Research Article Open Access
Analysis of Pesticides in Water Samples and Removal of Monocrotophos by γ-Irradiation
Ismail M1,2*, Murtaza Sayed1, Hasan M Khan1 and William J Cooper21National Center of Excellence in Physical Chemistry, University of Peshawar, Pakistan
2Department of Civil and Environmental Engineering, University of California, USA
- *Corresponding Author:
- Muhammad Ismail
NCE in Physical Chemistry
University of Peshawar
Peshawar 25120, Pakistan
Tel: 0092-91-9216766
Fax: 0092-91-9216671
E-mail: ismail_wazirs@yahoo.com
Received date: December 04, 2013; Accepted date: January 11, 2014; Published date: January 14, 2014
Citation: Ismail M, Sayed M, Khan HM, Cooper WJ (2014) Analysis of Pesticides in Water Samples and Removal of Monocrotophos by γ-Irradiation. J Anal Bioanal Tech 5:181. doi: 10.4172/2155-9872.1000181
Copyright: © 2014 Ismail M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The analysis of 20 organochlorine pesticides has been carried out in surface water samples collected from Peshawar, KPK, Pakistan, using SPME-GC-ECD method. This method showed good liner response with R2 values in the range of 0.9933 to 0.9999 for all pesticides. Percent recoveries at 1 μg L-1 for all the pesticides ranged from 89.9 to 106.1%. A total of 59 surface water samples were tasted for 20 organochlorin pesticides residues, of which, 37 samples were found contaminated with γ-BHC, 12 samples with β-BHC, 25 samples with heptachlor and 25 samples with aldrin with a maximum concentration of 0.092, 0.053, 0.089 and 0.036 μg L-1, respectively. However other pesticides were found in lesser no. of water samples. The Gamma irradiation decomposition of monocrotophos aqueous solution at various concentrations (60-150 mg L-1) was carried out and their removal efficiency was investigated. Gamma irradiation showed 100% degradation for a 60 mg L-1 solution at an absorbed dose of 1200 Gy. The dose constants investigated in this study ranged from 1.4×10-3 to 3.0×10-3 Gy-1. The effect of saturated solutions of N2 and N2O, and radical scavengers such as tert-butanol and iso-propanol, on the degradation of monocrotophos were also studied. The results showed that degradation of monocrotophos mainly proceeded through oxidative •OH radical. The inorganic by-products NO3-, NH4+ and PO43- were quantitatively determined by ion chromatography. A detail mechanism pathway of monocrotophos degradation by irradition has been developed.
Keywords
Organochlorine pesticides; Monocrotophos; SPME-GCECD; IC
Introduction
Pesticides are chemical or biological products which are used in direct form, in aqueous solutions or as mixtures for preventing, fighting and killing a variety of pests, such as weeds, insects, rodents, and fungi [1]. The widespread occurrence of pesticides in natural waters is due to their widespread application in agriculture throughout the world [2]. Human being is constantly exposed to a large number of pesticides present in the environment.
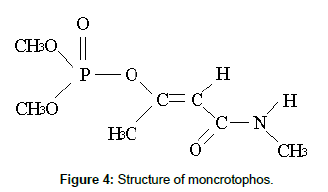
Surface and ground water contamination due to extensive use of pesticide for agricultural purposes is a serious threat to the environment. Most important among them is pollution due to OCPs, because of their high toxic effect and persistence for a long time in the environment. Pollution by some common pesticides, such as organophosphorous pesticides (OPPs) due to mistreatment, direct run off, empty containers careless discarding, leaching, and utensils and apparatus washings, etc. is also important [3]. Chlorinated pesticides have fatal toxic effects for aquatic life forms such as fish. These pesticides are counted hazardous for the environment and human beings as well, due to bioaccumulation and biomagnifying effects in the food chain and because they may produce reproductive and carcinogenic effects in animals and human beings [4]. The Guideline values recommended by WHO for various pesticide residues in drinking water are 2 μg L-1 (Lindane), 0.03 μg L-1 for (Aldrin/dieldrin), 2 μg L-1 (DDT), 0.2 μg L-1 (Chlordane), 0.03μg L-1 (Heptachlor + epoxide) and 20 μg L-1 (Methoxychlor) [5]. Monocrotophos (C7H14NO5P) is an organophosphorus compound and used as a systemic insecticide and acaricide. It prevents pests on a variety of crops, such as sugarcane, cotton, rice and fruits and vegetables etc. Monocrotophos is commonly used for agriculture purposes in Pakistan to control boring, chewing and sucking insects (such as aphids, Helicoverpaspp, caterpillars, moths, mites, jassids, budworm, scale and stem borer, as well as locusts) for a large range of crops. Unfortunately, the extensive use and easy approach of monocrotophos has resulted in enhanced utilization for homicidal and suicidal poisoning cases.
Monocrotophos is highly toxic compound and inhibits cholinesterase. It is very dangerous by all routes of exposure. Monocrotophos can be absorbed following ingestion, inhalation and skin contact. Monocrotophos can cause various diseases such as the damage of respiratory system, trigger runny nose, tears, pain, chest discomfort, coughing, localized sweating and involuntary muscle contractions, blurred vision and pupil constriction [6]. It is very toxic to bees, birds, mammals and aquatic invertebrates. The acute oral lethal dose (LD50) of monocrotophos is 21 mg kg-1 for rats [7]. According to World Health Organization, the ingestion of monocrotophos (120 mg) may be fatal to humans [6]. Monocrotophos is classified a highly hazardous pesticide (Class Ib) according to World Health Organization 2004 edition of the recommended classification of pesticides by hazard [8]. OPPs are a large group of pesticides, which replaced organochlorine pesticides due to their higher acute toxicity, lower accumulation in the environment and relatively higher decomposition rate. They are widely used since 1970s for pest control in agriculture and in households.
The widespread occurrence of pesticides in natural waters is due to their widespread application in agriculture throughout the world [9]. Several studies have showed that organophosphorus pesticides have endocrine-disrupting effects [10], mutagenicity [11], cytotoxicity [12] and immunosuppressive effects [13]. In addition, it is necessary to mention that the joined consequences of different pesticides may cause more toxic effects on human health [14]. Therefore, more concentration should be necessary for the removal of organophosphorus pesticides from the drinking water.
