Case Report Open Access
Analysis of the Genomic Profile in Disseminated Peritoneal Leiomyomatosis: Three cases
Viviani V1, Cavillon V1*, Boudier E1, Averous V2, Croce S3, Sananes N1, Baldauf JJ1, Langer B1 and Akladios CY11Department of Obstetrics and Gynaecology, Hopitaux Universitaires de Strasbourg, Avenue Molière, France
2Department of Pathological Anatomy, Hopitaux Universitaires de Strasbourg, Avenue Molière, Strasbourg, France
3Department of Pathological Anatomy, Institut Bergonie, France
- Corresponding Author:
- Cavillon V
Department of Obstetrics and Gynaecology
Hopitaux Universitaires de Strasbourg
Avenue Molière 67200 Strasbourg France
Tel: 33388127503
E-mail: victor.cavillon@chru-strasbourg.fr
Received date: April 19, 2017; Accepted date: April 28, 2017; Published date: April 30, 2017
Citation: Viviani V, Cavillon V, Boudier E, Averous V, Croce S, et al. (2017) Analysis of the Genomic Profile in Disseminated Peritoneal Leiomyomatosis: Three cases. J Preg Child Health 4:319. doi:10.4172/2376-127X.1000319
Copyright: © 2017 Viviani V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Disseminated peritoneal leiomyomatosis (DPL) is a rare disease entity belonging to the category of smooth muscle tumours of uncertain growth. It is characterized by proliferation of multiple smooth muscle nodules in the peritoneal cavity mimicking a malignant process such as peritoneal carcinomatosis but which when studied histologically proves to be of benign nature. Its origin is still unknown. Genomic analysis of DPL cases is of interest in order to understand its pathogenesis and subsequent course, but there are few extant studies. In this article we set out the genomic profiles, analysed by array-based comparative genomic hybridization (array-CGH), of peritoneal and uterine lesions in two cases of DPL detected after previous uterine morcellation for fibroids, as well as a "sporadic" case of DPL. Array-CGH findings revealed in all three cases a flat genomic profile. It is not possible to establish a genetic lineage between two lesions on the basis of the genomic profiles alone, owing to the absence of an unbalanced rearrangement. CGH is not conclusive enough in this type of disorder. Analysis of the exome could provide us with fresh information, not least about driving events in the cancerogenesis of these tumours.
Keywords
Disseminated peritoneal leiomyomatosis; Array-CGH; Genomic profile
Introduction
Disseminated peritoneal leiomyomatosis (DPL) is a rare disease, which mimics peritoneal carcinomatosis. This disease entity, which essentially affects young women in their childbearing years, has been described in less than 200 cases in the literature. Generally speaking, the lesions are asymptomatic and detected fortuitously on imaging or during a surgical intervention. They may sometimes give rise to abdominal or pelvic pain, metrorrhagia or more rarely rectorrhagia. This pathological entity nevertheless remains difficult to diagnose (whether clinically or radiologically). Histological analysis is accordingly absolutely essential in order to confirm its benign nature. The natural history of the condition is generally towards spontaneous regression but some cases can develop into pelvic obstruction or lowgrade leiomyosarcoma [1-4]. There is no consensus about its management. If treatment is necessary, it should be determined by symptoms, the patient’s hormone status and her wish for future pregnancies. Treatment is based on blocking the hormonal stimulus by chemical castration (using a LH-RH analogue) or by administration of anti-oestrogens such as the aromatase inhibitors. Surgical management by nodule resection can be considered if medical treatment is not effective [5-7]. Although several aetiological hypotheses have been raised (hormonal, genetic, meta-plastic, etc.), our aim was to study by array-CGH the secondary implantation of myometrial cells during resection procedures in view of the fact that this lesion is commonly associated with the post-phase of myomectomy.
Material and Methods
We studied cases of disseminated peritoneal leiomyomatosis occurring between 2013 and 2016 at the Strasbourg University Teaching Hospitals. Leiomyomatosis was confirmed by histological analysis. Genomic profiles of peritoneal and uterine lesions (in cases where uterine fibroma morcellation had been carried out) were collated by array-CGH using microarrays (Agilent® 8 × 60 k microarrays, analysed with Agilent Cytogenomic software 3.0.6.6) using material fixed in formol and embedded in paraffin.
