Research Article Open Access
Analysis of Microbial Resistance and Prescription Preferences using Antibiograms
Inderpal Kaur*, Inderpal singh grover, Jasmeet Singh, Kunwar Harsh Upveja and Sukrita PaulGovernment Medical College, Amritsar, India
- *Corresponding Author:
- Dr. Inderpal Kaur MD
Department of Pharmacology, Government Medical College, Amritsar, India
Tel: +919501004136
E-mail: inderpalpharma@gmail.com
Received date: September 29, 2016; Accepted date: October 24, 2016; Published date: October 26, 2016
Citation: Kaur I, Grover IS, Singh J, Upveja KH, Paul S (2016) Analysis of Microbial Resistance and Prescription Preferences using Antibiograms. J Infect Dis Ther 4:302. doi:10.4172/2332-0877.1000302
Copyright: © 2016 Kaur I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Introduction: Hospital antibiogram is a periodic summary of antimicrobial susceptibilities of local bacterial isolates submitted to the hospital's clinical microbiology laboratory. It not only aids clinicians to select the most appropriate empiric therapy, but also in monitoring resistance trends within an institution, thereby optimizing treatment.
Aims: To analyze the susceptibility trends of microbes by using antibiograms; assess the modification in prescribing empirical therapy and examine application of the susceptibility report in clinical practice.
Settings and design: A retrospective study of culture sensitivity reports and indoor prescriptions from departments of Medicine, Pulmonary medicine, Surgery, Orthopaedics, Obstetrics and Gynaecology and Intensive Care Unit.
Methods and material: Culture sensitivity reports of samples collected from these specialties were analyzed for the susceptibility pattern of antibiotics. In addition, prescriptions were analysed for the prescribing patterns for antimicrobials.
Statistical analysis used: The data was tabulated using Microsoft Office Excel 2010 and were later compiled to make an antibiogram. Chi-square values were calculated using online software Graphpad Quickcalcs.
Results: After analysing the data it was found that the most common infecting organisms were Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus susceptible to amikacin, piperacillin/tazobactam and linezolid. However the prescriptions analysed revealed that the commonly prescribed drugs were ceftriaxone and amoxycillin/clavulanic acid.
Conclusions: Antibiogram is useful in predicting and monitoring the trends of antimicrobial resistance. The survey revealed a clear mismatch between the sensitivity reports and the prescribing trends which can lead to evolution of multi-drug resistant organisms.
Keywords
Drug and therapeutic committee; Empiric therapy; Susceptibility pattern
Introduction
Discovery of antibiotics was one of the most celebrated achievements of modern medicine in 20th century. With the advent of ‘Golden era of antibiotics’, human life-expectancy has significantly increased by cure of previously fatal infections. However, almost half a century after introduction of these ‘Wonder drugs’, the emergence of stubborn, resistant microbes is the biggest threat we are facing right now. Antimicrobial resistance is defined as decrease in susceptibility of a microorganism to an antimicrobial agent to which it was previously sensitive. As a result, standard treatments become ineffective and infections persist and may transmit to others [1]. It’s a matter of global concern since it possesses a significant clinical and financial burden. It is estimated that US$ 30 billion is spent on the cumulative effects of antimicrobial resistance each year including multiple drug regimens, extra hospital days, additional medical care and lost productivity. Studies show that mortality, duration of hospital stay and healthcare costs for patients with methicillin-resistant Staphylococcus aureus (MRSA) infections was higher as compared to methicillin sensitive S.aureus infections [2].