AOPs are very effective in destroying organic pollutants and overcome issues of air pollution in aeration stripping or regenerating the sorbent(s) used in conventional processes. AOPs are used in addition to the conventional processes, as advanced processing of treated wastewater also. Irradiation of aqueous solutions by ionizing radiation produces three primary transient species: eaq , •OH and •H that can oxidize or reduce contaminants. When using ionizing radiation as an AOP, pollutants can be oxidized or reduced in most cases to less toxic and in some cases to more biodegradable compounds that do not pose adverse environmental consequences [1]. Several studies have been reported using AOPs for degradation of various contaminants including pesticides and organic pollutants [15-20]. Organic pollutants are ultimately converted to carbon dioxide and water by AOPs [21]; however, the economic feasibility of this approach is often not acceptable. To evaluate the application of AOPs it is necessary to obtain fundamental data on degradation and formation of reaction by-products.
Among sources of environmental pollution, pesticides contamination is wide spread and is source of concern all over the world. Therefore, in this research work efforts has been made to determine the concentration levels of selected chlorinated pesticides contamination in water samples of selected areas of Khyber Pukhtoon Khwa, to provide useful information for the growing public awareness about chlorinated pesticides exposure, diseases and adverse effects caused due to pesticides contamination.
The present study focused on to degrade monocrotophos using gamma radiations as an AOP. The main objectives of present research work can be summarized as follow: (1) to study decomposition of monocrotophos solution in aqueous media by gamma radiation; (2) to determine the monocrotophos decomposition kinetics and effect of initial concentration on decomposition process; (3) to investigate the effects of different radical promoters and scavengers on the degradation of monocrotophos by gamma irradiation; (4) to determine pesticides contamination levels in water samples of selected areas.
Experimental
Extraction of pesticides from water
Normally, pesticides contamination in water is very low. Therefore, for the analysis of pesticides requires first good extraction and concentration. The solid-phase micro-extraction (SPME) is a fresh extraction method from liquid samples. This method needs no solvent and no need of complicated equipment. It includes the immersion of a fused silica rod coated with a stationary phase. Pesticides are adsorbed on the fiber first and then desorbed to the GC injector (for gas chromatography) or by liquid removing (for HPLC) [22].
Standards and reagents
The chlorinated pesticide mixture and monocrotophos (standards) were obtained from Supelco (Bellefonte, PA, USA) with >99% purity. The methanol and acetonitril (HPLC grade) was purchased from Merck (Darmstadt, Germany). All of the chemical reagents used (perchloric acid, sodium hydroxide, tert-butanol and iso-propanol) were highpurity analytical grade reagents purchased from Sigma-Aldrich. All aqueous solutions were prepared in deionized water. Milli-Q water purification system (Millipore, Bedford, MA, USA) was used for deionized water. High purity nitrogen gas (99.99% purity) was used in the present study.
Solutions preparation
10 PPM stock solution containing all the 20 organochlorine pesticides was prepared in methanol from 2000 PPM standard solution (Supelco, 47426-U, Bellefonte, PA, USA) and stored at 4°C. A fresh standard working solutions of various concentrations (0.01, 0.05, 0.1, 0.3, 0.5, 1 and 2 μg L-1) containing all 20 pesticides were prepared from 10 PPM stock solution in deionized water for calibration curves which were used for quantitative analysis of pesticides in water samples.
1000 ppm monocrotophos stock solution was prepared by dissolving solid monocrotophos in Milli-Q water. 60 mg L-1 aqueous solutions of monocrotophos were prepared from stock solution in deionized water for gamma radiolysis study. Different concentrations of various additives (tert-butanol and iso-propanol) were added into 60 mg L-1 monocrotophos aqueous solution to study their effects on decomposition of monocrotophos by gamma radiation. Aqueous solutions of different concentrations of monocrotophos (60-150 mg L-1) were prepared to examine the effect of initial concentration on degradation efficiency of monocrotophos in the same gamma irradiation absorbed dose.
Samples collection
The Peshawar district was selected for analysis of pesticides in water samples, which is an agricultural area. A total of 59 water samples (from fields as well as canals) were collected from various locations of district Peshawar. Figure 1 indicates various water samples sites of Peshawar area. All water samples were collected in washed and clean plastic bottles. To remove particulate matter from the samples, water samples were first filtered with wattman filter paper and then with 0.45 μm filter paper. After filtration samples were stored in 20 ml dark glass vials for GC determination. A DVB-PDMS fiber was used for pesticides extraction from water samples.
Irradiation process
Cesium-137 source (J. L. Shepherd Mark I Model A68 Irradiator) was used for gamma radiolysis. It has a fixed Cesium-137 source central rod in a cavity 30 cm in diameter and 33 cm high. The Cesium-137 gamma radiation source was available in the University of California, Irrvine, CA, United States.
A Cesium-137 gamma radiation source was calibrated by Fricke dosimetry. The dose rate of source was 2 kGy h-1. All the aqueous monocrotophos solutions were placed in 20 mL air tight glass vials for desired series of gamma radiation doses. All experiments were performed at room temperatures.
Analytical instrumentation
A DVB-PDMS fiber was used for pesticides extraction from water samples obtained from Supelco (Bellefonte). Chromatographic analysis was performed on an Agilent gas chromatograph (6890 N series, Agilent Technologies, Palo, CA, USA) equipped with (ECD) electron capture detector (63Ni) and split/splitless injector. Column used was Hp-5 fused-silica capillary column, 30 m×0.32 mm i.d. and 0.25 μm film thicknesses from Agilent. The operating conditions for GC were as follows: starting oven temperature was 80°C for 4 m, then it is increased at 15°C min−1 to 215°C and remains constant for 30 s. Then it is increased again at 2°C min−1 to 230°C and remains constant for 3 m at this temperature. After this it is increased at 10°C min−1 and reached to 260°C. The detector temperature was 300°C and injector temperature was 250°C. A single GC run was completed in 28 min. N2 gas (99.999% purity) was used as a make-up and carrier gas at a flow rate of 45 ml min−1 and 1 ml min−1, respectively. The extraction time for fiber was (45 min), desorption time (2 min), and agitator speed (250 rpm) were selected.