Results
Case 1
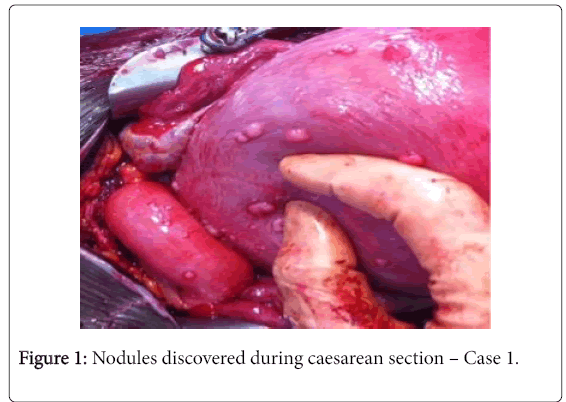
Patient A, aged 22, G2 P0, presented in spontaneous labour at 35 weeks’ gestation (WG) in March 2013. Her only previous history was a spontaneous miscarriage at 6 WG in 2012. A segmental transverse Caesarean was performed because of failure to engage on complete dilatation and an abnormal foetal heart rate. During the procedure, we discovered multiple disseminated nodules that resembled a peritoneal carcinomatosis (Figure 1).
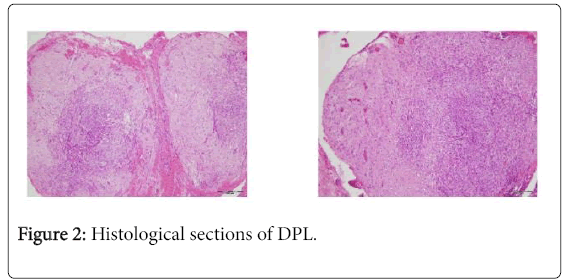
Fresh-mount and confirmatory microscopic study revealed a proliferation of spindle-shaped cells arranges in bundles without atypical or mitotic changes, which was suggestive of a disseminated peritoneal leiomyomatosis (Figure 2). The post-partum course was uncomplicated. Subsequently, the patient spontaneously became pregnant in 2014 (voluntary termination of pregnancy) followed by a twin pregnancy in 2015, which was monitored in our centre until 33 WG. The patient was thereafter lost to follow-up.
Case 2
The second patient B was a nulliparous primigravida aged 41 who had a Caesarean at 38 WG before onset of labour owing to uterine scarring and foetal myelomeningocoele. Her medical history included a hysteroscopic myomectomy 5 years before hand, and cystectomy for an ovarian endometrioma. During the Caesarean, we found multiple pelvic and mesenteric peritoneal nodules measuring about a centimetre. Histological analysis concluded that this was a DPL with some decidual zones.
Case 3
The third patient C, aged 42, presented at the emergency service with abdominal pain associated with diarrhoea and hot flushes. CTscan of the chest, abdomen and pelvis was performed and found a 2 cm sized isolated nodule in the small intestine suggestive of an intestinal neuroendocrine tumour. Octreotide scan to confirm this diagnosis did not reveal any somatostatin receptors. The patient underwent exploratory laparotomy which found multiples peritoneal nodules measuring about 3 cm. Histological analyses concluded that these were peritoneal leiomyomas in association with endometriosis.
This patient’s medical history included a subtotal hysterectomy with morcellation on account of bulky leiomyomas (1007 g) and hysteroscopic myomectomies, respectively 3 and 11 years beforehand.
Array-CGH analysis
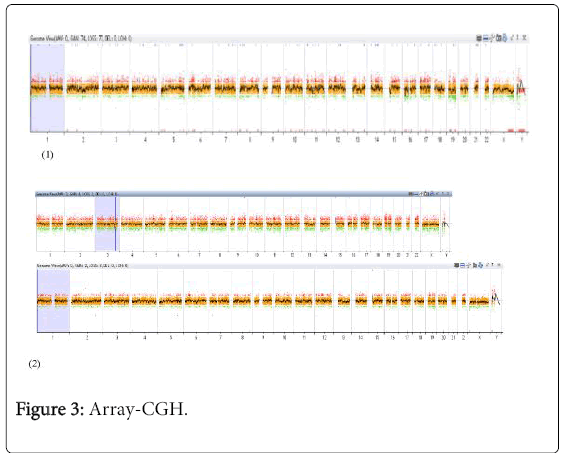
We conducted an array-CGH study of the peritoneal leiomyomas from these three patients. The objective was to compare the genomic profile of the uterine leiomyomas resected from patients B and C in order to verify whether there was a genetic lineage between these socalled “iatrogenic” cases, i.e. patients who had had a previous surgical procedure and in whom DPL was detected during surgery. We also studied the genomic profile of the “sporadic” case of DPL in patient A who had no history of previous surgery (Figure 3).
