Special Issue Article Open Access
Analysis of Maize Photosyntheis Parameters and Whole Plant Oxidative Damage Under Long-term Drought
| Alyne Oliveira Lavinsky*, Paulo César Magalhães, Roniel Ávila, Carlos César Gomes-Jr and Newton Portilho Carneiro | ||
| Embrapa Milho e Sorgo, Rodovia MG-424, Km 45 Caixa Postal: 285 ou 151 CEP: 35701-970, Sete Lagoas, MG, Brazil | ||
| Corresponding Author : | Alyne Oliveira Lavinsky Embrapa Milho e Sorgo Rodovia MG-424, Km 45 Caixa Postal: 285 ou 151 CEP: 35701-970 Sete Lagoas, MG, Brazil Tel: 3027-1100 E-mail: alynelavinsky@gmail.com |
|
| Received April 12, 2015; Accepted May 20, 2015; Published May 22, 2015 | ||
| Citation: Lavinsky AO, Magalhães PC, Ávila R, Gomes-Jr CC, Carneiro NP (2015) Analysis of Maize Photosyntheis Parameters and Whole Plant Oxidative Damage Under Long-term Drought. Adv Crop Sci Tech S1:007. doi: 10.4172/2329-8863.1000S1-007 | ||
| Copyright: © 2015 Lavinsky AO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
We test if maize maintain yield under long-term drought throught improvement of photosyntheis (A) coupled with up-regulation of the antioxidant system induced by increase in levels of abscisic acid (ABA). Four maize genotypes with constrasting drought tolerance: BRS1010 and 2B710 (sensitive) and DKB 390 and BRS1055 (tolerant) in two soil water levels, field capacity (FC) and water deficit (WD) were used. WD was applied at the pre-flowering stage for 12 days, and oxidative damage was measured as malondialdehyde (MDA) accumulation in whole plant. Plants from tolerant genotypes DKB390 and BRS1055 showed higher A and had no signal of oxidative damage compared to sensitive genotypes 2B710 and BRS1010 under WD, resulting in a higher yield attributes. For our surprising, it was dissociated from up-regulation of the antioxidant system ABA-mediated. In turn, plants from two sensitive genotypes under WD showed compared to FC consistent reduction of A due to mesophyll conductance (gm) limitation. Only WD plants from sensitive genotype BRS1010 presented leaf ABA levels increased related to its counterparts under FC; however, due to the inactivation of catalase activity the oxidative damage control was not effective, resulting a hardly MDA acumulation in both leaves and roots. The maize tolerance under long-term drought is linked to scape of gm decline.
| Keywords | |
| Antioxidant system; Mesophyll conductance; Malondialdehyde; Photorespiration; Abscisic acid | |
| Introduction | |
| The world’s most important crops in terms of total yield in 2014/15 is maize (Zea mays), with 1014 Mt [1], and its productivity is greatly constrained by drought with depending upon the genotype, growth stage, duration and intensity of stress [2,3]. The period in which maize is particularly sensitive to water stress is one week before to two weeks after flowering [4]. Plant breeders and major seed companies have developed maize genotypes with enhanced yields in water deficient environments, and phenotypic traits, such as anthesis silking interval, yield, grain number, carbon allocation to roots, leaf rolling and leaf chlorophyll content, has been used to select drought tolerant maize germplasm [4]. Successful drought resistant genotypes improved commercial maize yields under water limiting conditions by up to 15% and, importantly, yields under water sufficient conditions were only marginally less than control genotypes [5,6]. Although we know a great deal about the agronomic performance of drought tolerant maize genotypes, much less is known about the physiological mechanisms that contribute to desiccation tolerance in these genotypes. | |
| Maize responses to drought usually includes stomata closure [7,8], a shift from shoot to root growth [7], decreasing photosynthetic activity [7,8] and altering carbohydrate [9] and amino acid metabolism [7,9]. Drought is also known to induce oxidative stress directly, by generating reactive oxygen species (ROS) during the conversion of its valence forms, or indirectly, by inactivating antioxidant system [10]. The ameliorative effect of A on tolerant maize genotypes exposed to drought is believed to occur, to a large extent, through counteracting oxidative stress via modulating antioxidant enzymes at leaf level, including superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR) and glutathione peroxidase (GPX), as well as antioxidant molecules [8,11]. Failure of the antioxidant defense system may result in leaf damage when metabolites and components of the cellular machinery react with ROS [8,11], resulting in lipid peroxidation [10], thus ultimately impairing A and yield [12]. | |
