |
| Norfentanyl-D5 | 238.2 | 84.1 | N/A |
| Ketamine | 238.1 | 125 | 89.1 |
| Phenobarbital-D5 | 238.1 | N/A | N/A |
| Methylphenidate | 234.2 | 84.1 | 56.2 |
| Normeperidine | 234.1 | 188.1 | 160.1 |
| Norfentanyl | 233.2 | 84.1 | 56 |
| Phenobarbital | 233.1 | N/A | N/A |
| Ritalinic acid-D10 | 230.2 | 93.1 | N/A |
| Butalbital-D5 | 230 | N/A | N/A |
| Meprobamate-D7 | 226.2 | 165.2 | N/A |
| Butalbital | 225 | N/A | N/A |
| Norketamine | 224.1 | 207.1 | 115.1 |
| Tapentadol | 222.1 | 107.1 | 77.1 |
| Ritalinic acid | 220.1 | 84.1 | 56.1 |
| Meprobamate | 219.1 | 158.1 | 69.1 |
| Methylone-D3 | 211.1 | N/A | N/A |
| Methylone | 208.1 | N/A | N/A |
| MDMA | 194.1 | 163.1 | 135 |
| Gabapentin-D10 | 182.1 | N/A | N/A |
| Mephedrone-D3 | 181.1 | N/A | N/A |
| Mephedrone | 178.1 | N/A | N/A |
| Gabapentin | 172.1 | N/A | N/A |
| Pregabalin-D6 | 166.2 | N/A | N/A |
| Pregabalin | 160.1 | N/A | N/A |
| Methamphetamine-D5 | 155.1 | 92.1 | N/A |
| Methamphetamine | 150.1 | 119.1 | 91.1 |
| Amphetamine-D5 | 141.1 | 124.1 | N/A |
| Amphetamine | 136.1 | 119.1 | 91.1 |
Table 1: Test menu, internal standards, precursor ions, product ion one and product ion two for monitoring pain medications arranged by m/z value of the precursor ion generated in the positive mode of ESi.
Chemicals and reagents
The following chemicals are employed to perform the quantification of substances in urine by LC-MS/MS analysis: Water, HPLC-grade (Fisher Scientific®, cat# WS-4), stable for 1 year at room temperature (RT); acetonitrile, LCMS-grade (Fisher Scientific®, Chromasolvbrand, cat# 34967). stable for 1 year at RT; methanol, HPLC-grade (Burdick and Jackson, cat# 230), stable for 1 year at RT; formic acid, ACS grade, 88% purity (EMD Science, cat# FX045-5), stable for 1 year at RT.
Analyte and deuterium-labeled internal standards are purchased from Cerilliant®Corporation, Round Rock, TX, USA. Each standard is certified by Cerilliant® to be the true, pure compound. See Table 1 for the full list of compounds analyzed.
The analytical methods used in this study have previously been described [22,24-26]. Briefly, an Agilent® 1200 series binary pump SL Liquid Chromatography system, well plate sampler, thermostated column compartment, paired with an Agilent® 6410 QQQ mass spectrometer and Agilent® MassHunter software was used for analysis of all substances. The method used an acetonitrile-aqueous formic acid gradient running at 0.4 mL/min. A 2.1 x 50 mm, Poroshell 120 EC-18 or SB-18 of particle size 2.7 micron column was used for chromatography. The column temperature was 50°C. Mobile phase A = 0.1% formic acid in water, B = 0.1% formic acid in acetonitrile. The Agilent® 6410 Triple Quadrupole mass spectrometer was used in the positive ESI mode. The nitrogen drying gas temperature was 350°C, and the flow was 12 L/ min, nebulizer gas (nitrogen) 40 psi, and the capillary voltage was 3000 V. Fragmentor voltage was optimized for each analyte. Dwell times were calculated by Agilent® MassHunter Acquisition, depending on the number of concurrent MRMs. HPLC water, acetonitrile, methanol, and formic acid HPLC grade were obtained from VWR, Westchester, PA, USA.
Cycle times were set to 500 milliseconds. In the MRM mode, 2 transitions were used to identify and quantitate a single compound (see Table 2). A quantitative transition was used to calculate concentration based on the quantifier ion, and a qualitative transition was used to ensure accurate identification of the target compound based on the ratio of the qualifier ion to the quantifier ion.
| Substance | Test Concentration (ng/mL) | Substance | Test Concentration (ng/mL) |
|---|---|---|---|
| 6-Monoacetylmorphine | 1,280 | Desmethylcitalopram | 250 |
| 11-Nor-9-carboxy THC | 512 | Desmethylclomipramine | 500 |
| 6-Acetyl morphine | 5 | Desmethylsertraline | 250 |
| 7-Amino clonazepam | 125 | Dextromethorphan | 500 |
| 7-Amino flunitrazepam | 125 | Diazepam | 500 |
| Acetaminophen | 25,000 | Dihydrocodeine | 100,000 |
| α-OH-alprazolam | 2,560 | Diltiazem | 500 |
| Alprazolam | 250 | Diphenhydramine | 500 |
| Amantadine | 250 | Doxepin | 500 |
| Amitiptyline | 500 | Doxylamine | 500 |
| Amoxapine | 600 | Duloxetine | 500 |
| Amphetamine | 12,800 | Ecgonine methyl esther | 500 |
| Antipyrine | 1000 | EDDP | 12,800 |
| Atomoxetine | 500 | Ephedrine | 100,000 |
| Benzocaine | 500 | Fentanyl | 256 |
| Benzoylecgonine | 3,200 | Flunitrazepam | 125 |
| Brompheniramine | 50 | Fluoxetine | 500 |
| Bupivacaine | 500 | Flurazepam | 125 |
| Buprenorphine | 1,280 | Hydrocodone | 6,400 |
| Bupropion | 500 | Hydromorphone | 6,400 |
| Bupropion metabolite | 1,000 | Ibuprofen | 25,000 |
| Butalbital | 5,000 | Imipramine | 500 |
| Carbamazepine | 5,000 | Ketamine | 500 |
| Carisoprodol | 6,400 | Lamotrigene | 5,000 |
| Chlordiazepoxide | 125 | Lidocaine | 1,000 |
| Chlorpheniramine | 500 | Lorazepam | 5,120 |
| Chlorpomazine | 500 | Loxapine | 250 |
| Citalopram | 250 | Maprotiline | 1,250 |
| Clomipramine | 125 | MCPP | 250 |
| Clonazepam | 500 | MDA | 100 |
| Clonidine | 250 | MDEA | 100,000 |
| Clozapine | 500 | MDMA | 12,800 |
| Cocaethylene | 100 | Meclizine | 600 |
| Cocaine | 100 | Meperidine | 500 |
| Codeine | 6,400 | Meprobamate | 6,400 |
| Cyclobenzaprine | 100 | Mesoridazine | 500 |
| Desalkyflurazepam | 500 | Methadone | 6,400 |
| Desipramine | 500 | Methamphetamine | 12,800 |