These profiles reveal lesions which have undergone very little rearrangement with few unbalanced elements.
On comparison of genomic profiles for each case, we were unable to observe either significant gains or losses, or an unbalanced rearrangement (e.g. HMGA2, HMGA1) between the uterine leiomyoma profile and the DPL profile. This finding would tend to support a genetic lineage between the resected tumour and DPL. We did not observe any major non-balanced difference between the profile for the first case, which corresponded to a so-called “sporadic” DPL and the profiles for the other two patients with “iatrogenic” DPL following uterine fibroma morcellation.
Discussion
Our study did not enable us to establish a genetic lineage between the two lesions based on their genomic profiles owing to the absence of an unbalanced rearrangement between the so-called “sporadic” case and the two cases observed in the post-phase of morcellation. This type of observation would not seem to uphold the hypothesis of peritoneal grafting.
Disseminated peritoneal leimyomatosis corresponds to an intraperitoneal proliferation of multiple nodules of smooth muscle cells which mimic peritoneal carcinomatosis. From the histological stand-point however, these cells have a mesenchymal and smooth muscle profile (desmin positive and alpha-smooth muscle actin) without atypia or necrosis, similar to that of fibroblasts and myofibroblasts, in which the mitotic index is low at less than 3 mitoses per 10 high power fields (HPF) [8]. These lesions may be observed over the entire peritoneal surface especially in the vicinity of the uterus and omentum as well as mesentery, colon, small intestine and pouch of Douglas.
In terms of pathogenesis, a hormonal origin has been suggested. In fact, most cases have been associated with a high level of oestrogen and progesterone (especially during pregnancy), with prolonged use of oral contraception or with secretory ovarian tumours [1,3]. Other studies would seem to suggest these lesions might develop from pluripotent mesenchymal cells on the peritoneal surface deriving from the Müllerian system [9,10]. Cytogenetic modifications have also been found in DPL. In their study, Quade et al. produced results supporting a monoclonal origin for DPL in view of the genetic impairment common to different nodules: inactivation of the X-chromosome, deteri-rations in chromosomes 7, 12 and 18 [11-13].
Ordulu et al. detected a case of DPL with the same chromosomal defects in different nodules: r(1)(p34.3q41),Del(3)(q23q26.33) and (12;14)(q14.3;q24.1) [14]. Wu et al. reported, in a case of re-current DPL following myomectomy, several deletions in chromosomes 7 and 22 and were able to confirm these deletions with array-CGH [15]. We did not find these types of chromosomal defects with array-CGH in our study, but it should be stressed that articles published to date have reported cytogenomic point mutations only: their recurrence and significance in tumour pathogenesis remains unknown [16-20].
Another hypothesis points towards secondary grafting of myometrial cells following laparoscopic myomectomy by morcellation or after hysterectomy because of multiple uterine fibroids [1,8]. These types of lesions have also been found at incision sites in the abdominal wall and are often associated with endometriosis [11]. This phenomenon of parasitic peritoneal leiomyomatosis, according to the literature, is due to the implantation and growth of viable leiomyoma particles disseminated inside the peritoneal cavity and is thought to be observed in about 0.9% of patients who undergo morcellation for removal of fibroids [21,22].
Parasitic leiomyomas are already known to be complication of laparoscopic morcellation of fibromas. Eleven cases of so-called “parasitic” DPL, i.e., occurring after morcellation, have been described in the literature.
The largest case series is a Chinese report with six affected patients [23]. In their article, the authors advance the hypothesis that iatrogenic DPL may be a distinct entity on account of the different nodule size, the failure of lesions to regress spontaneously and the few number of cases detected during pregnancy. This presupposes pathophysiological mechanisms different from those involved in “sporadic” DPL.