Methicillin-resistant Staphylococcus aureus (MRSA) was first reported in 1961 and became endemic in many hospitals worldwide by 1980s. With the widespread emergence of MRSA, glycopeptide antibiotics such as vancomycin have been more frequently used in the clinical practice. This has led to sporadic cases of glycopeptides resistance. In the 90s, fluoroquinolone resistance in E. coli became prominent. The situation is still very volatile as infections caused by antimicrobial-resistant pathogen continue to haunt the clinicians. Furthermore, several highly resistant gram-negative bacteria- namely Acinetobacter species, multidrug-resistant (MDR) P. aeruginosa , and carbapenem-resistant Klebsiella species and Escherichia coli , are emerging as significant pathogens in both the United States and other parts of the world. Our therapeutic options for these pan-antibiotic resistant microorganisms are so extremely limited that clinicians are forced to re-introduce older, previously discarded drugs, such as colistin, that are associated with significant toxicity and for which there is a lack of robust data to guide selection of dosage regimen or duration of therapy [3]. To emphasize on their rising danger and the matter of fact that these pathogens conveniently ‘escape’ the effects of anti-bacterial agents, they were collectively termed ESKAPE group of organisms; where ESKAPE stands for Enterococcus faecium, Staphylococcus aureus , Klebsiella pneumoniae , Acinetobacter baumanii , Pseudomonas aeruginosa , and Enterobacter species [4]. Recently, Clostridium difficile too has been added to the list. Lately Indian subcontinent has been in the spotlight for Superbug containing the New Delhi metallo-β-lactamase 1 (NDM-1), an enzyme which makes the bacteria resistant to β-lactam antibiotics including Carbapenem group. So, it can be said that bacterial infections are becoming increasingly resistant to existing antibiotics, and, ironically, as the number of patients succumbing to these infections rise, the number of newer antimicrobial agents in the pipeline are dwindling.
One of the most important reasons for development of antimicrobial resistance is indiscriminate use of antibiotics. For example, antibiotics were prescribed in 68% cases of acute respiratory tract infections and of those, 80% were unnecessary according to CDC guidelines [5]. There may be many contributory factors to it like demands from patients, peer pressure, fancy perks from pharmaceutical industries leading to overuse of a particular type of antibiotic, diagnostic uncertainty, pressure to keep hospital-stay short and last but not least, physicians’ lack of knowledge about the local susceptibility patterns which has been cited as one of the top causes. This is where cumulative antibiogram comes to the rescue.
Cumulative Antibiogram is defined as report generated by analysis of isolates from particular institution in a defined period of time that reflects percentage of 1st isolate per patient of given specie that is susceptible to each of antimicrobial agents routinely tested [6]. It is a pre-requisite for any antibiotic policy, to steer the physicians to select the most appropriate empiric antibiotic therapy. For instance, it is known that for patients in ICUs, mortality rises if the empiric antibiotic therapy chosen does not cover the pathogens causing the infection. Kollef et al. showed that infection-related mortality was 17.7% in those patients who received appropriate empiric therapy and 42% in those who received inapt empiric antibiotic therapy [7]. The most common reason for the unsuitability of the chosen empiric antibiotic therapy was the resistance of bacteria to the antibiotic selected. In an effort to improve the adequacy of antibiotic selection, Ibrahim et al. reviewed the antibiogram for their ICU and created a clinical guideline for antibiotic selection in that unit. The adequacy of empiric antibiotic selection for ventilator-associated pneumonia for patients in their ICU increased from 48.0% before the creation of antibiotic guidelines to 94.2% with the use of their guidelines [8]. Antibiograms are regarded as cost-effective and convenient method of assessment of local susceptibility rates and monitor resistance trends overtime in institutions.
The compilation and presentation of an antibiogram is generally initiated by the clinical microbiology laboratory with collaboration from clinicians, pharmacologists and infection control personnel. This document demonstrates recent, precise, and clinically useful data in an organized manner. The development of sophisticated computer programs like WHO-NET software and improvements in laboratory information systems assist in this process.
The objective of our study was to analyze antimicrobial susceptibility trends by using Antibiograms and compare the susceptibility rates with the antibiotic prescribing patterns across the institution. This analysis was then used to orient the clinicians in attempt to rationalize their antibiotic prescribing habits and contain the emergence and spread of resistance.