The monocrotophos solutions were analyzed by HPLC (Agilent Technologies) equipped with a Phenomenex Gemini C18 column (4.6×250 mm). The mobile phase used was the mixtures of acetonitrile and water (20:80%) at a flow rate of 1.2 mL min-1. UV detector at λmax of 210 nm was used. The HPLC/MS system equipped with an 1100 HPLC Pump (Agilent) and a Waters LCT Classic Mass Spectrometer. The Mass Spectrometer equipped with an electrospray ionization source. The by-product formed during gamma irradiation of monocrotophos was analyzed by Ion Chromatography. Metrohm Ion- Chromatograph (IC) equipped with electro chemical anion self-regenerating suppressor, conductivity detector, analytical column Metrosep Assup-7 was used for analysis of by-product.
Results and Discussions
Pesticide residues in water samples of Peshawar
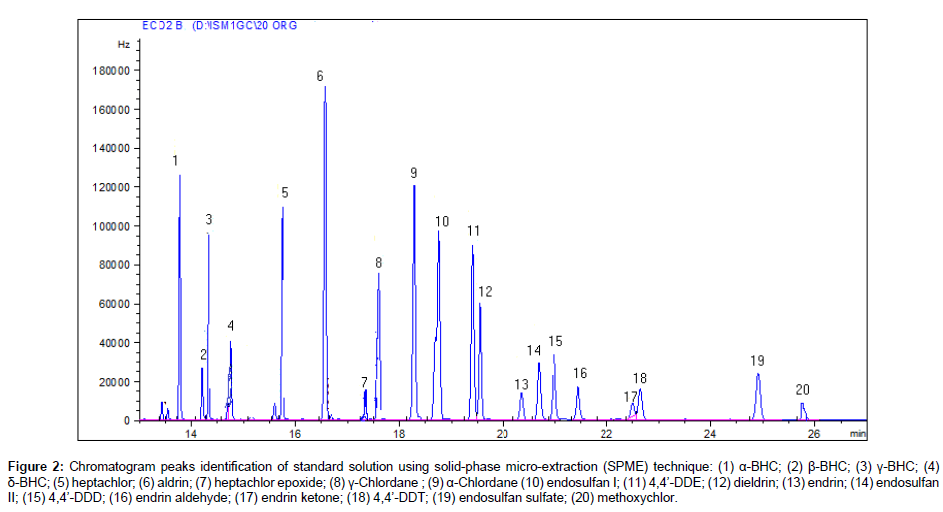
The chromatogram using solid-phase micro-extraction (SPME) of the 20 organochlorine pesticides standard is shown in Figure 2. All the organochlorines are eluted within a very reasonable time, about 28 min and well resolved under the optimized GC conditions. The extractions of pesticides from water were performed in 20 mL dark glass vials at room temperature with the SPME fiber immersed in the solution. To investigate an SPME procedure for the analysis of organochlorine pesticides, optimization of several variables related to the extraction and desorption steps are required to achieve maximum efficiency. The parameters used were extraction time (45 m), desorption time (90 s), pH of solution (6.2), agitation and stirring speed (250 rpm). After extraction of pesticides by SPME fiber, the analytes were desorbed into the hot injector of the GC by thermal desorption. All studies were carried out in triplicate and average values were calculated.
Figure 2: Chromatogram peaks identification of standard solution using solid-phase micro-extraction (SPME) technique: (1) α-BHC; (2) β-BHC; (3) γ-BHC; (4) δ-BHC; (5) heptachlor; (6) aldrin; (7) heptachlor epoxide; (8) γ-Chlordane ; (9) α-Chlordane (10) endosulfan I; (11) 4,4’-DDE; (12) dieldrin; (13) endrin; (14) endosulfan II; (15) 4,4’-DDD; (16) endrin aldehyde; (17) endrin ketone; (18) 4,4’-DDT; (19) endosulfan sulfate; (20) methoxychlor.
The analytical parameters evaluated to validate the method were linearity, selectivity, accuracy, precision and detection limits. The method used to quantify the analytes of interest was using calibration curves obtained by plotting peak area versus pesticide concentration. The linearity was evaluated by linear regression analysis, which was calculated by the least square regression method and linearity was observed at different concentrations. The calibration curves of 20 organochlorine compounds at various concentrations (0.01, 0.05, 0.1, 0.3, 0.5, 1 and 2 μg L-1) were obtained by plotting peak areas verses concentration. A good linear correlation between the chlorinated pesticides concentrations and peak areas were found with R2 values in the range of 0.9933 to 0.9999 (Table 1). Lower limits of detection were calculated based on a signal-to-noise ratio (S/N) 3:1. The R2 values and lower limits of detection are shown in Table 1. The lower limits of detection for all 20 organochlorine pesticides were found very less than their maximum contamination level.
| No | Compound | Linear range (µg L-1) | R2 | LOD (µg L-1) | Guideline value (µg L-1) |
|---|---|---|---|---|---|
| 1 | α-BHC | 0.01-1 | 0.9995 | 0.003 | - |
| 2 | β-BHC | 0.05-1 | 0.9996 | 0.006 | - |
| 3 | γ-BHC | 0.01-1 | 0.9998 | 0.002 | 2, 0.2b |
| 4 | δ-BHC | 0.01-1 | 0.9995 | 0.003 | - |
| 5 | Heptachlor | 0.01-1 | 0.9996 | 0.004 | 0.03, 0.4b |
| 6 | Aldrin | 0.01-1 | 0.9999 | 0.002 | 0.03 |
| 7 | Heptachlor epoxide | 0.05-2 | 0.9980 | 0.02 | 0.03, 0.2b |
| 8 | γ-Chlordane | 0.01-1 | 0.9997 | 0.003 | 0.2, 2b |
| 9 | α- Chlordane | 0.01-1 | 0.9995 | 0.002 | 0.2, 2b |
| 10 | Endosulfan I | 0.01-1 | 0.9998 | 0.002 | 0.05a |
| 11 | 4,4'-DDE | 0.01-1 | 0.9997 | 0.004 | - |
| 12 | Dieldrin | 0.01-1 | 0.9998 | 0.002 | 0.03 |
| 13 | Endrin | 0.01-1 | 0.9944 | 0.004 | 2b |
| 14 | Endosulfan II | 0.01-1 | 0.9998 | 0.003 | 0.05a |
| 15 | 4,4'-DDD | 0.01-1 | 0.9957 | 0.005 | - |
| 16 | Endrin aldehyde | 0.01-1 | 0.9976 | 0.004 | - |
| 17 | Endrin ketone | 0.05-1 | 0.9968 | 0.003 | - |
| 18 | 4,4'-DDT | 0.05-1 | 0.9955 | 0.009 | 2 |
| 19 | Endosulfan sulfate | 0.01-1 | 0.9999 | 0.002 | - |
| 20 | Methoxychlor | 0.05-2 | 0.9933 | 0.03 | 20 |
WHO guideline value, a: Australia guideline value, b: U.S. EPA maximum permissible level LOD: Lower limits of detection
Table 1: The Linear range, R2 values and lower limits of detection for selected pesticides.