| Desmethyldoxepin | 500 | Methylphenidate | 50 |
| Metoclopramide | 500 | Pentazocine | 500 |
| Midazolam | 125 | Phencyclidine | 640 |
| Midazolam | 100 | Phenobarbital | 5,000 |
| Mirtazepine | 250 | Phenylpropanolamine | 100,000 |
| Morphine | 6,400 | Phentermine | 100,000 |
| Phenytoin | 5000 | ||
| Naproxen | 100,000 | Promethazine | 500 |
| Morphine-3-glucuronide | 250 | Propoxyphene | 12,800 |
| Norbuprenorphine | 1,280 | Psuedoephedrine | 100,000 |
| Norcodeine | 100,000 | Quetiapine | 250 |
| Nordiazepam | 5,120 | Sertraline | 500 |
| Norfentanyl | 1,024 | Strychnine | 500 |
| Norfluoxetine | 500 | Temazepam | 6,400 |
| Normeperidine | 500 | Thioridazine | 500 |
| Normorphine | 100,000 | Topiramate | 5,000 |
| Norpropoxyphene | 12,800 | Tramadol | 3,200 |
| Nortriptyline | 500 | Trazodone | 1,000 |
| Norverapamil | 500 | Triazolam | 125 |
| O- desmethylvenlafaxine | 500 | Trimethobenzamide | 500 |
| Olanzapine | 600 | Trimethoprim | 500 |
| Oxezepam | 5,120 | Varapamil | 500 |
| Oxycodone | 6,400 | Venlafaxine | 500 |
| Paraxanthine | 100,000 | Zolpidem | 250 |
| Paroxetine | 500 | Zopiclone | 125 |
Table 2: Substances tested for interference in LC-MS/MS with test concentrations.
Ion suppression and ion enhancement were evaluated by tabulating the deuterated internal standard (ISTD) responses within 96-well plate batches. The reference (i.e. no ion suppression) values for ISTD area responses were defined as the mean ISTD area responses for the eight blank specimens included in each batch.
Methanolic standards were obtained from Cerilliant® Corporation. The methanolic standards for each substance were spiked into synthetic urine.Specimens and calibrators were prepared for injection by incubating 25 μL of urine with 50 units of β-glucuronidase. The efficiency of the hydrolysis procedure was determined by use of a morphine glucuronide control. Hydrolysis of the control material was considered acceptable if the value of the recovered morphine was above 90% of the nominal concentration.
Five microliters of specimens, controls and calibrators were injected.
Method Validation and Assay Performance
The upper limit of linearity (ULOL) was determined by the replicate analysis (N =3) of progressively increasing high level standards against the production calibration curve. The ULOL is the highest concentration tested that can be identified and quantified within 20% of the target value. The lower limit of quantitation (LLOQ) was determined by the replicate analysis (N=8) of progressively decreasing low level standards against the production calibration curve. The LLOQ is the lowest concentration tested that can be identified and quantified within 20% of the target value. Assay performance data are listed in Table 3. The criteria for identification are transition ratios within 20% and relative retention times within 2% of the midrange calibrator with acceptable chromatography.
| ISTD | Maximum Ion Suppression (%) | Median Ion Suppression (%) | Maximum Ion Enhancement (%) |
|---|---|---|---|
| Morphine-D6 | 76.1 | 39.2 | 10 |
| Oxymorphone-D3 | 63.5 | 29.1 | 16.3 |
| Hydromorphone-D6 | 72.6 | 27.2 | 10.3 |
| Amphetamine-D5 | 47.8 | 8.7 | 7.7 |
| Codeine-D6 | 49.1 | 17.4 | 9.2 |
| Oxycodone-D6 | 57.2 | 23 | 13.3 |
| Methamphetamine-D5 | 79.4 | 8.9 | 3.4 |
| Oxycodone-D6 | 57.2 | 23 | 13.3 |
| Hydrocodone-D6 | 55.4 | 13.3 | 9.3 |
| Methamphetamine-D5 | 79.4 | 8.9 | 3.4 |
| Hydrocodone-D6 | 55.4 | 13.3 | 9.3 |
| 6-Acetylmorphine-D6 | 52.8 | 5.7 | 14 |
| Ritalinic acid-D10 | 56.2 | 7.9 | 14.7 |
| 7-NH2-clonazepam-D7 | 88.3 | 37.9 | 16.3 |
| Ketamine-D4 | 56.9 | 5.6 | 8.9 |
| Benzoylecgonine-D3 | 59.4 | 9.6 | 6.2 |
| Ketamine-D4 | 56.9 | 5.6 | 8.9 |
| Norfentanyl-D5 | 56.9 | 10 | 13.2 |
| Methylphenidate-D9 | 48.3 | 5.6 | 5.7 |
| Tramadol-13CD3 | 53.1 | 5.1 | 6.4 |
| Tramadol-13CD3 | 53.1 | 5.1 | 6.4 |
| Normeperidine-D4 | 42.8 | 6 | 3.9 |
| Meperidine-D4 | 44.2 | 4.9 | 5 |
| Meprobamate-D7 | 57 | 16.8 | 8.4 |
| Norbuprenorphine-D3 | 49.8 | 16.6 | 13.2 |
| Phencyclidine-D5 | 28.3 | 3.6 | 7.3 |
| Fentanyl-D5 | 24.9 | 3.3 | 4.5 |
| Doxepin-D3 | 33.8 | 7 | 9.2 |
| EDDP-D3 | 26.1 | 2.7 | 4.9 |
| Buprenorphine-D4 | 41 | 5.4 | 4.6 |
| Amitriptyline-D3 | 31.9 | 4.2 | 8.6 |
| Amitriptyline-D3 | 31.9 | 4.2 | 8.6 |
| Amitriptyline-D3 | 31.9 | 4.2 | 8.6 |
| α-OH-alprazolam-D5 | 22.8 | 3 | 16.8 |
| Amitriptyline-D3 | 31.9 | 4.2 | 8.6 |
| Oxazepam-D5 | 31.4 | 15.2 | 12.2 |
| Propoxyphene-D5 | 30.8 | 2.6 | 5.6 |
| Amitriptyline-D3 | 31.9 | 4.2 | 8.6 |
| Propoxyphene-D5 | 30.8 | 2.6 | 5.6 |
| Methadone-D3 | 19.4 | 2.9 | 5.5 |
| Oxazepam-D5 | 31.4 | 15.2 | 12.2 |
| Temazepam-D5 | 27.5 | 11.7 | 9.9 |
| Carisoprodol-D7 | 40.6 | 9.1 | 6.8 |
| Temazepam-D5 | 27.5 | 11.7 | 9.9 |
The test analytes are listed in the order of their elution. Ion suppression and ion enhancement were evaluated by tabulating the deuterated internal standard (ISTD)responses within 96-well plate batches. The reference (i.e. no ion suppression) values for ISTD area responses were defined as the mean ISTD area responses for the eight blank specimens included in each batch. Maximum ion suppression (%) for each ISTD was calculated using the formula: 100 – (mean blank ISTD area – minimum intra-batch ISTD area * 100) Median ion suppression (%) for each ISTD was calculated using the formula: 100 – (mean blank ISTD response – median intra-batch ISTD area * 100) Maximum ion enhancement was calculated using the formula:(Maximum intra-batch ISTD area – mean blank ISTD area) – 100