| The higher yield in a maize genotype tolerant to drought was coupled with up-regulation of the ABA- mediated antioxidant system at leaf level, mainly CAT [10]. However, like leaves, roots exposed to drought are potential producers of ROS, and thus could counteract oxidative stress via modulating antioxidant enzymes [13]. To the best of our knowledge, little attention was paid to how ABA- mediated antioxidant system antioxidant in root affects A in successful drought tolerant genotypes. It is therefore tempting to speculate that signaling ABA pathways are tightly interregulated with antioxidant system at the whole-plant level to increase water uptake, in a manner that allows the maintenance of higher A and productivity. | |
| The aim of this study was to test if long-term drought tolerant genotypes could maintain yield under water limiting conditions through improvements of the A coupled with up-regulation of ABAmediated antioxidant system at whole plant level. | |
| Materials and Methods | |
| Plant material, cultivation conditions and experimental design | |
| The experiment was conducted in a greenhouse at the National Maize and Sorghum Research Center (19º28’ S, 44º15’08’’ W, 732 m a.s.l.), and the plant material consisted of four open-pollinated maize genotypes with contrasting drought tolerance: two tolerant (DKB 390 and BRS1055) and two sensitive (BRS1010 and 2B710). The choice of genotypes was based on results of previous field experiments performed by researchers from the breeding program of the National Maize and Sorghum Research Center, who over the years, has accumulated experience in maize phenotyping for drought tolerance. Under WD, DKB390 and BRS1055 showed higher flowering synchronization and yield compared to BRS1010 and 2B710 [14,15]. | |
| Plants were grown in plastic pots containing 20 kg of typical dystrophic Red Latosol soil. The water content in the soil was monitored daily between 9:00 a.m. and 3:00 p.m., with a moisture sensor (GB Reader N1535; (Measurement Engineering, Australia) installed at the center of each pot with the aid of a screw auger at a depth of 20 cm. These sensors detect the water content in the soil based on electrical resistance and are coupled to digital meters. Water replacement by irrigation was based on the data obtained with the sensor and water was added to reach FC during the period preceding the imposition of the treatments. The water replacement calculations were performed with a spreadsheet and based on a soil water retention curve. In parallel, all necessary cultural and phytosanitary treatments were performed. | |
| At the pre-flowering growth stage, half of each initial treatment was subjected to WD the other half continued to receive daily irrigation in order to maintain soil moisture close to FC, with a soil water tension of −18 kPa. WD was imposed by daily provision of 50% of the total available water until the soil water tension reached at least −138 kPa. After twelve days under these conditions, the leaf gas exchange and chlorophyll a fluorescence were measured in ear leaf with an infrared gas analyzer equipped with a fluorometer (LI-6400-40; LI-COR, USA) [16]. Samples of corn ear-leaves and roots tips (2 cm lenght) washed from the soil were collected at beginning of silking. Subsequently, samples were stored in liquid nitrogen for determination of antioxidant enzymes activity, levels of ABA, as well as cellular damage based on MDA accumulation. The water supply was then restored and maintained at optimum levels until physiological maturity. At harvest, the agronomic parameters associated with productivity were analyzed according to the methodology detailed in the “Agronomic parameters” section. The experimental unit was the pot containing two plants, with six replications per treatment. | |