Such a hypothesis could be supported if the genomic profile of the parent tumour cells proved to be identical with that of the DPL cells. Array-CGH analysis enables global, high-resolution analysis of the genome by means of a rapid, sensitive and readily extrapolated method as well as detection of infracytogenetic anomalies. It serves as a pangenomic diagnostic test in clinical genetics [12]. This automatable method enables a very high number of genome regions to be tested by competitive hybridisation. Analyses of the genomic profiles that we undertook in the sporadic case (A) and in the cases following morcellation did not enable us to establish an indisputable genetic lineage between the lesions owing to the absence of unbalanced rearrangement in the genomic profiles of the sporadic case A (no previous medical history) and the genomic profiles of cases B and C.
In our study, array-CGH did not show unbalanced translocation. As is often the case, the profiles were therefore flat or had undergone little remodelling. It may be the case that another driving event underlies these pathologies (as, for instance, in desmoid tumours). Array-CGH is not conclusive in this type of pathology. It provides a comparative evaluation by means of hybridisation between the DNA which is being studied (tumour, leiomyomatosis, etc.) and a reference DNA or DNA pool. This technique assesses the logarithmic difference in the amount of DNA for different test probes. It is unable to demonstrate a lack of variation in the amount of DNA which might be present in a mutation, in a balanced rearrangement (balanced translocation) or a major intragenic rearrangement (in a gene exon). This “profiling” is a measurement, a partial viewpoint which does not register other genome events. The “whole genome” (sequencing introns and exons) or the exome which sequences lesional DNA exons may allow demonstration of mutations, intragenic deletions which are beyond the resolution power of array-CGH (e.g. 60 kbase).
Analysis of the exome could therefore provide new information, in particular concerning the driving events in the cancerogenesis of these tumours.
Hypothetical iatrogenic DPL due to tissue implantation can be suspected only clinically or by analysis of clonality.
Eight cases of malignant transformation to leiomyosarcoma have been reported in the literature. An interval of between 3 and 18 months is described between the occurrence of DPL and degeneration into a leiomyosarcoma [16]. Becker et al. have suggested that cases of DPL occurring when there is no hormonal stimulation, no hormonal receptor expression and no previous history of fibroma may correspond to a distinct entity in patients at high risk of malignant transformation [17]. Monitoring by imagery (tomodensitometry) is essential for at least a year owing to the small but existent risk of degeneration into a leiomyosarcoma. It is however not at all clear whether the malignant form of smooth muscle proliferation actually derives from the benign form. There is no ideal immunohistochemical study to distinguish a uterine leiomyoma from a leiomyosarcoma. Array-CGH studies have shown that 18 out of 26 uterine leiomyosarcomas were associated with leiomyoma-like morphological areas that were usual-type, symplastic or cellular in nature [18].
Ordulu et al. in a study on intravenous leiomyomatosis suggested that this entity might be an inter-mediate stage between benign smooth muscle tumours and malignant tumours [19]. His team hypothesised that unbalanced HMGA2 translocation with the supernumerary oncogenic derivative might promote passage of the leiomyoma into the blood vessels, thus manifesting a kind of vascular tropism. This is an additional hypothesis concerning the pathogenesis of disseminated peritoneal leiomyomatosis that remains to be tested.
Studying DPL is likely to take on importance owing to the rise in surgical treatment for fibroids as well as to the rise in hormonally induced pregnancies especially among patients receiving assisted reproductive techniques. Analysis of the genomic profile of these rare tumours may help medical practitioners to define appropriate methods for patient follow-up.
Conclusion
Genomic analysis of disseminated peritoneal leiomyomatosis by array-CGH was unable to establish a genetic lineage between two lesions based on their genomic profiles owing to the absence of unbalanced rearrangement between the so-called de novo case and the two cases observed in the post-phase of morcellation. Analysis of the exome could provide us with new items of information, in particular concerning the driving events in the cancerogenesis of these tumours.
References
- Al-Talib A, Tulandi T (2010) Pathophysiology and possible iatrogenic cause of leiomyomatosis peritonealis disseminata. Gynecol Obstet Invest 69: 239-244.
- Papadatos K (1996) CT of leiomyomatosis peritonealis disseminata mimicking peritoneal carcinomatosis. AJR Am J Roentgenol 167: 475-476.
- Tavassoli C (1982) Peritoneal leiomyomatosis: A clinic-pathologic study of 20 cases with ultrastructural observations. Int J Gynecol Pathol 1: 59-74.
- Altinok A (2000) Disseminated peritoneal leiomyomatosis. A benign entity mimicking carcinomatosis. Arch Gynecol Obstet 264: 54-55.