Table 3: Ion suppression as a function of elution.
Deuterated internal standards were used to improve quantitative accuracy and minimize the effects of ion suppression that can occur at high analyte concentrations or with the coelution of other concentrated substances or contaminants. Suppression effects were measured by comparing the response of the internal standard at the cutoff with the response at the ULOL. When deuterated internal standards are used, quantitative accuracy can usually be obtained with a 50% suppression of the internal standard response at the ULOL.
Integration peak area thresholds were established for each target compound by a user-defined script in MassHunter Quantitative Analysis, based on compound-specific calibrator responses and potentials for ion suppression. Four calibrators, four QC specimens, and eight blanks were run as part of each 96-well plate. Data quality for each batch was verified by calibrator and QC accuracy, calibration curve linearity and r2, ISTD response, and visual review of chromatography for all positive results.
It was made certain that the chromatography could separate any potential isobaric interfering compounds. This was accomplished by establishing that retention time differences between the isobaric pairs were greater than 0.1 minute. We eliminated another possible interference from compounds with atomic mass units that were within 1 dalton of any internal standard listed in Table 1. This was accomplished by adjusting the chromatography times and gradients so that there was no overlap between these compounds and the internal standards. This is important because of the isotope cascade (a+1 effect), where the contribution of naturally occurring 13C can contribute to the area counts of a compound one mass unit higher if they coelute [27]. The analyses were optimized for groups of substances. A single injection for all the substances was not used.
A list of potentially interfering medications was generated from the proposed test menu (Table 1) and a list of commonly prescribed medications [28] (Table 2). These compounds were added to a control specimen to show that all of the analytes of interest were quantified to within +/- 20% of target concentrations.
Carryover was evaluated by the analysis of synthetic negative urine following the ULOL standards.Carryover limit (CL) was determined in the following manner: single analyte specimens were prepared from reference standards in blank urine. Certified Cerilliant® and Sigma® standards of either 1,000,000 ng/ml or 100,000 ng/ml were diluted in synthetic urine to achieve the spiked concentrations shown in Table 4. Blanks containing internal standards were injected between each ULOL specimen to determine if analyte carryover was occurring. This was repeated 3 times. The results of the carryover experiments are presented in columns 4,5, and 6 of Table 4. Carryover was deemed to have occurred, if a substance was detected, and the qualifier ions were acceptable within 20% of the expected values. The average percent carryover is presented in the last column of Table 4.
| Analyte | Limit of Quantitation | Upper Limit of inearity | Analyte | Limit of Quantitation | Upper Limit of Linearity |
|---|---|---|---|---|---|
| 6-Acetylmorphine | 10 | 5,000 | Methylone | 10 | 25,000 |
| 7-Amino-clonazepam | 20 | 50,000 | Methylphenidate | 50 | 50,000 |
| Alpha-hydroxyalprazolam | 20 | 50,000 | Morphine | 50 | 100,000 |
| Amitriptyline | 50 | 50,000 | Naltrexol | 10 | 50,000 |
| Amphetamine | 50 | 100,000 | Naltrexone | 10 | 50,000 |
| Benzoylecgonine | 50 | 100,000 | Norbuprenorphine | 20 | 5,000 |
| Buprenorphine | 10 | 5,000 | Nordiazepam | 40 | 50,000 |
| Butalbital | 200 | 100,000 | Norfentanyl | 8 | 5000 |
| Carisoprodol | 50 | 100,000 | Norfluoxetine | 25 | 100,000 |
| Codeine | 50 | 100,000 | Norketamine | 50 | 50,000 |
| Cyclobenzaprine | 50 | 50,000 | Normeperidine | 50 | 100,000 |
| Desipramine | 50 | 50,000 | Norpropoxyphene | 50 | 100,000 |
| Desmethylvenlafaxine | 100 | 100,000 | Nortriptyline | 50 | 50,000 |
| Doxepin | 50 | 50,000 | Oxazepam | 50 | 50,000 |
| Duloxetine | 25 | 100,000 | Oxycodone | 50 | 100,000 |
| EDDP | 100 | 100,000 | Oxymorphone | 50 | 100,000 |
| Fentanyl | 2 | 2,000 | 4-OH-3-OMe-paroxetine | 25 | 100,000 |
| Fluoxetine | 25 | 100,000 | Paroxetine | 25 | 100,000 |
| Gabapentin | 100 | 100,000 | Phencyclidine | 50 | 100,000 |
| Hydrocodone | 50 | 100,000 | Phenobarbital | 200 | 100,000 |
| Hydromorphone | 50 | 100,000 | Pregabalin | 100 | 100,000 |
| Imipramine | 50 | 50,000 | Propoxyphene | 50 | 100,000 |
| Ketamine | 50 | 50,000 | Ritalinic acid | 50 | 50,000 |
| Lorazepam | 40 | 50,000 | Secobarbital | 200 | 100,000 |
| MDMA | 50 | 100,000 | Tapentadol | 50 | 100,000 |
| MDPV | 10 | 25,000 | Temazepam | 50 | 50,000 |
| Meperidine | 50 | 100,000 | THC | 15 | 50,000 |
| Mephedrone | 10 | 25,000 | Tramadol | 100 | 100,000 |
| Meprobamate | 50 | 100,000 | Venlafaxine | 100 | 100,000 |
| Methadone | 50 | 100,000 | Zolpidem | 10 | 50,000 |
| Methamphetamine | 50 | 100,000 | Zolpidem-COOH | 10 | 50,000 |
Table 4: Lower limits of quantitation and upper limits of linearity.