As the solid-phase micro-extraction (SPME) technique is a nonexhaustive extraction procedure, the relative recovery, which is defined as the ratio of GC peak areas of spiked ground water sample extracts to spiked Milli-Q water extracts, was employed. Relative recovery tests were carried out spiking the real sample with standards (1 and 2 μg L-1) and values are shown in Table 2. The Percent recovery found at 1 μg L-1 and at 2 μg L-1concentration of pesticides ranged from 89.87 ± 3.9 to 106.05 ± 2.3 and 88.50 ± 2.6 to 109.22 ± 2.9, respectively. All These results show that SPME-GC-ECD technique is an excellent and valid method for the analysis of chlorinated pesticides in real water samples.
| No | Compound | Retention times | 1 µg L-1 | 2 µg L-1 |
|---|---|---|---|---|
| 1 | α-BHC | 13.78 | 96.19 ± 0.9 | 97.39 ± 97.39 |
| 2 | β-BHC | 14.18 | 106.05 ± 2.3 | 109.22 ± 2.9 |
| 3 | γ-BHC | 14.34 | 98.21 ± 1.7 | 102.89 ± 2.4 |
| 4 | δ-BHC | 14.78 | 97.32 ± 3.5 | 98.08 ± 1.8 |
| 5 | Heptachlor | 15.74 | 90.56 ± 4.3 | 89.36 ± 3.4 |
| 6 | Aldrin | 16.57 | 98.14 ± 2.8 | 96.79 ± 1.9 |
| 7 | Heptachlor epoxide | 17.33 | 89.87 ± 3.9 | 91.81 ± 2.7 |
| 8 | γ-Chlordane | 17.60 | 95.36 ± 1.2 | 96.02 ± 3.1 |
| 9 | α-Chlordane | 18.30 | 92.78 ± 2.5 | 88.50 ± 2.6 |
| 10 | Endosulfan I | 18.77 | 93.52 ± 2.8 | 95.10 ± 4.1 |
| 11 | 4,4'-DDE | 19.43 | 91.35 ± 4.6 | 90.25 ± 3.8 |
| 12 | Dieldrin | 19.57 | 96.89 ± 1.8 | 98.39 ± 2.9 |
| 13 | Endrin | 20.35 | 101.08 ± 2.2 | 97.04 ± 1.7 |
| 14 | Endosulfan II | 20.72 | 103.78 ± 0.8 | 107.90 ±3.2 |
| 15 | 4,4'-DDD | 21.02 | 94.81 ± 2.4 | 95.70 ± 2.8 |
| 16 | Endrin aldehyde | 21.45 | 98.18 ± 0.6 | 99.53 ± 0.7 |
| 17 | Endrin ketone | 22.53 | 103.72 ± 1.9 | 105.95 ± 2.3 |
| 18 | 4,4'-DDT | 22.64 | 87.51 ± 3.7 | 90.15 ± 4.1 |
| 19 | Endosulfan sulfate | 24.91 | 97.98 ± 1.2 | 96.75 ± 3.3 |
| 20 | Methoxychlor | 25.81 | 96.89 ± 1.5 | 95.02 ± 3.9 |
Table 2: Percent recovery of selected pesticides at two concentration levels by using SPME-GC-ECD technique.
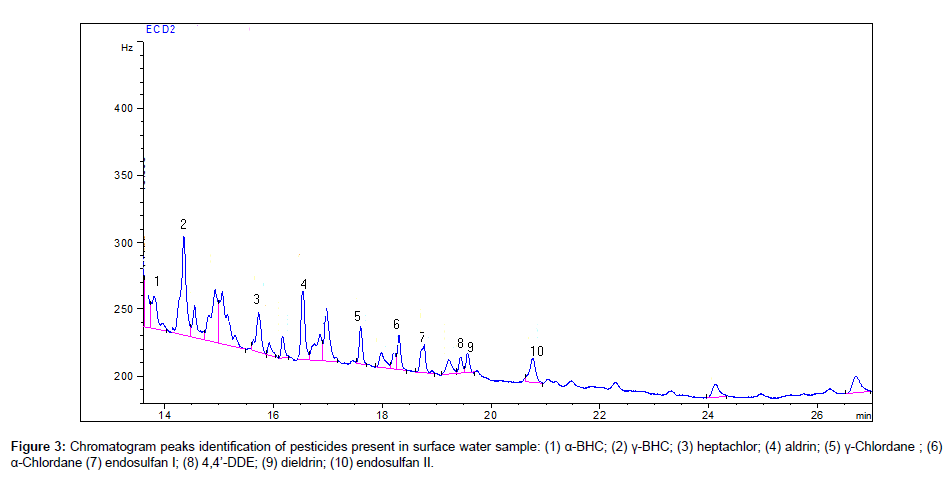
The results of determination of pesticides in the surface water samples collected from Peshawar area are summarized in Table 3. A total of 59 surface water samples were tasted for 20 organochlorin pesticides residues, of which most of the samples were found contaminated with various pesticides. Among the various pesticides analyzed, β-BHC, γ-BHC, heptachlor, aldrin, α-chlordane, endosulfan I, 4,4’-DDE and dieldrin were detected in most of the water samples. The representative chromatograms of 20 organochlorine pesticides and some pesticides contamination in surface water samples from Peshawar area are given in Figures 2 and 3. The identification of each chlorinated pesticide present in the water samples were confirmed by comparing their retention times with the standards of chlorinated pesticides.