Materials and Methods
Study location
The present study was conducted in Guru Nanak Dev Hospital, Amritsar; adjoined to Government Medical College, Amritsar. It is a 1000 bedded tertiary care health institution. Departments included were Medicine, Pulmonary medicine, Surgery, Orthopedics, and Obstetrics and Gynecology.
Study period
For antibiogram preparation: From1st February, 2013to 31st July, 2013
Prescription analysis: From 1st May, 2013 to 31st July, 2013
Study population
All the patients admitted to aforementioned departments during the period of the study were included.
Inclusion criteria
1. Susceptibility reports of only Indoor patients were taken into consideration.
2. To prepare the antibiogram 1st diagnostic isolate of given specie per patient per analysis period was included, irrespective of body site, antimicrobial susceptibility profile or other phenotypic characters [9].
3. Blood, urine and pus cultures were included.
4. Prescriptions of adult patients above 18 years with antibiotic medications were collected for analysis.
Exclusion criteria
1. Antibiotic sensitivity test reports from laboratories other than Microbiology department of our institute were excluded
2. While preparing the antibiogram, the following isolates were excluded:
a. Duplicate bacterial isolates [9]
b. Surveillance culture and screening isolates [9]
c. Isolates of the colonizers [9]
d. Strains which show intermediate susceptibility
e. CSF isolates
3. Patients already on antibiotics were also excluded
The approval of the ethics committee of the institution was obtained
Data collection and analysis
1. To prepare antibiogram: Culture sensitivity reports for the aforementioned departments (Table 1) were collected and since least no of culture sensitivity reports were from Pulmonary medicine (423) therefore, we evaluated 400 culture sensitivity reports from each department. Antibiograms were prepared by plotting the number of isolates of a particular micro-organism against the antibiotic to which they were found susceptible.
| Sr. No. | Department | No. of culture sensitivity reports |
|---|---|---|
| 1 | Medicine | 528 |
| 2 | Pulmonary medicine | 423 |
| 3 | Surgery | 451 |
| 4 | Orthopedics | 469 |
| 5 | Obstetrics and Gynecology | 448 |
Table 1: Culture sensitivity reports for the aforementioned departments.
For prescription analysis
a) Prescriptions for antibiotic empiric therapy from each of the aforementioned departments were collected during the study duration specified previously and all the prescriptions satisfying the inclusion criteria were included in the study (Table 2). Prescriptions were analyzed for empirical antibiotic therapy received, which was compared with the sensitivity pattern in the antibiogram.
| Sr. No. | Department | No. of prescriptions |
|---|---|---|
| 1 | Medicine | 346 |
| 2 | Pulmonary medicine | 286 |
| 3 | Surgery | 325 |
| 4 | Orthopedics | 316 |
| 5 | Obstetrics and Gynecology | 314 |
Table 2: Prescriptions for antibiotic empiric therapy from each of the aforementioned departments.
b) To construct the antibiograms and prepare the final results, Microsoft Office Excel 2010 software was used. Statistical analysis of antibiotic prescription against organism susceptibility was done by Chi-square test (χ2). Chi-square values were calculated using online software Graphpad Quickcalcs.
Results
Data from 2000 Culture sensitivity reports was piled up to prepare antibiogram and 1587 Prescriptions were evaluated for prescribed drug against its organism susceptibility for particular antibiotic in percentage. Most common infecting organism isolated in samples from various departments was as given in Table 3.
| Sr.No. | Department | Most common organism |
|---|---|---|
| 1 | Medicine | E. coli |
| 2 | Pulmonary medicine | P. aeruginosa |
| 3 | Surgery | S. aureus |
| 4 | Orthopedics | S. aureus |
| 5 | Obstetrics and Gynecology | S. aureus |
Table 3: Most common infecting organism isolated in samples from various departments.