| For the statistical analysis, the results were submitted to variance analysis and the means were compared by the Scott-Knott test at 5% probability. | |
| Enzymatic assays | |
| The activity of the enzymes of the antioxidant system, named dismutase SOD (EC 1.15.1.1), CAT (EC 1.11.1.6), and APX (EC 1.11.1.11) were determined from plant material extracted in a medium containing potassium phosphate buffer 0.1M (pH 6.8), 0.1 mM EDTA, 1 mM DTT, 1 mM PMSF and 1% PVPP (w/v). Total SOD activity was determined by measuring the ability of this enzyme to inhibit the photochemical reduction of p-nitro-blue-tetrazolium chloride by superoxide at 560 nm. The activity of CAT was estimated by measuring the rate of decomposition of H2O2 at 240 nm, while total APX activity was determined by monitoring the decline in absorbance at 290 nm. Additional details are described in ref. [10]. The levels of ABA was performed using immunoenzymatic assay kits (Phytodetec ABA Enzyme Immunoassay Test Kit—Sigma-Aldrich). The MDA accumulation was estimated as the content of total 2-thiobarbituric acid-reactive substances [10]. | |
| Photosynthetic gas exchange measurements | |
| The leaf gas-exchange parameters A, stomatal conductance to water vapor (gs), internal CO2 concentration (Ci) and transpiration rate (E) were measured simultaneously chlorophyll a fluorescence parameters from 10:00 a.m. to 1:00 p.m., when A is at its maximum, under artificial PPFD of 1500 μmol photons m-2s-1 at the leaf level, 400 mol CO2 mol air-1 and 21% O2. During the measurements, the leaf-toair vapor pressure deficit was ca. 1.0 kPa and a leaf temperature of 25ºC. Based on relationship between A and E, the water use efficiency (WUE) was calculated. | |
| The equipment was programed to make curves A/Ci, varying sequentially CO2 partial pressure: 40, 30, 20, 10, 5, 40, 60, 80, 100, 120, 140 and 160 Pa. Estimations of gm were performed using the combined gas exchange/fluorescence data [15]. A–Ci curves were converted into A–Cc curves for estimation of the maximum rate of carboxylation of Ribulose 1,5 bisphosphate (Rubisco, Vcmax) and phospoenolpyruvate and pyruvate orthophosphate dikinase (PEPc and PPDK, Vpmax), as well as the maximum rate of carboxylation limited by electron transport (Jmax) [16]. The maximum efficiency of photosystem II (FvFm) was determined through a fluorometer (Plant Efficiency Analyser. Hansatech Instruments King’s Lynn, UK) in leaves adapted to dark. Leaf conditioning was carried out with the help of leaf clips with the light intensity in the sensor being 60 % of the equipment’s total capacity, for a period of 5 s at each reading. Rates of ATP and NADPH consumption, as well as H+ requirement were estimated based on ref. [17]. | |
| Additionally, nitrogen (N) allocated in the photosynthetic machinery was accessed as ref. [18], including the N partition between fractions involved in carboxylation enzymes (NRubisco, NPEPc and NPPDK), light harvesting (Ni) and bioenergy (Nb). | |
| Agronomic parameters | |
| Total leaf area per plant (LA) was measured with an area meter (LI- 3100; LI-COR, USA), in six plants per treatment. The plants were then partitioned into roots, stems, leaves, tassel, ears (cob, husk, and grains), and dried in an oven with forced air circulation at 70 °C for 72 h. Based on the dry weights of the different parts, the dry grain biomass (DGB), total dry biomass (TDB), harvest index (HI) were estimated. | |
| Additionally, a group of 50 kernels was soaked overnight in ethylenediamine (10%, w/v) and longitudinally cut with a knife to evaluate possible changes in embryo size, depending on the treatment. Photographs were obtained using a stereoscopic microscope and the Image J program was used to calculate the ratio between the areas of the endosperm and the embryo (EM: E). | |
| Results and Discussion | |
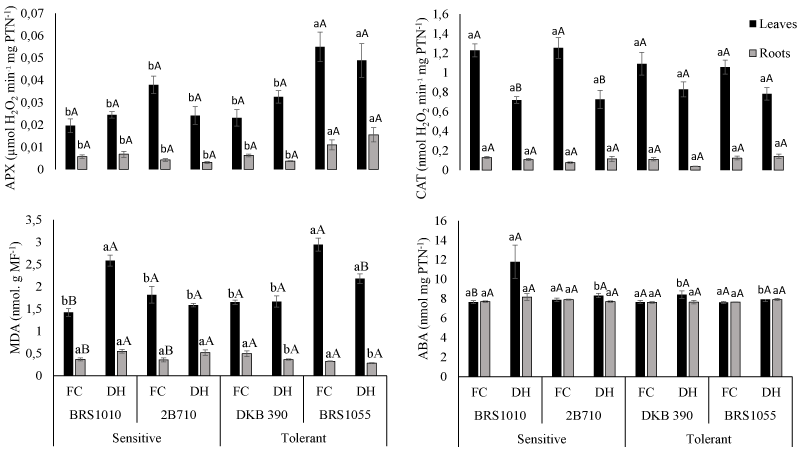