| No | Compound | Total samples tested | Contaminated samples | Range (µg L-1) |
|---|---|---|---|---|
| 1 | α-BHC | 59 | 2 | Nd - 0.022 |
| 2 | β-BHC | 59 | 12 | Nd - 0.053 |
| 3 | γ-BHC | 59 | 37 | Nd - 0.092 |
| 4 | δ-BHC | 59 | 5 | Nd - 0.087 |
| 5 | Heptachlor | 59 | 25 | Nd - 0.089 |
| 6 | Aldrin | 59 | 15 | Nd - 0.036 |
| 7 | Heptachlor epoxide | 59 | 0 | - |
| 8 | γ-Chlordane | 59 | 0 | - |
| 9 | α-Chlordane | 59 | 6 | Nd - 0.013 |
| 10 | Endosulfan I | 59 | 8 | Nd - 0.014 |
| 11 | 4,4'-DDE | 59 | 12 | Nd - 0.036 |
| 12 | Dieldrin | 59 | 17 | Nd - 0.011 |
| 13 | Endrin | 59 | 0 | - |
| 14 | Endosulfan II | 59 | 5 | Nd - 0.028 |
| 15 | 4,4'-DDD | 59 | 0 | - |
| 16 | Endrin aldehyde | 59 | 2 | Nd - 0.026 |
| 17 | Endrin ketone | 59 | 0 | - |
| 18 | 4,4'-DDT | 59 | 0 | - |
| 19 | Endosulfan sulfate | 59 | 1 | Nd - 0.007 |
| 20 | Methoxychlor | 59 | 0 | - |
Table 3: Brief description of selected pesticides contamination in surface water samples of Peshawar area.
In 59 surface water samples of Peshawar, 37 samples were found contaminated with γ-BHC, 12 samples with β-BHC, 25 samples with heptachlor and 25 samples with aldrin with a maximum concentration of 0.092, 0.053, 0.089 and 0.036 μg L-1, respectively. γ-BHC (Lindane) has been used to treat food crops, seed treatment, soil treatment, forestry products and to treat livestock and pets. It was used widely in cultivating cocoa, cotton, sugar cane, coffee and others crops. BHC isomers can undergo long-range transport and be deposited into aquatic systems, where it can bioaccumulate in the food chain. Heptachlor has been used to kill soil insects and termites, to control grasshoppers, cotton insects and some crop pests, particularly infecting corn.6 samples of α-chlordane, 8 samples of endosulfan I, 12 samples of 4,4’-DDE and 17 samples of dieldrin were found contaminated with a maximum concentration of 0.013, 0.014, 0.036 and 0.011 μg L-1, respectively. The endosulfan I was predominant than the other endosulfans. The 4,4’-DDE is the byproduct of DDT degradation and is more stable in the environment. In surface water samples of Peshawar, the pesticides heptachlor epoxide, γ-chlordane, endrin, 4,4’-DDD, endrin ketone, 4,4’-DDT and methoxychlor were not detected in any samples in the present study. However, α-BHC, δ-BHC, endosulfan II, endrin aldehyde and endosulfan sulfate were found only in 2, 5, 5, 2 and 1 surface water samples of Peshawar, respectively.
Decomposition of monocrotophos aqueous solution by gamma radiation
In the present study gamma irradiation of aqueous solutions of monocrotophos was investigated as a surrogate for the application of advanced oxidation processes, such as the electron beam process [23]. The structure of monocrotophos is given in Figure 4. Gamma irradiation of aqueous media results in the formation of three reactive species, the hydrated electrons e-aq, hydroxyl radicals •OH, hydrogen radicals •H, and less reactive species, which are given by the following equation [24];
H2O ~~~~>e-aq (0.27) + H•(0.06) + •OH(0.28) + H2 (0.05) + H2O2 (0.07) + H3O+(0.27) (1)
where, the numbers in parenthes are radiation yield (G value) of each species in μmol J-1 absorbed energy. The three reactive species can react with each other or with other solutes present in the solution [25].
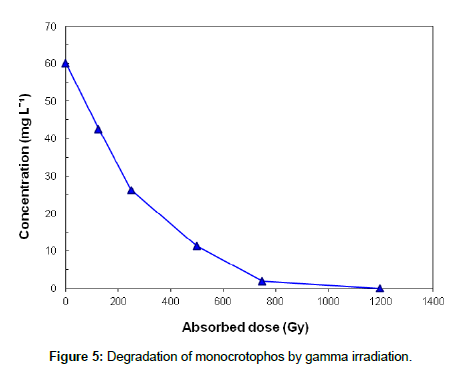
A decay curve of monocrotophos by gamma radiation is shown in Figure 5, where the solute was almost completely degraded at 1200 Gy. The degradation of monocrotophos follows first order kinetics with respect to dose.
Calculation of percent degradation efficiency (η)
Percent degradationefficiency(η) was calculated by the following formula [1]:
η = [(C0-CD)/C0] ×100 (2)
where, C0 is the initial concentration of monocrotophos at zero dose and CD is the concentration at a specified dose.
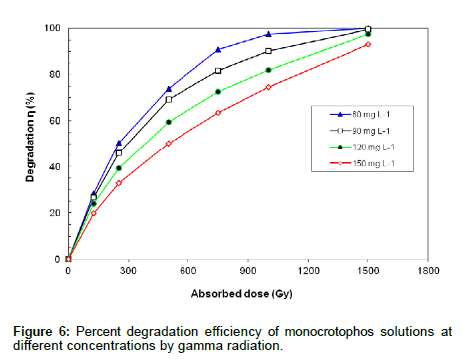
Different concentrations of monocrotophos (60-150 mg L-1) were prepared in deionized water and irradiated up to 1500 Gy. All aqueous solutions were saturated with N2 prior to gamma irradiation and η was calculated. Figure 6 shows the % degradation efficiency of monocrotophos solutions at different concentrations at various doses of gamma radiation.The data shows that η increased with increased radiation dose. At a given dose, the η decreased as the concentration of monocrotophos increased, presumably due to competition for the reactive species by the reaction by-products produced.