Total number of prescriptions analyzed for various departments are given in Table 2. Antibiotic prescription versus organism susceptibility, along with their χ2-value is given in the form of bar graph. All χ2- values were found to be significant at p<0.0001.
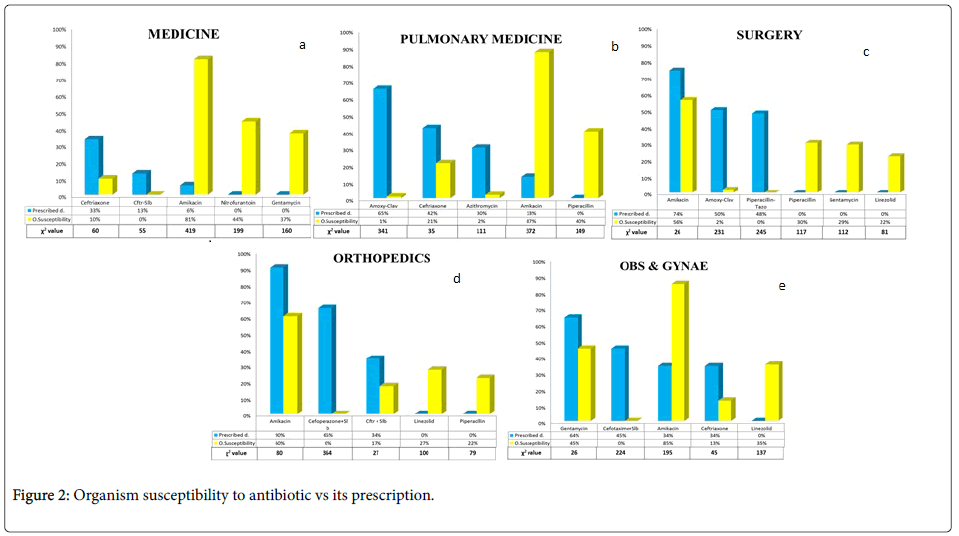
Antibiograms and organism susceptibility to antibiotic vs its prescription are shown in Figures 1 and 2, respectively.
Culture reports from medicine department showed that the organism susceptibility was maximum to Amikacin-81% (325) followed by Nitrofurantoin-44% (176) and Gentamycin-37% (150) but among the most prescribed drugs were ceftriaxone-33% (114) and Ceftriaxone/Sulbactum-13% (45). In Pulmonary Medicine organisms were most susceptible to Amikacin-87 % ( 347) and Piperacillin 40% (161) but Amoxicillin/Clauvulinic acid-65% (186); was frequently prescribed followed by ceftriaxone-42% (120) and Azithromycin-30% (86). Reports from surgery showed organism sensitivity to Amikacin-56% (225) followed by Piperacillin-30% (118) and Gentamycin-29% (116) and and the commonly prescribed drugs were Amikacin-74% (240) followed by Amoxycillin/Clauvulinic acid-50% (162) and Piperacillin/Tazobactum-48% (1). Cultures from Orthopedics showed the organism susceptiblity to Amikacin-60% (241) followed by Linezolid-27% (108) and Piperacillin-22% (91). Amikacin-90% (283) was the most commonly prescribed drug followed by Cefoperazone/Sulbactum-65% (205) and Ceftriaxone/ Sulbactum-34% (107). Obstetrics and Gynecology antibiogram showed highest sensitivity to Amikacin-84% (339) followed by Gentamycin-45% (181) and Linezolid-35% (141) and the most commonly prescribed drugs were Gentamycin-64% (201) followed by Cefotaxime/Sulbactum-45% (141).
Discussion
Antibiogram is a versatile document which, besides exhibiting the antibiotic susceptibility pattern across the institution, presents a clear picture of the most common disease-causing organisms in various units of the hospital. Nosocomial infections are a major public health concern these days and a cause of considerable mortality and morbidity for hospitalized patients. They occur among 7-12% of the hospitalized patients globally, with more than 1.4 million people suffering from the infectious complications acquired in the hospital [10].