| Analyte | Test Concentration | Limit of Detection | Measured Concentration for BLANK- A | Measured Concentration for BLANK- B | Measured Concentration for BLANK- C | % Carryover |
|---|---|---|---|---|---|---|
| 6-acetylmorphine | 20,000 | 5 | 0 | 0 | 0 | 0 |
| Buprenorphine | 20,000 | 3 | 0 | 0 | 0 | 0 |
| Carboxy-THC | 20,000 | 5 | 61.71 | 0 | 9.235 | 0.03 |
| Fentanyl | 20,000 | 1 | 0 | 3.8273 | 0 | 0.012 |
| Norbuprenorphine | 20,000 | 3 | 0 | 0 | 0 | 0 |
| Norfentanyl | 20,000 | 2 | 0 | 0 | 0 | 0 |
| 7-amino-clonazepam | 50,000 | 10 | 0 | 0 | 0 | 0 |
| Alpha-hydroxyalprazolam | 50,000 | 10 | 0 | 0 | 0 | 0 |
| Amitriptyline | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Benzoylecgonine | 50,000 | 15 | 0 | 0 | 0 | 0 |
| Cyclobenzaprine | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Desipramine | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Doxepin | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Imipramine | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Lorazepam | 50,000 | 10 | 0 | 15.5281 | 0 | 0.003 |
| Meperidine | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Nordiazepam | 50,000 | 10 | 0 | 0 | 0 | 0 |
| Normeperidine | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Norpropoxyphene | 50,000 | 50 | 0 | 0 | 0 | 0 |
| Nortriptyline | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Oxazepam | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Phencyclidine | 50,000 | 15 | 0 | 0 | 0 | 0 |
| Propoxyphene | 50,000 | 25 | 0 | 0 | 0 | 0 |
| Temazepam | 50,000 | 10 | 0 | 0 | 0 | 0 |
| Amphetamine | 50,000 | 25 | 0 | 0 | 0 | 0 |
| Amphetamine | 100,000 | 25 | 0 | 0 | 0 | 0 |
| Carisoprodol | 50,000 | 25 | 0 | 0 | 0 | 0 |
| Carisoprodol | 100,000 | 25 | 0 | 0 | 0 | 0 |
| Codeine | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Codeine | 100,000 | 20 | 0 | 0 | 0 | 0 |
| EDDP | 50,000 | 50 | 0 | 0 | 0 | 0 |
| EDDP | 100,000 | 50 | 0 | 0 | 0 | 0 |
| ETG | 100,000 | 100 | 366.822 | 0 | 617.22 | 0.061 |
| ETS | 100,000 | 100 | 149.591 | 153.301 | 150.605 | 0.015 |
| Hydrocodone | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Hydrocodone | 100,000 | 20 | 0 | 0 | 0 | 0 |
| Hydromorphone | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Hydromorphone | 100,000 | 20 | 0 | 0 | 0 | 0 |
| MDMA | 50,000 | 25 | 0 | 0 | 0 | 0 |
| MDMA | 100,000 | 25 | 0 | 0 | 0 | 0 |
| Meprobamate | 50,000 | 50 | 0 | 0 | 0 | 0 |
| Meprobamate | 100,000 | 50 | 0 | 0 | 0 | 0 |
| Methadone | 50,000 | 50 | 0 | 0 | 0 | 0 |
| Methadone | 100,000 | 50 | 0 | 0 | 0 | 0 |
| Methamphetamine | 50,000 | 25 | 0 | 0 | 0 | 0 |
| Methamphetamine | 100,000 | 25 | 0 | 0 | 0 | 0 |
| Morphine | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Morphine | 100,000 | 20 | 0 | 0 | 0 | 0 |
| Norhydrocodone | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Norhydrocodone | 100,000 | 20 | 0 | 0 | 0 | 0 |
| Noroxycodone | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Noroxycodone | 100,000 | 20 | 0 | 0 | 0 | 0 |
| Oxycodone | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Oxycodone | 100,000 | 20 | 0 | 0 | 0 | 0 |
| Oxymorphone | 50,000 | 20 | 0 | 0 | 0 | 0 |
| Oxymorphone | 100,000 | 20 | 0 | 0 | 0 | 0 |
| Tapentadol | 50,000 | 50 | 0 | 0 | 0 | 0 |
| Tapentadol | 100,000 | 50 | 0 | 0 | 0 | 0 |
| Tramadol | 50,000 | 50 | 0 | 0 | 0 | 0 |
| Tramadol | 100,000 | 50 | 0 | 0 | 0 | 0 |
Table 5: Carryover limit.