Effect of N2-saturated N2O-saturated and dissolved oxygen gases on monocrotophos degradation
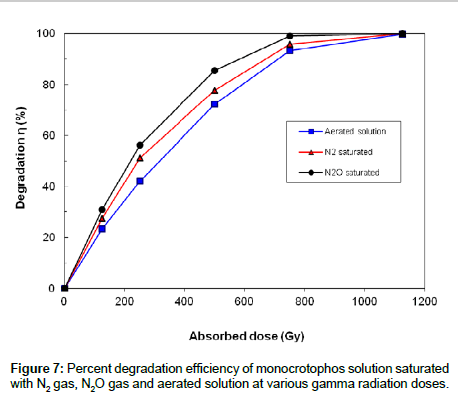
Experiments were performed to study effect of various gases on the decomposition of monocrotophos solution by gamma radiation. Aqueous solutions of 60 mg L-1 monocrotophos saturated with N2, N2O and dissolved oxygen were irradiated with doses of 0, 125, 250, 500, 750 and 1125 Gy in 20 ml glass vials by gamma radiation. Figure 7 shows the effect of dissolved oxygen, N2-saturated and N2O-saturated gases on monocrotophos degradation by gamma irradiation. Figure 7 indicates that monocrotophos was degraded in all the three cases and with the higher absorbed dose, η were increased. However the η in the N2O saturated solution was higher than that in the N2 saturated solution. The η in the N2 saturated solution was higher than that in aerated solution.
In solutions of monocrotophos saturated with N2O, the e-aq and H• atoms are converted to •OH making the concentration of •OH radicals more than double as shown below [25];
e-aq + N2O + H2O → •OH + N2 + OH- k= 9.1 x 109 L/mol.s (3)
H. + N2O → •OH + N2 k= 2.1 x 106 L/mol.s (4)
In nitrous oxide saturated condition, the •OH is the major radical that attack on the monocrotophos. Whereas in nitrogen saturated solution of monocrotophos, all the three radicals i.e. e-aq, •OH and •H may attack on monocrotophos. In aerated solution, oxygen can react with e-aq and •H, and forming O2•–and HO•2 by following equation;
H. + O2 → HO•2 k = 2.1×1010 L/mol.s (5)
e-aq + O2 → O2•– k = 1.9×1010 L/mol.s (6)
Due to this reason some decrease occurs in the degradation efficiency of monocrotophos. It shows that •OH is the major radical that play the significant role in the degradation process of monocrotophos. The other radical species (•H and e-aq) play role in the degradation process of monocrotophos but have lesser contribution.
Determination of radiation chemical yield (G-value)
Radiation chemical yield (G–value) is defined as the number of species of products formed or of the reactants destroyed with the absorption of 100 eV of radiation energy. It can be calculated using the following equation [26];
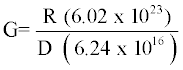 (7)
(7)
Where R is the change in the concentration of pollutant [M]. D is the absorbed dose (Gy), 6.02×1023 is the Avogadro’s number and 6.24×1016 is the conversion factor from Gy to 100 eV/L.
Table 4 shows the G-values of monocrotophos solution saturated with N2 gas, N2O gas and aerated solution at various gamma radiation doses. The results indicate that the G-values of monocrotophos solution ranged from 2.305-6.432, 2.305-5.716, and 2.301-4.824 molecules/100 eV for N2O saturated, N2 saturated and aerated solution, respectively, at various doses of gamma radiation. At all condition a decrease in G-values with increasing irradiation doses were observed. It is probably owing to the presence of intermediate reaction by products and their possible competition for different reactive species with parent compound.However, G-values were found somewhat higher for N2O saturated, followed by N2 saturated and lastly for aerated solution at the same given dose. It is due to higher degradation rate of monocrotophos in N2O saturated solution than that in the N2 saturated solution and in aerated solution.
| Absorbed dose (Gy) | G-values (molecules /100 eV) | ||
|---|---|---|---|
| Aerated | N2 sat urated | N2O sa turated | |
| 0 | - | - | - |
| 125 | 4.824 | 5.716 | 6.432 |
| 250 | 4.364 | 5.322 | 5.827 |
| 500 | 3.746 | 4.035 | 4.436 |
| 750 | 3.227 | 3.312 | 3.422 |
| 1125 | 2.301 | 2.305 | 2.305 |
Table 4: G-values of monocrotophos solution saturated with N2 gas, N2O gas and aerated solution at various gamma radiation doses.
Determination of dose constant and kinetic study
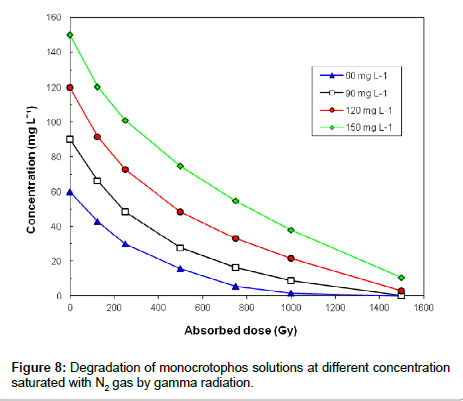
The dose constant, k, was obtained by plotting the natural logarithm (ln) of the monocrotophos concentration [M] versus dose (Gy). Figure 8 shows gamma radiation degradation of monocrotophos solution saturated with N2 gas at four different concentrations i.e. 60, 90, 120 and 150 mg L-1. At 1200 Gy dose of gamma irradiation, 60 μg L-1 aqueous solution of monocrotophos was completely degraded. It is clear from the Figure 8 that concentration of monocrotophos decreases exponentially with γ-irradiation absorbed doses, which can be represented by equation [27].