| The activity of enzyme SOD was not significantly different among different genotypes and water levels (data not shown). The activity of APX was higher in BRS1055 compared to other genotypes independent of water level while CAT activity was decreased only in sensitive genotypes under WD, compared to FC (Figure 1). In WD plants from BRS1010, the decrease in CAT activity was acompained by increase in leaf level of ABA (Figure 1), as well as increase in MDA levels (Figure 1), both in leaves and roots. In WD plants from 2B710 the increase in MDA levels was verified only at root level, dissociated of changes in antioxidant enzyme activity enzyme in this organ (Figure 1). The WD plants from DKB390 and BRS1055 didn’t change activity of antixidant system enzymes nor ABA levels compared to its counterparts under FC (Figure 1), and even the counteraction of oxidative stress via modulating antioxidant enzymes ABA-mediated was not active, the MDA levels with remained unchanged related to FC. | |
| Antioxidant enzymes activity has been reported to increase in plants exposed to various environmental stresses, including drought [11,10]. As a result, the activity of these enzymes has been used as an indirect selection criterion for screening drought-resistant plant materials. The protective effect of ABA on A is with due to its the ability to enhance the elimination system for ROS, as measured in terms of antioxidant enzymes such as SOD, CAT and APX [10]. The ABA aplication has no influence on antioxidant enzymes in maize under early-term drought, with exception of CAT activity, which, with ABA application had its activity elevated resulting in higher values, especially in tolerant hybrid DKB 390 [10]. In addition, the activity of antioxidant enzymes at the beginning of stress was high, while at the tenth day under drought the enzymatic activity decreased [10], corroborating our results. Maybe, both CAT and ABA were deactivated due long exposure to drought, and yet A and yield in DKB 390 and BRS1055 was influenced in less extent than BRS1010 and 2B710 under WD, demonstrating the effectiveness of the oxidative stress control in these genotypes under long-term was due to another tolerance mechanism that not drought-related enhancement of the antioxidant defense capacity ABA-mediated. | |
| Only plants from sensitive genotype BRS1010 after 12 days under WD presented higher ABA levels than its counterparts under FC (Figure 1); however, due to the inactivation of ABA-mediated antioxidant system and absense of another tolerance mechanism the stress control was not effective. In this genotype, the CAT activity in leaves decreased and lipid peroxidation increased, as shown by higher MDA concentrations in both leaves and roots (Figure 1). With the increase of water stress, H2O2 participation in the Haber–Weiss/Fenton reaction as free radical attacking the cell membranes [19]. This way, scavenging to diminish these molecules becomes necessary, but this capability was not found in the sensitive genotype BRS1010 because it was not able to diminish the H2O2 content. | |
| Regardless genotype, there was a significant reduction of A and gs in plants exposed to WD compared to with well irrigated plants (Table 1). By the way, there was a strong correlation between these two variables (data not shown) while Ci and Cc values increased (Table 1). Plants of the tolerant genotypes DKB390 and BRS1055 under WD showed A and gs values of, respectively, 70.33 % and 64.66 % higher, as well as larger values of E, compared to those values observed in plants from the sensitive genotypes 2B710 and BRS1010 grown in the same condition (Table 1). The N partition between fractions involved in NRubisco, NPEPc and NPPDK, Ni and Nb were significantly affected by the soil water level, with lower values on WD compared to those obtained under FC (Table 2). Such information helps to explain the declines in Vp,max, Vc,max and Jmax in WD plants (Table 1), demostrating lower CO2 use by the enzymes PEPc and Rubisco. In contrast, the PR was slightly increased (Table 1), in parallel decreases in rate of ATP and NADP comsumption, as well as H+ requeriment. | |
| The carbon fixation, which generally represents the main sink for absorbed light in chloroplasts, was found to be depressed in all genotypes under drought by photoinhibition, mainly BRS1010 (Table 1). Drought resistant genotypes with high yield can release more water through the stomata openings, which in turn promotes a higher canopy cooling to escape from photoinhibition [10]. Plants from BRS1010 under WD restrict latent heat loss by E, with possible increased leaf temperature, leading photoinhibition, which in turn, impair A. As a result of the decrease in both A and E values, the WUE was severely decreased in WD plants of BRS1010 (Table 1). Notably, the A values in WD plants of BRS1010 genotype was 7.81 times lower than genotype 2B710, while E was only 2.2 times lower, which explain the quite similar WUE values of 2B710 WD compared to those verified in DKB 390 and BRS 1055 genotypes under same condition. | |