Our study deals with the analysis of culture-sensitivity reports of only indoor patients, thus, the chances of coming across nosocomial or Hospital Acquired Infections (HAI) were high. In our study, the antibiograms of various departments indicated that most common pathogens isolated were gram negative bacilli (Medicine 72%, Pulmonary medicine 76%, Surgery 63%, Orthopedics 68% and Obstetrics and Gynecology 48%). Among the array of gram negative organisms, the Enterobacteriaceae family was the most frequently identified group overall. Recent data from the U.S. National Healthcare Safety Network indicate that gram negative bacteria are accountable for more than 30% of hospital-acquired infections [11]. Among the HAIs caused by Gram negative bacilli, urinary tract infections were most prevalent. Klevens et al. have reported in their study that UTI accounts for more than 30% of infections reported by acute care hospitals [12]. Most of it has been found to be related with catheterization, generally known as Catheter-associated Urinary tract infection (CAUTI). Urinary catheters are used routinely in the wards of our hospital, usually for frequent and accurate monitoring of urinary output. And this can lead to increase in number of isolates of Gram negative pathogens.
In Pulmonary medicine ward, the most common individual organism was Pseudomonas aeruginosa (35%). This finding is in agreement with reports from USA which suggest that P. aeruginosa is the most frequent bacterium isolated from the respiratory tract (31.6%) [13]. This can be attributed to patients of Community and Hospital-acquired pneumonia admitted in that ward. Hospitalacquired pneumonia by P. aeruginosa may also be iatrogenic. Being an extremely adaptable organism it can survive and multiply even with minimal nutrients, if moisture is available. Thus, equipment such as respirator and bronchoscopes can be frequently contaminated.
On analysis of culture-sensitivity reports from Surgical units i.e. Surgery and Orthopedics, it was observed that Staphylococcus aureus (27% and 29%, respectively) was most commonly encountered individual bacterial specie. This finding is supported by data from CDC which states that Staph. aureus is one of most prevalent organism associated with surgical wound infections [14]. This is also in accord with a study done by Kollef on surgical nosocomial infections which reported 31.1% isolates of Gram-positive bacteria [15]. Furthermore, S. aureus was the major pathogen from patients in Gynecology and Obstetrics wards (45%) and most commonly isolated bacteria from patients who underwent emergency type of surgery which may be due to surface contamination by this bacterium on the skin and environment causing nosocomial infections.
Antibiotics are one of the pillars of modern medicine and play a vital role both as the prophylaxis and management of infectious diseases. Successful treatment of patients with bacterial infection relies on the identification of bacterial pathogens and on the selection of an antibiotic effective against that particular organism. The issue of their availability, cautious selection and rational use are of critical importance to the global community [16].
In the present study, on carefully comparing the prescriptions for empiric antibiotic therapy with the antibiograms, we observed that in Medicine, the most prescribed drugs were Ceftriaxone (33%), followed by Ceftriaxone-Sulbactam combination (13%) but the organism susceptibility of these antimicrobial agents were only 10% (χ2=60) and 1% (χ2=55), respectively. On the other hand, Amikacin and Gentamycin showed a remarkable organism susceptibility, 77% and 36%, respectively, but these drugs were rarely prescribed (Amikacin=7% prescription rate (χ2=419) and Gentamycin=nil (χ2=160)). Likewise, in Pulmonary Medicine, the antibiotics which were most frequently prescribed i.e. Amoxicillin-clavulanic acid (65%), Ceftriaxone (42%) and Azithromycin (30%), showed a very dismal (Amoxicillin-clavulanic acid=3% (χ2=341)) or no (Ceftriaxone (χ2=35) and Azithromycin (χ2=111)) organism susceptibility at all. But, Amikacin, with exceptionally high organism susceptibility (86%), was uncommonly prescribed (χ2=372).