The frequency at which parent substance-metabolite pairs were observed was scored. For the purposes of this calculation the LCMS/ MS cutoff concentrations of the parent substance and metabolite were set at the lower limit of quantitation. The analytical limit of quantitation or LOQ was used as the cutoff point for both parent substance and metabolite. If the concentration of either the substance or the metabolite was below the LOQ then that pair was not used in the calculation. The numbers of specimens tested for each analyte are presented in the summary Tables 6a and 6b.
| High Positive Concentration, X (ng/mL) | Following Specimen Concentration, Y (ng/mL) | Conclusion |
|---|---|---|
| X > 100,000 | Y > 50 | Repeat analysis after blank |
| 100,000 > X ≥ 50,000 | Y < 50 | No action required |
| 100,000 > X ≥ 50,000 | 50 < Y < 200 | Repeat analysis after blank |
| 100,000 > X ≥ 50,000 | Y > 200 | No action required |
Table 6: Carryover evaluation criteria for benzoylecgonine. Values for other medications will vary. The ULOL and carryover limit for benzoylecgonine is 100,000 ng/mL.
The analysis was carried out using the following method: the parent substance was considered to be present if it was above the cutoff concentration. Similarly the metabolite was considered to be present if it was above the gap should be there The number of times the metabolite failed this scoring procedure was noted and those parent substance values were separated for review.
When both parent substance and metabolite were present (i.e. above the cutoff concentration) then the pairing was scored as positive. When the parent substance was present but metabolite was not found to be present (below cutoff concentration), the observation was scored as negative (Table 7a).
| Substance | LC-MS/MS Cutoff (ng) | Metabolite | LC-MS/MS Cutoff (ng) | Positive Substance Count | Number of Times Observed For Each Substance | Number of Times Not Observed For Each Observed | Median Substance Concentration When Observed | Median Substance Concentration When Not Observed | Percent Matching |
|---|---|---|---|---|---|---|---|---|---|
| Methamphetamine | 100 | Amphetamine | 100 | 62 | 55 | 7 | 6,589 | 701 | 89 |
| Methadone | 50 | EDDP | 50 | 803 | 781 | 22 | 2,269 | 355 | 97 |
| Buprenorphine | 10 | Norbuprenorphine | 20 | 108 | 105 | 3 | 65 | 131 | 97 |
| Fentanyl | 2 | Norfentanyl | 8 | 711 | 698 | 13 | 44 | 10 | 98 |
| Carisoprodol | 50 | Meprobamate | 50 | 598 | 588 | 10 | 457 | 174 | 98 |
| Hydrocodone | 50 | Hydromorphone | 50 | 3,005 | 2,076 | 929 | 1,540 | 341 | 69 |
| Oxycodone | 50 | Oxymorphone | 50 | 2,129 | 1,972 | 157 | 2,139 | 450 | 93 |
Table 7a: Observations on the occurrence of parent medication and metabolite (concentration in ng/mL).
To obtain additional information we conducted another analysis to determine how often metabolite was found when parent substance was not observed (see Table 7b). The cutoffconcentrations were the same as for the previous analysis.
| Metabolite | LC-MS/MS Cutoff (ng/mL) | Substance | LC-MS/MS Cutoff (ng/mL) | Positive Metabolite Count | Number of Times Metabolite Found With Parent Medication | Number of Times Metabolite Found Without Parent Medication | Median Metabolite Concentration With Parent Medication | Median Metabolite Concentration Without Parent Medication | Percent Metabolite Without Parent |
|---|---|---|---|---|---|---|---|---|---|
| EDDP | 50 | Methadone | 50 | 810 | 781 | 29 | 3,960 | 96 | 3.5 |
| Norbuprenorphine | 20 | Buprenorphine | 10 | 131 | 105 | 26 | 323 | 58 | 20 |
| Norfentanyl | 8 | Fentanyl | 2 | 752 | 698 | 54 | 304 | 18 | 7.1 |
| Meprobamate | 50 | Carisoprodol | 50 | 993 | 588 | 405 | 24,448 | 3,815 | 40.7 |
Table 7b: Observations on the occurrence of metabolite without parent medication (concentration in ng/mL).
Results
Table 1 lists the parent substances and metabolites that were tested in this cohort as well as the precursor ions and product ions. Table 2 lists the commonly prescribed medications that were tested to ensure there was no interference during analysis. There was no interference from any of the compounds listed in Tables 1 and 2.
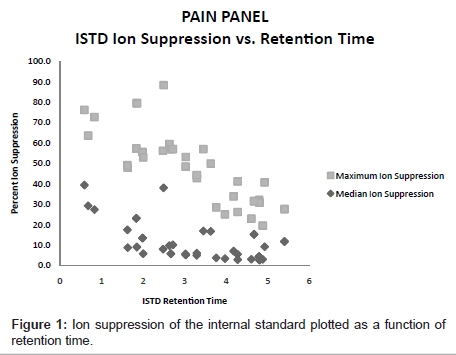
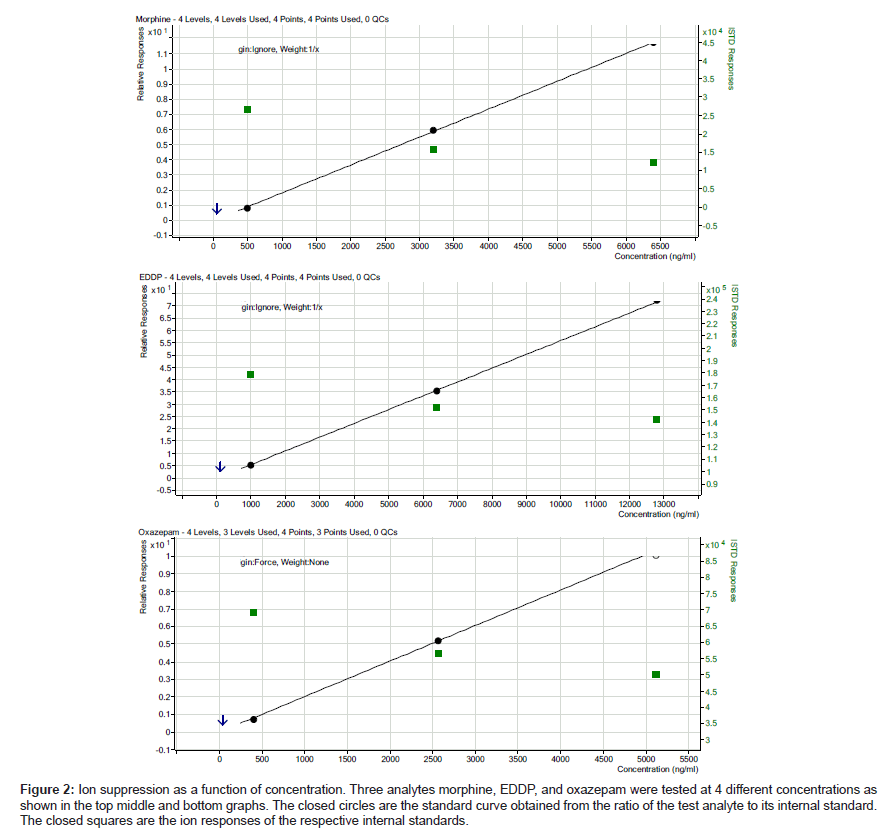
The ion suppression varied depending on the test analyte (Table 3).In general, there was more ion suppression in the earlier eluting peaks than the later eluting ones (Figure 1). Also, there was more ion suppression in the more highly concentrated specimens. This is shown in Figure 2. Instrument response and the effect of ion suppression did not affect the ability of the method to give linear results over a wide range of concentrations (Table 4).