| Adjustment of light capture, use and dissipation is required to provide photoprotection to the photosynthetic apparatus [20]. In our study we showed that Chl a fluorescence parameters declined under WD, but plants of BRS1055 genotype under drought had Fv/Fm values higher than the others genotypes, corroborating the highest Ni value (Tables 1 and 2). It may be suggested that photoprotection of the PSII reaction center by xanthophyll engaged in sustained thermal energy dissipation are likely to have occurred in this genotype, which in turn, delays the degradation of the D1 protein, the main polypeptide of PSII reaction center. In addition, the p,max, Vc,max and Jmax ,NRubisco, ATP, NADP and H+ values declined in all genotypes under drought in parallel to increases in Ci, Cc and PR (Table 1). | |
| In fully hydrated leaves, the CO2/O2 ratio in bundle sheath cells of C4 species is three to six times higher than in mesophyll cells under atmospheric levels of CO2 and O2 [21]. Therefore, the oxygenase activity of Rubisco, and consequently the PR, is low [22]. Under drought conditions, the Ci may decrease because of decreased gs and should cause PR increase [22]. However, PR has been shown to neither increase nor contribute to the limitation of A in C4 grasses under drought stress [22]. In contrast, this study demonstrated a slight increase in PR even when Ci is substantially increased under drought, due partial deactivation of carboxylation capacity at Rubisco catalytic site. Adjustments to even mild disturbances in redox status, caused by a deficiency in ascorbate, AOX or chloroplastic NADP-malate dehydrogenase, comprise increases in photorespiratory components such as catalase, P-protein of glycine decarboxylase complex and glycine content [23]. Therefore, a strong interaction between the chloroplast redox status and PR to induce scape mechanism against ROS formation defense under severe drought is not surprising. Overall, these evidences conffirm that carboxylation capacity at Rubisco catalytic site at least was partially deactivated in WD plants. | |
| Recently Centritto et al. [24] have posited that under drought conditions, gm also plays an important role in determining A because rice genotypes with inherently higher gm are capable of maintaining a higher A. To the best of our knowledge, the current study is the first to report a direct effect of sink strength on gm in a C4 cereal species. The goal of present study was finding effects of long-term drought on photosynthetic resource economy in maize were associated with a mesophyll and nor a biochemical limitation. As the role of gm was never taken into account in C4 species, previous studies have considered that non-stomatal due to a decrease in carboxylation capacity overrides in maize genotypes under under long-term drought. | |
| Changes in gm were significantly correlated with the droughtinduced change in WUE, what proves the importance of gm in optimizing resource use under water restriction period [25]. The ABA has long-lasting effects on plant hydraulic properties via aquaporin activity, which contributes to the maintenance of a favorable plant water status; if so, such decrease in gm only in drought sensitive genotypes might be linked to impaired root aquaporin activity, as these proteins are an important component controlling gm in herbaceuos plants such as maize [26]. When root aquaporin activity is affected by WD, leaf elongation rate decreases and becomes more sensitive to changes in evaporative demand [27], and only the two sensitive genotypes under WD showed lower LA values compared to FC (Table 3). These assumptions might provide a mechanistic link to at least partially explain the ameliorative effects of drought tolerant genotypes DKB390 and BRS1055 on A