Cd=C0e-kD (8)
Where Cd is the concentration of monocrotophos after radiation dose, C0 is the initial concentration of monocrotophos, k is the dose constant and D is the absorbed dose. This equation is similar to pseudo-firstorder reaction.The above equation can also be modified as;
-ln (Cd/C0) =kD (9)
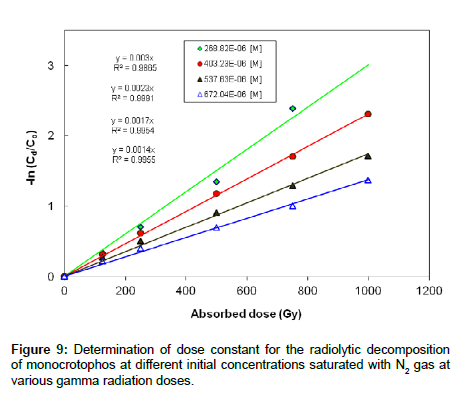
Experiments were carried out to investigate the effect of initial concentration on the decomposition of monocrotophos using γ-irradiation. The aqueous monocrotophos solutions saturated with N2 gas were irradiated to various doses. Figure 9 shows the decomposition kinetics of monocrotophos by γ-irradiation as a function of adsorbed dose, for initial concentrations of 60,90, 120 and 150 mg L-1(268.8×10-6, 403.2×10-6, 537.6×10-6 and 672.0×10-6 M, respectively) at a dose rate of 2 kGy h−1. The kinetic experiments were performed in triplicate for each radiation dose. It is clear that decomposition was more efficient at lower concentrations than at higher concentration suggesting that the formation of reaction by-products competed with the initial degradation of monocrotophos.
The dose constant decreased with the higher initial concentration of monocrotophos and increased with the lower initial concentration of monocrotophos as shown in Figure 9. It is clear that dose constant markedly decreased with higher initial monocrotophos concentration, from 3.0×10-3 Gy−1 for a concentration of 268.82×10-6 M to 1.4×10-3 Gy−1 for a concentration of 672.04×10-6 M. This reveals that monocrotophos can be degraded at higher rate at smaller concentrations as compared to larger concentration. It can also be explained that higher radiation dose will be required with the increasing concentration of monocrotophos. These results are comparable with the earlier reported results in literature [1,27,28].
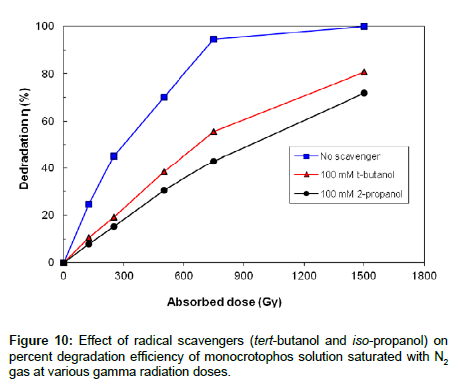
Effect of iso-propanol and tert-butanol additives on degradation of monocrotophos
Iso-propanol and tert-butanol were used as radical scavengers to investigate that which species play the main role in the gamma radiation degradation of monocrotophos. Figure 10 shows the effect of iso-propanol and tert-butanol on the irradiation degradation process of monocrotophos solution saturated with nitrogen gas. Iso-propanol has very high rate constant with •OH and •H radical (1.9×1010 L/mol.s and 7.4×107 L/mol.s, respectively). It can scavenge •OH and •H and less amount of these radicals will be available for target compound in the present of this scavenger [25]. Whereas tert-butanol also reacts very fast with •OH (6.0×108 L/mol.s) and scavenging •OH as shown below;
•OH + (CH3)2 CHOH -------> (CH3)2• COH+H2O (10)
H• + (CH3)2 CHOH -------> (CH3)2• COH +H2 (11)
•OH + CH3C (CH3)2 OH ------> •CH2C (CH3)2OH + H2O (12)
In the presence of iso-propanol, e-aq plays the key role while in the presence of tert-butanol, H• and e-aq play the predominant role. Figure 10 shows that iso-propanol and tert-butanol greatly inhibits the irradiation degradation process of monocrotophos, as both additives scavenging •OH radical, however, the degradation rate is somewhat lower in iso-propanol solution as compared to the tert-butanol solution because iso-propanol has high rate constant with •OH and H• as compared to the tert-butanol. So it is clear from the Figure 10 that •OH plays significant role while H• and e-aq also take part in the degradation process but play a minor role in the gamma degradation process of monocrotophos.
By-product analysis
Monocrotophos solution can be effectively degraded by gamma irradiation. The by-product formed during gamma irradiation of monocrotophos were analysed by Ion Chromatography. Metrohm Ion- Chromatograph (850 Professional IC, 872 Extension Module with 858 Professional sample Processor) equipped with electro chemical anion self-regenerating suppressor, conductivity detector and analytical column Metrosep Assup-7 (250/4.0 mm) was used for analysis of byproduct. The eluent used was 0.36 M sodium carbonate and suppressor reagent solution 0.1 M H2SO4 was used with a flow rate of 0.8 ml/min.
The inorganic reaction by-products of monocrotophos degradation, Nitrate and phosphate anions were determined at various irradiation doses. Some low molecular weight organic acids i.e., formic acid and acetic acid were also determined as reaction by-products of monocrotophos degradation at various irradiation doses. Figure 11 summarizes the formation of reaction by-products of monocrotophos degradation i.e. Nitrate, phosphate, formate, acetate, NH4+ and CH3NH2. The quantitative conversion of the organic phosphorus was observed at 8 KGy. These results suggest that nearly complete degradation of monocrotophos was achieved to inorganic anions at dose of about 8 KGy.
Conclusions
The SPME-GC-ECD technique is an excellent and reliable method for the determination of various pesticides in water samples. This method showed good liner response with R2 values in the range of 0.9933 to 0.9999 and good percent recoveries for all 20 organochlorin pesticides. The results of present study demonstrated that the gamma irradiation treatment was very effective in the degradation of aqueous monocrotophos solution. 100% degradation η of 60 mg L-1 monocrotophos solution saturated with N2 gas was achieved at 1200 Gy dose of gamma radiation. The kinetic study showed that the gamma irradiation degradation of monocrotophos followed pseudo first-order reaction. The results showed that monocrotophos degradation takes place mainly due to •OH radicals, because the maximum % degradation η was achieved when the production of •OH radicals was enhanced in the solution.
The G-value decreased with higher doses of gamma irradiation. The effect of N2O and N2 gases showed that decomposition rate of monocrotophos in the N2O saturated solution was greater than those saturated with N2. The study of various radical scavenger experiments indicated that oxidative radical •OH, were mainly responsible for irradiation decomposition of monocrotophos. However, e-aq and •H, has less contribution to the decomposition of monocrotophos. In conclusion the application of radiation technology is very effective and promising to destroy and remove pesticides and organic pollutants from contaminated water.