Figure 2: Ion suppression as a function of concentration. Three analytes morphine, EDDP, and oxazepam were tested at 4 different concentrations as shown in the top middle and bottom graphs. The closed circles are the standard curve obtained from the ratio of the test analyte to its internal standard. The closed squares are the ion responses of the respective internal standards.
Evaluations for carryover were identified using the data presented in Table 5 and resulted in the following rule set:
1.A specimen above the measured ULOL (upper limit of linearity) followed by a positive of any value: re-inject specimen after a blank run. If the re-injected specimen is still positive, then rerun a fresh aliquot of the specimen. This will eliminate any needle carryover contaminating the specimen.
2.A high level positive (defined for each substance) that is below the ULOL followed by a result below the LOQ: The low level may be the result of carryover, but, as the level is below the LOQ the carryover incident is moot and the specimen is reported as negative. No further action is required.
3. A high level positive (level defined for each substance) that is below the ULOL, followed by a specimen above the LOQ and below a level defined for each substance. Re-inject the specimen after blank.
4. A high level positive (level defined for each substance) that is below the ULOL, followed by a specimen above a level defined for each substance. Carryover may have added to the second high level; however the addition is a nominal percentage of the measured level (i.e. less than 20%), and as such, the second high level specimen is a true positive and no further action is required.
A small number of specimens (about 1%) had excreted concentrations of analyte (particularly morphine and oxycodone) that exceeded 100,000 ng/mL. These observations indicated the necessity of having a method to detect and address carryover. The established method to minimize the possibility of carryover is summarized in Table 6.
Tables 7a and 7b show the correlation between seven parent substance and metabolite pairs observed in urine drug tests. Between one and five percent of the specimens did not show the presence of the metabolite. Table 8 shows the prevalence with which non-listed medications were present in this population’s urine specimens. The most prevalent non-listed medication class was benzodiazepines (23%). The second most widely used non-listed medication class was opiates (19.2%).
| Medication/ Medication Class | Observed Frequency |
|---|---|
| Amphetamine | 1.4% |
| Benzodiazepines | 23.0% |
| Buprenorphine | 1.0% |
| Carisoprodol | 4.6% |
| Fentanyl | 1.5% |
| Meperidine | 0.4% |
| Methadone | 2.2% |
| Opiates | 19.2% |
| Propoxyphene | 2.7% |
| Tapentadol | 0.2% |
| Tramadol | 4.1% |
Table 8: Prevalence non-reported medications observed in 290,627 specimens.
Discussion
The validation procedure for any assay should establish accuracy, precision, analytical sensitivity, analytical specificity, and the reportable range, as well as determination of calibration and control procedures [17]. These procedures are laid out in the Clinical Laboratory Standards Institute (CLSI) document on mass spectrometry in the clinical laboratory [23].
Analyses for pain medications as well as illicit substances typically involve dozens of analytes. This differs from other types of analyses commonly utilized by physicians that may test for only one or two analytes (e.g., immunosuppressive agents, cyclosporine and tacrolimus). Table 1 presents a list of the medications commonly requested by pain management clinicians. But there are additional substances that the pain population could be taking. The analyses showed that testing only for the listed prescribed medications and illicit substances would not provide a complete picture of what substances a patient was taking.
Despite the ability of LC-MS/MS technology to be highly specific (and considering the wide range of medications and other substances that must be tested for to accommodate pain clinicians’ needs), the laboratory must identify any substance that could potentially produce analytical interference, no matter how remote, in order to assure optimal accuracy. The logical place to begin ruling out interference is with those medications listed in Table 1.
The analytical challenge of monitoring this many substances was met by use of deuterated internal standards and elimination of potential isobaric interferences using appropriate chromatography.
Isobaric interferences can be minimized by reviewing the molecular characteristics of commonly prescribed medications. A list of these medications is presented in Table 2. A more complete list along with other properties including molecular weights are found in the previously mentioned CLSI document [23]. As a general rule, it is not necessary to test those commonly prescribed medications that are not isobaric.
Medications other than those can potentially cause interference, of course. Their identification can be more difficult and may entail running a significant number of patient specimens to identify them. In some cases, testing for the presence of both parent substance and metabolite can identify interference. For example, when a specimen is positive for a metabolite such as normeperidine, but the parent substance (in this case, meperidine) is absent, and the parent substance was not listed as a prescribed medication, further analysis specifically for normeperidine may show that interference had occurred.