- Butnor KJ, Burchette JL, Robboy SJ (1999) Progesterone receptor activity in leiomyomatosis peritonealis disseminata. Int J Gynecol Pathol 18: 259-264.
- Hales HA, Peterson CM, Jones KP, Quinn JD (1992) Leiomyomatosis peritonealis disseminata treated with a gonadotropin-releasing hormone agonist. A case report. Am J Obstet Gynecol 167: 515-516.
- Parente JT, Levy J, Chinea F, Espinosa B, Brescia MJ (1995) Adjuvant surgical and hormonal treatment of leiomyomatosis peritonealis disseminate: A case report. J Reprod Med 40: 468-470.
- Bucher M, Pusztaszeri M, Bouzourene H (2006) Leiomyomatosis peritonealis disseminata: Immunohistochemical profile and origin. Ann Pathol 26: 207-210.
- Taubert HD (1965) Leiomyomatosis peritonealis disseminata, unusual complication of genital leiomyomata. Obstet Gynecol 25: 561-574.
- Batt RE, Smith RA, Buck Louis GM, Martin DC, Chapron C, et al. (2007) Müllerianosis. Histol Histopathol 22: 1161-1166.
- Ayano Toriyama (2013) Leiomyomatosis peritonealis disseminata co-existing with endometriosis within the same lesions: A case report with review of the literature. Int J Clin Exp Pathol 6: 2949-2954.
- Andrieux J (2008) Puces à ADN (CGH-array) Application pour le diagnostic de déséquilibres cytogénétiques cryptiques. Pathologie Biologie 56: 368-374.
- Quade BJ, McLachlin CM, Soto-Wright V, Zuckerman J, Mutter GL et al. (1997) Disseminated peritoneal leiomyomatosis: Clonality analysis by X chromosome inactivation and cytogenetics of a clinically benign smooth muscle proliferation. Am J Pathol 150: 2153-2166.
- Zehra O, Paola PC, Wilson WSC, Wai KC, Charles L, et al. (2010) Disseminated peritoneal leiomyomatosis after laparoscopic supracervical hysterectomy with characteristic molecular cytogenetic findings of uterine leiomyoma. Genes, Chromosomes Cancer 49: 1152-1160.
- Wu YT, Wu Y, Chen SC, Zhou F, Yang CB, et al. (2015) A novel molecular cytogenetic fin-ding of leiomyomatosis peritonealis disseminata. Gynecol Obstet Invest, p: 23.
- S. Fulcher RA, Szucs A (1998) Leiomyomatosis peritonealis disseminata complicated by sarcomatous transformation and ovarian torsion: Presentation of two cases and review of the litera-ture. Abdom Imaging 23: 640-644.
- Bekkers RL (1999) Leiomyomatosis peritonealis disseminata: does malignant transformation occur? A litterature review. Gynecol Oncol 75: 158-163.
- Mittal KR (2009) Molecular and immunohistochemical evidence for the origin of uterine leiomyosarcomas from associated leiomyoma and symplastic leiomyoma-like areas. Mod Pathol 22: 1303-1311.
- Ordulu Z, Nucci MR, Dal Cin P, Hollowell ML, Otis CN, et al. (2016) Intravenous leiomyomatosis: An unusual intermediate between benign and malignant uterine smooth muscle tumors. Mod Pathol 29: 500-510.
- Cucinella G, Granese R, Calagna G, Somigliana E, Perino A (2011) Parasitic myomas after laparoscopic surgery: An emerging complication in the use of morcellator? Description of four cases. Fertil Steril 96: e90-e96.
- Larraín D, Benoit R, Khoo CK, Botchorishvili R, Canis M, et al. (2010) Latrogenic parasitic myomas: Unusual late complication of laparoscopic morcellation procedures. J Minim Invasive Gynecol 17: 719-724.
- Tan-Kim J, Hartzell KA, Reinsch CS (2015) Uterine sarcomas and parasitic myomas after laparoscopic hysterectomy with power morcellation. Am J Obstet Gynecol 212: 594-594.
- Lu B, Xu J, Pan Z (2016) Latrogenic parasitic leiomyoma and leiomyomatosis peritonealis disseminata following uterine morcellation. J Obstet Gynaecol Res 42: 990-999.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 2740
- [From(publication date):
April-2017 - Dec 18, 2024] - Breakdown by view type
- HTML page views : 2064
- PDF downloads : 676