via scape of gm decline under WD (Table 1). As gs was reduced in all WD plants, and only plants from sensitive genotypes BRS1010 and 2B710 declined gm values in parallel, we believe that gm compensates reductions in gs in tolerant genotypes. We showed that the N was invested in the photosynthetic apparatus, including carboxylation enzymes, electron transport and light harvesting declined under drought in all genotypes. The N is required for building aquaporins or other proteins that contribute to gm, and ongoing costs of maintaining such proteins [28]. Only WD plants in sensitive genotypes gm was limited by lower N investment. Perhaps, the tolerant genotypes just declined gs from the need to limit E and to prevent runaway xylem embolism, which in turn, favoured a shift of N from carboxylation enzymes to gm. | |
| The oxidative damage whole plant effect in A and yield attributes caused by WD is remarkable. Under WD, the genotypes DKB 390 and BRS 1055 showed similar values of A and TDB, but the DGB was 28% higher in the DKB 390, resulting in a higher HI when compared to BRS 1055 (Table 3). At first instance, the lower HI values in BRS1055 would be interpreted as a low tolerance for WD. However, when compared to its counterpart under FC a decrease of only 9.3% occurred in HI for BRS 1055 under WD. The sensitive genotypes BRS 1010 and 2B710 under WD presented reductions of 22 and 24% in HI, respectively (Table 3). In fact, plants from BRS 1010 and 2B710 presented LA, EM:E and DGB reduced under WD compared to FC (Table 3), indicating the occurrence of a low photoassimilate flow to the grain in these two maize genotypes under WD, compared to genotypes DKB 390 and BRS 1055. The results found in DKB390 and BRS1055 for HI confirmed its higher tolerance to drought compared to BRS1010 and 2B710. | |
| Conclusion | |
| The failure of the antioxidant defense system ABA-mediated in WD plants result in oxidative damage only when genotypes doesnt present another drought tolerance mechanism, resulting in lipid peroxidation increase, thus ultimately impairing A and yield. The unknown tolerance mechanism in tolerant genotypes under long-term drought is linked to scape of gm decline. | |
| Acknowledgements | |
| This research was supported by the Foundation for Research Assistance of Minas Gerais State, Brazil (FAPEMIG, Grant BPD-00477-13) granted to AOL. | |
| References | |
References
- value="1" id="Reference_Titile_Link">FAO. http://faostat.fao.org/site/339/default.aspx. 2014.
- value="2" id="Reference_Titile_Link">Li Y, Sun C, Huang Z, Pan J, Wang L et al. (2009) Mechanisms of Progressive Water Deficit Tolerance and Growth Recovery of Chinese Maize Foundation Genotypes Huangzao 4 and Chang 7-2, Which are Proposed on the Basis of Comparison of Physiological and Transcriptomic Responses. Plant Cell Physiol 50: 2092-2111.
- value="3" id="Reference_Titile_Link">Lopes MS, Araus JL, van Heerden PD, Foyer CH (2011) Enhancing drought tolerance in C(4) crops. J Exp Bot 62: 3135-3153.
- value="4" id="Reference_Titile_Link">Araus JL, Serret MD, Edmeades GO (2012) Phenotyping maize for adaptation to drought. Front Physiol 3: 305.
- value="5" id="Reference_Titile_Link">Harrigan GG, Ridley WP, Miller KD, Sorbet R, Riordan SG, et al. (2009) The forage and grain of MON 87460, a drought-tolerant corn hybrid, are compositionally equivalent to that of conventional corn. J Agric Food Chem 57: 9754-9763.
- value="6" id="Reference_Titile_Link">Tollefson J (2011) Drought-tolerant maize gets US debut. Nature 469: 144.
- value="7" id="Reference_Titile_Link">Barnaby JY, Kim M, Bauchan G, Bunce J, Reddy V, et al. (2013) Drought responses of foliar metabolites in three maize hybrids differing in water stress tolerance. PLoS One 8: e77145.
- value="8" id="Reference_Titile_Link">Souza TC, Castro EM, Magalhães PC, Albuquerque PEP, Marabesi MA (2013a) The influence of ABA on water relation, photosynthesis parameters, and chlorophyll fluorescence under drought conditions in two maize hybrids with contrasting drought resistance. ActaPhysiol Plant 35: 515-527.
- value="9" id="Reference_Titile_Link">Mohammadkhani N, Heidari R (2008) Effects of Drought Stress on Soluble Proteins in two Maize Varieties. Turk J Biol 32: 23-30.