The Surgical branches demonstrated a healthier scenario regarding prescription of Aminoglycosides. In Surgery, Amikacin showed 57% organism susceptibility and was prescribed in 74% cases (χ2=26). In Orthopedics, Amikacin demonstrated highest organism susceptibility (58%) and was prescribed as an empiric therapy in 90% patients (χ2=80). In Obstetrics and Gynecology, even though, Amikacin displayed the highest organism susceptibility at 84%, Gentamycin, with 45% organism susceptibility, was the most prescribed antibiotic (64%, χ2=26). However, the second most frequently prescribed antibiotics i.e. Amoxicillin-clavulanic acid (50%) in Surgery, Cefoperazone - Sulbactam combination (65%) in Orthopedics and Cefotaxim - Sulbactam combination (45%) in Obstetrics and Gynecology, showed very low or nil organism susceptibility. Thus, as evident by the χ2 values (>200), there were greater discrepancies between second most prescribed antibiotics and organism susceptibilities.
Therefore to summarize, there was gross disparity between sensitivity pattern and the antibiotic prescribing trend in various wards. Irrational use of antimicrobials is the biggest contributing factor to the growing peril of resistance especially in low-income countries [17]. In our study, we observed a clear mismatch between culturesensitivity pattern and antibiotic prescribing trend. Linezolid is a synthetic antimicrobial agent of the oxazolidinone class [18]. In this study, Linezolid illustrated an organism susceptibility rate of 23%, 27% and 38% in Surgery, Orthopedics and Obstetrics- Gynecology, respectively. However in all the three departments, it was not prescribed routinely. The most probable rationale behind this observation can be the fact that Linezolid is considered to be a ‘Reserve Drug’ , set aside as an alternative agent for treatment of infections caused by multi-drug resistant strains like vancomycin-resistant E. faecium , nosocomial pneumonia caused by methicillin-resistant strains of S. aureus , complicated skin and skin-structure infections caused by MRSA [19].
In the present study, antibiograms were segregated on the basis of different units where patients were admitted. Unit–specific antibiograms gives a superior picture of the organism susceptibility spectrum and thereby, the resistance trends since it is known that patterns of resistance to antibiotic vary widely between as well as within healthcare institutions. In the same way, unit-wise antibiogram prepared in our study clearly depicted the variations in isolated microorganisms, susceptibility trend and the antibiotic prescribing practices, as mentioned above. It also illustrated the odd resistance patterns in specific areas of the hospital. Institution-wide antibiograms may conceal important differences in susceptibility data across units within the institution. These differences may be significant, not only for selecting most effective empirical antimicrobial therapy for a patient in that unit but also for monitoring the emerging patterns of antimicrobial resistance specific to certain units within the institution [20].
Limitations of the Study and Challenges of Antibiogram
The present study had the following deficiencies:-
1. The antibiogram susceptibilities in our study may not forecast the best empiric drug combination because of unpredictable cross resistance. Cross-resistance should be taken into consideration to choose the initial combination regimens for serious gram negative infections, especially, P. aeruginosa [21].
2. Many culture sensitivity tests are frequently outsourced to the laboratories, other than our Institution’s own Microbiology department. Hence, it is difficult to generalize the inferences of our findings for all the patients admitted.
3. Intensive care unit (ICU) was not included in our study since very few specimens were sent for culture sensitivity tests to the Microbiology department. It is because ICU is not an independent unit in our institution. The requests for culture sensitivity tests are sent by the respective departments under which the patient is admitted.