The classic false positive involves identifying methamphetamine in the presence of large concentrations of pseudoephedrine by GC-MS [29]. In this example, the presence of 500,000 ng/mL of pseudoephedrine caused a false positive methamphetamine result. The interference occurred on the GC separation by distorting chromatography, (peak shape or retention times), and by the formation of methamphetamine from the breakdown of the derivatized pseudoephedrine in the hot injection port of the GC. In LC-MS/MS,in-source fragmentation or rearrangement can occur before the first quadrupole, and can only be evaluated by experimentation. Compounds that are not isobaric generally will not cause a false positive, but can cause a false negative by preventing ionization of the analyte of interest. Experiments should include the interferants at levels expected in urine, which can be very high for some medications.
The liquid chromatography component of the analysis should be optimized so that the retention times of isobaric compounds do not overlap, that is, that there is chromatographic separation. It is possible to have the precursor ions generate the same m/z product ions. However, these generally have different product ion ratios. If this occurs, a good practice would be to use different transitions. It is also essential to monitor ion ratios when performing this type of analysis.
In the case of isobaric compounds which are not in the target compound list, but could conceivably appear in specimens, it is also accepted practice to demonstrate non-interference of such a compound both for identification (presence of the isobar does not affect qualifier ratios for the target compounds) and quantification (presence of the isobar does not affect the measured concentration of the target compound). The interference study is typically carried out with the possible interfering isobar at a concentration substantially above that which might be expected. Some examples of isobaric pairs are ephedrine/pseudoephedrine, morphine/hydromorphone, codeine/ hydrocodone, and methamphetamine/phentermine.
Ion suppression is another important variable that needs to be evaluated during method development. Ion suppression can occur when the ionization process is saturated, or when electrospray droplet formation and/or evaporation are altered. Deuterium labeled internal standards help correct for ion suppression and are an essential component of “dilute and shoot” methods. By monitoring the intensity of the internal standard area counts, analysts can monitor ion suppression.
One way to explain why LC-MS/MS is inherently free of interference is to consider the probability of an interfering substance having the same properties as the test compound. For example, if the chromatography separates compounds into sixty 0.1 minute segments, this may be considered a 60 fold separation, If the first quadrupole separates compounds by 1 amu, then for compounds in the molecular weight range of 100 to 600 amu, this is a 500 fold discrimination. If the second quadrupole also discriminates based on collision energy and the third quadrupole separates the two product qualifier ions by 1 amu each, this is additional 200 fold discrimination. Finally, the ratio of the productions (qualifier ratio) adds 10 to 20 fold discrimination. In total this adds up to at least 60 X 500 X 200 X 10 = 60,000,000 fold discrimination ability. That is the odds are less than 1 in 60,000,000 that there would be a match by another compound.
For monitoring of patients on chronic opioid therapy, quantifying the presence of the metabolites of the prescribed medications is very useful. The data can be used to show the patient has taken the medication and is likely to be metabolizing the medication is a manner consistent with other patients. Unexpected results may also possibly indicate that the patient is a fast or slow metabolizer, information that can help the clinician provide optimal care.
The observation that some specimens only contained the parent drug and no metabolite indicated the possibility of abnormal metabolism or potential attempts by patients to make the physician believe that they were taking the medication when they were not. This would have been done by “shaving” a small piece of prescription medication into the urine specimen. In these instances, the patient would have been unaware that actually ingesting the medication facilitates metabolism of the substance and therefore produces the drug’s metabolite [30-34].
The LC-MS/MS instrumentation provides quantitative data over the potential range of concentrations needed to monitor these patients (Table 3). Patients can excrete a wide range of concentrations of medication. Fortunately, LC-MS/MS techniques have a wide dynamic range (up to 10,000 fold). This high sensitivity and specificity may produce positive results for certain substances that are not necessarily medically relevant but are merely trace level impurities in pharmaceutical preparations. Table 9 shows the percentage of allowable impurities in various opioid medications [35-38]. Thus the analyst must be aware of true positive results caused by medication impurities.
| Formulation | Process Impurities | Allowable Limit (%) | Typically Observed (%) |
|---|---|---|---|
| Codeine | Morphine | 0.15 | 0.01-0.1 |
| Hydrocodone | Codeine | 0.15 | 0-0.1 |
| Hydromorphone | Morphine Hydrocodone | 0.15 0.1 | 0-0.025 0-0.025 |
| Morphine | Codeine | 0.5 | 0.01-0.05 |
| Oxycodone | Hydrocodone | 1 | 0.02-0.12 |
| Oxymorphone | Hydromorphone Oxycodone | 0.15 0.5 | 0.03-0.1 0.05-0.4 |
Table 9: Known impurities in medication formulations (30-33).
Conclusions
Laboratories using LC-MS/MS as a tool for monitoring patients on polymedication therapy must address a number of considerations to assure that they provide the highest level of accuracy possible. These considerations include 1) avoiding isobaric interferences, 2) determining that ancillary medications do not interfere, 3) evaluating the extent and impact of matrix effects, including ion suppression and ion enhancement, and 4) developing procedures to minimize potential carryover and procedures to identify and deal with any likely carryover. When taking these potential issues into consideration, LC-MS/MS is an accurate and reliable method of analysis.
References
- Federation of State Medical Boards of the United States, Inc. (2004) Model Policy for the Use of Controlled Substances for the Treatment of Pain.
- Trescot AM, Boswell MV, Atluri SL, Hansen HC, Deer TR, et al. (2006) Opioid guidelines in the management of chronic non-cancer pain. Pain Physician 9: 1-40.
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, et al. (2009) Opioids for chronic noncancer pain: Prediction and identification of aberrant drug-related behaviors: A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 10: 131-146.
- Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS (2008) What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med 9: 444-459.
- Fishman SM, Wilsey B, Yang J, Reisfield GM, Bandman TB, et al. (2000) Adherence monitoring and drug surveillance in chronic opioid therapy. J Pain Symptom Manage 20: 293-307.
- Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD (2007) Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain 8: 573-582.
- Gourlay DL, Heit HA, Almahrezi A (2005) Universal precautions in pain medicine: A rational approach to the treatment of chronic pain. Pain Med 6: 107-112.
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, et al. (2004) Predicting aberrant drug behavior in patients treated for chronic pain: Importance of abuse history. J Pain Symptom Manag 28: 250-258.
- Manchikanti L, Damron KS, McManus CD, Barnhill RC (2004) Patterns of illicit drug use and opioid abuse in patients with chronic pain at initial evaluation: A prospective, observational study. Pain Physician 7: 431-437.