- value="10" id="Reference_Titile_Link">Souza TC, Magalhães PC, Castro EM, Carneiro NP, Padilha FA et al. (2014) ABA application to maize hybrids contrasting for drought tolerance: changes in water parameters and in antioxidant enzyme activity. Plant Growth Reg 73: 205-217.
- value="11" id="Reference_Titile_Link">Ramachandra Reddy A, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161: 1189-1202.
- value="12" id="Reference_Titile_Link">Souza TC, Castro EM, Magalhães PC, Lino LO, Alves ETet al. (2013b) Morphophysiology, morphoanatomy, and grain yield under field conditions for two maize hybrids with contrasting response to drought stress. ActaPhysiol Plant 35: 3201-3211.
- value="13" id="Reference_Titile_Link">Devi R, Kaur N, Gupta AK (2012) Potential of antioxidant enzymes in depicting drought tolerance of wheat (Triticumaestivum L.). Indian J BiochemBiophys 49: 257-265.
- value="14" id="Reference_Titile_Link">Guimarães LJ, Mendes FF, Parentoni SN, Guimarães PEO, Pacheco CAP et al. (2012) Desempenho de Híbridos de Milhoquanto à Tolerância à Seca, XXIX CongressoNacional de Milho e Sorgo, Águas de Lindóia.
- value="15" id="Reference_Titile_Link">Harley PC, Loreto F, Di Marco G, Sharkey TD (1992) Theoretical considerations when estimating mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98: 1429-1436.
- value="16" id="Reference_Titile_Link">Massad RS, Tuzet A, Bethenod O (2007) The effect of temperature on C(4)-type leaf photosynthesis parameters. Plant Cell Environ 30: 1191-1204.
- value="17" id="Reference_Titile_Link">Du N, Fan J, Chen S, Liu Y (2008) A hydraulic-photosynthetic model based on extended HLH and its application to Coast redwood (Sequoia sempervirens). J TheorBiol 253: 393-400.
- value="18" id="Reference_Titile_Link">Niinemets Ü, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20: 845-866.
- value="19" id="Reference_Titile_Link">Queval G, Hager J, Gakière B, Noctor G (2008) Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J Exp Bot 59: 135-146.
- value="20" id="Reference_Titile_Link">Xu Z, Zhou G, Shimizu H (2009) Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J Exp Bot 60: 3737-3749.
- value="21" id="Reference_Titile_Link">Kiirats O, Lea PJ, Franceschi VR, Edwards GE (2002) Bundle sheath diffusive resistance to CO(2) and effectiveness of C(4) photosynthesis and refixation of photorespired CO(2) in a C(4) cycle mutant and wild-type Amaranthusedulis. Plant Physiol 130: 964-976.
- value="22" id="Reference_Titile_Link">Carmo-Silva AE, Powers SJ, Keys AJ, Arrabaça MC, Parry MA (2008) Photorespiration in C4 grasses remains slow under drought conditions. Plant Cell Environ 31: 925-940.
- value="23" id="Reference_Titile_Link">Voss I, Sunil B, Scheibe R, Raghavendra AS (2013) Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol (Stuttg) 15: 713-722.
- value="24" id="Reference_Titile_Link">Centritto M, Lauteri M, Monteverdi MC, Serraj R (2009) Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J Exp Bot 60: 2325-2339.
- value="25" id="Reference_Titile_Link">Hommel R, Siegwolf R, Saurer M, Farquhar GD, Kayler Z, et al. (2014) Drought response of mesophyll conductance in forest understory species--impacts on water-use efficiency and interactions with leaf water movement. Physiol Plant 152: 98-114.
- value="26" id="Reference_Titile_Link">Parent B, Hachez C, Redondo E, Simonneau T, Chaumont F, et al. (2009) Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiol 149: 2000-2012.
- value="27" id="Reference_Titile_Link">Tardieu F, Parent B, Simonneau T (2010) Control of leaf growth by abscisic acid: hydraulic or non-hydraulic processes? Plant Cell Environ 33: 636-647.
- value="28" id="Reference_Titile_Link">Cowan IR (1986) Economics of carbon fixation in higher plants. Givnish TJ. On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, pp.133-176.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 19487
- [From(publication date):
specialissue-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 13985
- PDF downloads : 5502
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