4. All the data was collected and analyzed manually. WHO NET software was not used.
Conclusion
The present study reveals a clear mismatch between susceptibility reports and prescribing trends which can give rise to antibiotic resistance. To rectify these discrepancies in prescribing pattern, Antibiotic policy and institutional Drug and Therapeutic Committee is one of the mandatory requirements for accreditation, and making an antibiogram is the first step before framing the antibiotic policy. With a collaboration between departments of pharmacology, microbiology and the clinicians, it will not only review the antibiograms but will also regulate the antibiotic prescribing thereby enhancing its efficacy and promoting its rational use.
References
- World Health Organization. WHO Global Strategy for the Containment of Antimicrobial Resistance. Switzerland: WHO, 2001.
- Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, et al. (2005) The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol 26: 166–174.
- National Nosocomial Infections Surveillance System Report (2007) Data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004; 32:470–85.) (Falagas ME, Bliziotis IA.Pandrug-resistant gram-negative bacteria: the dawn of the post-antibiotic era? Int J Antimicrob Agents 29: 630–636
- Rice LB (2008) Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 197: 1079–1081
- Scott JG, Cohen D, DiCicco-Bloom B, Orzano AJ, Jaen CR, et al. (2001) Antibiotic use in acute respiratory infections and the ways patients pressure physicians for a prescription. J Fam Pract 50: 853-858.
- CLSI (2009) Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data: Approved Guideline. Third edition. CLSI document M39-A.
- Kollef MH, Sherman G, Ward S, Fraser VJ (1999) Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115: 462-474
- Ibrahim EH, Ward S, Sherman G, Schaiff R, Fraser VJ, et al. (2001) Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. Crit Care Med 29: 1109-1115.
- Clinical and Laboratory Standards Institute (CLSI) (2009) Analysis and presentation of cumulative antimicrobial susceptibility test data. 3rd ed. Approved guideline M39-A3. Wayne PA.
- Kamat US, Ferreira V, Savio R (2008) Antimicrobial resistance among nosocomial isolates in a teachinghospital in Goa. Indian J Community Med 33: 89-92
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, et al. (2009) NHSN annual update: antimicrobial-resistantpathogens associated with healthcareassociatedinfections: annual summary ofdata reported to the National HealthcareSafety Network at the Centers for DiseaseControl and Prevention, 2006-2007. Infect Control Hosp Epidemiol 29: 996-1011.
- Klevens RM, Edward JR, Richards CL Jr, Horan TC, Gaynes RP, et al. (2007) Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Reports 122: 160-166.
- NeuhauserMM, Weinstein RA, Rydman R, Danziger LH, Karam G, et al. (2003) Antibiotic resistance among gram negative bacilli in US intensive care units: implications for fluoroquinoloneuse. JAMA 289: 88 –888.
- CDC NNIS System (1996) National Nosocomial Infections Surveillance (NNIS) Semiannual Report from October 1986-April 1996. Am J Infect Control 24: 380-388.
- Kollef MH, Sharpless L, Vlasnik J, Pasque C, Murphy D, et al. ( 1997) Theimpact of nosocomial infections on patient outcomes following cardiac surgery. Chest 112: 666-675.
- Abula T, Kedir M (2004) The pattern of antibiotic usage in surgical in-patient of ateaching hospital, northwest Ethiopia. Ethiop J HealtDev 18: 35-38.
- Gaash B (2008) Irrational use of Antibiotics. Indian J Prescribing Doctor 5: 56-59.
- Diekema DI, Jones RN (2000) Oxazolidinones: a review. Drugs 59: 7-16.
- Clemett D, Markham A (2000) Linezolid. Drugs 59: 815-827.
- Binkley S, Fishman NO, LaRosa LA (2006) Comparison of unit-specific and hospital-wide antibiograms: potential implications forselection of empirical antimicrobial therapy. Infect Control Hosp Epidemiol 27: 682–687.
- Safdar N, Handelsman J, Maki DG (2004) Does combination antimicrobial therapy reduce mortality in gram-negative bacteremia? A meta-analysis. Lancet Infect Dis 4: 519-527.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 13155
- [From(publication date):
October-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 12290
- PDF downloads : 865