- Manchikanti L, Manchukonda R, Pampati V, Damron KS (2005) Evaluation of abuse of prescription and illicit drugs in chronic pain patients receiving short-acting (hydrocodone) or long-acting (methadone) opioids. Pain Physician 8: 257-261.
- Cone EJ, Caplan YH, Black DL, Robert T, Moser F (2008) Urine drug testing of chronic pain patients: Licit and illicit drug patterns. J Anal Toxicol 32: 530-543.
- Gourlay DL, Heit HA, Caplan YH (2010) Urine drug testing in clinical practice: The art and science of patient care. California Academy of Family Physicians. Stamford, CT: PharmaCom Group, Inc.
- Pesce A, West C, Egan-City C, Clarke W (2012) Diagnostic Accuracy and Interpretation of Urine Drug Testing for Pain Patients: An Evidence-Based Approach. In: Acree B (ed) Toxicity and Drug Testing. InTech, Croatia.
- Mikel C, Almazan P, West R, Crews B, Latyshev S, et al. (2009) LC-MS/MS extends the range of drug analysis in pain patients. Ther Drug Monit 31: 746-748.
- Depriest A, Heltsley R, Black DL, Cawthon B, Robert T, et al. (2010) Urine drug testing of chronic pain patients. III. Normetabolites as biomarkers of synthetic opioid use. J Anal Toxicol 24: 444-449.
- Ullucci PA, Cadoret R, Stasiowski PD, Martin HF (1978) A comprehensive GC/MS drug screening procedure. J Anal Toxicol 2: 33-38.
- Department of Health and Human Services (2008) Mandatory Guidelines and Revised Mandatory Guidelines for Federal Workplace Drug Testing Programs. Substance Abuse and Mental Health Services Administration, HHS 73: 228.
- Reisfield GM, Bertholf RL (2008) "Practical guide" to urine drug screening clarified. Mayo Clin Proc 83: 848-849.
- Reisfield GM, Goldberger BA, Bertholf RL (2009) 'False-positive' and 'false-negative' test results in clinical urine drug testing. Bioanalysis 1: 937-952.
- Dahn T, Gunn J, Kriger S, Terrell AR (2009) Quantitation of morphine, codeine, hydrocodone, hydromorphone, oxycodone, oxymorphone, and 6-monoacetylmorphine (6-MAM) in urine, blood, serum, or plasma using liquid chromatography with tandem mass spectrometry detection. In: Garg U, Hammler-Stabler C (eds). Clinical Applications of Mass Spectrometry: Methods and Protocols. Humana Press, New York.
- Mikel C, Pesce A, West C (2010) A tale of two drug testing technologies: GC-MS and LC-MS/MS. Pain Physician 13: 91-92.
- Pesce A, Rosenthal M, West R, West C, Crews B, et al. (2010) An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectrometry versus immunoassay drug testing in pain patients. Pain Physician 13: 273-281.
- Chace DH, Barr JR, Duncan MW, Matern D, Morris MR, et al. (2007) Mass Spectrometry in the Clinical Laboratory: General Principles and Guidance; Approved Guideline. Clinical and Laboratory Standards Institute (CLSI). 27: 24
- Crews B, Mikel C, Latyshev S, West R, West C (2009) 6-Acetylmorphine detected in the absence of morphine in pain management patients. Ther Drug Monit 31: 749-752.
- Mohsin S, Yang Y, Zumwalt M (2007) Quantitative analysis of opiates in urine using RRHT LC/MS/MS. Agilent Technologies, Inc.
- West R, Pesce A, West C, Crews B, Mikel C, et al. (2010) Comparison of clonazepam compliance by measurement of urinary concentration by immunoassay and LC-MS/MS in pain management population. Pain Physician 13: 71-78.
- Fitzgerald RL, Griffin TL, Yun YM, Godfrey RA, West R, et al. (2012) Dilute and shoot: Analysis of drugs of abuse using selected reaction monitoring for quantification and full scan product ion spectra for identification. J Anal Toxicol 36: 106-111.
- BlueCross BlueShield of Texas (2011) Most commonly prescribed drugs.
- Hornbeck CL, Carrig JE, Czarny RJ (1993) Detection of a GC/MS artifact peak as methamphetamine. J Anal Toxicol 17: 257-263.
- Barakat NH, Atayee RS, Best BM, Pesce AJ (2012) Relationship between the concentration of hydrocodone and its conversion to hydromorphone in chronic pain patients using urinary excretion data. J Anal Toxicol 36: 257-264.
- Hughes MM, Atayee RS, Best BM, Pesce AJ (2012) Observations on the metabolism of morphine to hydromorphone in pain patients. J Anal Toxicol 36: 250-256.
- Leimanis E, Best BM, Atayee RS, Pesce AJ (2012) Evaluating the relationship of methadone concentrations and EDDP formation in chronic pain patients. J Anal Toxicol 36: 239-249.
- Tse SA, Atayee RS, Best BM, Pesce AJ (2012) Evaluating the relationship of carisoprodol concentrations and meprobamate formation in pain patients. J Anal Toxicol 36: 221-231.
- Yee DA, Best BM, Atayee RS, Pesce AJ (2012) Observations on the urine metabolic ratio of oxycodone to oxymorphone in pain patients. J Anal Toxicol 36: 232-238.
- Evans M, Kriger S, Gunn J, Schwilke G (2009) Effective monitoring of opiates in chronic pain patients. Pract Pain Manage 9: 32-33.
- Haddox JD, Kupper RJ, Cone EJ (2011) The 27th Annual Meeting of the American Academy of Pain Medicine, Washington, DC.
- West R, Crews B, Mikel C, Almazan P, Latyshev S, et al. (2009) Anomalous observations of codeine in patients on morphine. Ther Drug Monit 31: 776-778.
- West R, West C, Crews B, Almazan P, Latyshev S, et al. (2011) Anomalous observations of hydrocodone in patients on oxycodone. Clin Chim Acta 412: 29-32
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16385
- [From(publication date):
specialissue-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11673
- PDF downloads : 4712