Acknowledgements
We are thankful to the Higher Education Commission of Pakistan for financial assistant and partial support funded by the National Science Foundation grant CBET-1034555 for this research work
References
- Ismail M, Khan HM, Sayed M, Cooper WJ (2013) Advanced oxidation for the treatment of chlorpyrifos in aqueous solution. Chemosphere 93: 645-651.
- Gao J, Liu L, Liu X, Zhou H, Lu J, et al. (2009) The occurrence and spatial distribution of organophosphorous pesticides in Chinese surface water. Bull Environ Contam Toxicol 82: 223-229.
- Sankararamakrishnan N, Kumar Sharma A, Sanghi R (2005) Organochlorine and organophosphorous pesticide residues in ground water and surface waters of Kanpur, Uttar Pradesh, India. Environ Int 31: 113-120.
- Di Bella G, Licata P, Bruzzese A, Naccari C, Trombetta D, et al. (2006) Levels and congener pattern of polychlorinated biphenyl and organochlorine pesticide residues in bluefin tuna (Thunnusthynnus) from the Straits of Messina (Sicily, Italy). Environ Int 32: 705-710.
- Hamilton DJ, Ambrus A, Dieterle RM, Felsot AS, Harris CA, et al. (2003) Regulatory limits for pesticide residues in water (IUPAC Technical Report). Pure Appl Chem 75: 1123-1155.
- World Health Organization (WHO 1993) International Programme on Chemical Safety: Monocrotophos: health and safety guide. Geneva, Switzerland.
- Janghel EK, Rai JK, Rai MK, Gupta VK (2006) A new and highly sensitive spectrophotometric determination of monocrotophos in environmental, agricultural and biological samples. J Chin Chem Soc 53: 343-347.
- World Health Organization (2004) The WHO recommended classification of pesticides by hazard and guidelines to classification. Geneva, Switzerland.
- Hildebrandt A, Guillamón M, Lacorte S, Tauler R, Barceló D (2008) Impact of pesticides used in agriculture and vineyards to surface and groundwater quality (North Spain). Water Res 42: 3315-3326.
- Kitamura S, Suzuki T, Ohta S, Fujimoto N (2003) Antiandrogenic activity and metabolism of the organophosphorus pesticide fenthion and related compounds. Environ Health Perspect 111: 503-508.
- Okamura A, Kamijima M, Shibata E, Ohtani K, Takagi K, et al. (2005) A comprehensive evaluation of the testicular toxicity of dichlorvos in Wistar rats. Toxicology 213: 129-137.
- Jacobsen H, Ostergaard G, Lam HR, Poulsen ME, Frandsen H, et al. (2004) Repeated dose 28-d oral toxicity study in Wistar rats with a mixture of five pesticides often found as residues in food: alphacypermethrin, bromopropylate, carbendazim, monocrotophos and mancozeb. Food Chem Toxicol 42: 1269-1277.
- Neishabouri EZ, Hassan ZM, Azizi E, Ostad SN (2004) Evaluation of immunotoxicity induced by diazinon in C57bl/6 mice. Toxicology 196: 173-179.
- Dolara P, Salvadori M, Capobianco T, Torricelli F (1992) Sister-chromatid exchanges in human lymphocytes induced by dimethoate, omethoate, deltamethrin, benomyl and their mixture. Mutat Res 283: 113-118.
- Basfar AA, Mohamed KA, Al-Abduly AJ, Al-Shahrani AA (2009) Radiolytic degradation of atrazine aqueous solution containing humic substances. Ecotoxicol Environ Saf 72: 948-953.
- Cole SK, Cooper WJ, Fox RV, Gardinali PR, Mezyk SP, et al. (2007) Free radical chemistry of disinfection byproducts. 2. Rate constants and degradation mechanisms of trichloronitromethane (chloropicrin). Environ Sci Technol 41: 863-869.
- Renbang Z, Huaying B, Lingyuna X (2009) γ-irradiation degradation of methamidophos. Chinese J Chem 27:1749-1754.
- Zhang J, Zheng Z, Luan J, Yang G, Song W, et al. (2007) Degradation of hexachlorobenzene by electron beam irradiation. J Hazard Mater 142: 431-436.
- Zhang Y, Hou Y, Chen F, Xiao Z, Zhang J, et al. (2011) The degradation of chlorpyrifos and diazinon in aqueous solution by ultrasonic irradiation: effect of parameters and degradation pathway. Chemosphere 82: 1109-1115.
- Khan JA, He X, Khan HM, Shah NS, Dionysiou DD (2013) Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82-/Fe2+ and UV/HSO5-/Fe2+ processes. A comparative study. Chem Eng J 218: 376-383.
- Almeida KCS, Oikawa H, Oliveria J, Duarte CI (2006) Degradation of petroleum hydrocarbons in seawater by ionizing radiation. J Radioanal Nucl Ch 270: 93-97.
- Boussahel R, Bouland S, Moussaoui KM, Baudu M, Montiel A (2002) Determination of chlorinated pesticides in water by SPME/GC. Water Res 36: 1909-1911.
- Nickelsen MG, Cooper WJ, Lin K, Kurucz CN, Waite TD (1994) High energy electron beam generation of oxidants for the treatment of benzene and toluene in the presence of radical scavengers. Water Research 28: 1227-1237.
- Woods RJ, Pikaev AK (1994) Applied Radiation Chemistry: radiation processing. John Wiley and Sons, Inc., New York, USA.
- Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms, and hydroxyl radicals in aqueous solution. J Phys Chem Ref Data 17: 513-886.
- Spinks JWT, Woods RJ (1990) Introduction to Radiation Chemistry.3rd edition, John Wiley and Sons Inc., New York, USA.
- Lee B, Lee M (2005) Decomposition of 2,4,6-trinitrotoluene (TNT) by gamma irradiation. Environ Sci Technol 39: 9278-9285.
- Ocampo-Perez R, Rivera-Utrilla J, Sanchez-Polo M, Lopez-Pe˜nalver JJ, Leyva-Ramos R (2011) Degradation of antineoplastic cytarabine in aqueous solution by gamma radiation. Chem Eng J 174: 1- 8.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 23849
- [From(publication date):
February-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 18641
- PDF downloads : 5208