Analysis of Factors Related to Head MRI Changes in Neonatal Hyperbilirubinemia and Effect on Neurodevelopmental Outcomes
Received: 23-Apr-2021 / Accepted Date: 07-May-2021 / Published Date: 14-May-2021 DOI: 10.4172/2572-4983.1000213
Abstract
Background and objective: This study aims to detect how hyperbilirubinemia affects cerebral structures by imagery (MRI and DWI) to prevent irreversible future brain damage. It will equally help us examine the relationship between imaging and neurodevelopment.
Research design and methods: In a retrospective, controlled study, 50 infants of 35 weeks gestation or more with hyperbilirubinemia were assigned to two groups based on abnormal or normal head imaging (MRI, DWI). The primary outcomes included peak bilirubin levels according to socio-demographic factors and how these levels influence MRI and DWI imaging. The secondary outcome was neurodevelopment at 1, 3, 6, 9 and 12 months for the imaging changes versus the normal imaging groups assessed by a combined formulaire based on the CDC’s developmental milestones and the Denver II Developmental Screening Test (DDST) by investigators.
Results: Peak total serum bilirubin levels (TSB) in the group with MRI changes was significantly higher than those in the group without MRI changes (342.5 ± 47.6 μmol/L vs. 284.3 ± 46.3 μmol/L), p=0.000. In addition, peak TSB values in the group with abnormal DWI was higher than those with normal DWI (314.9 ± 19.5 μmol/L vs. 302.2 ± 55.3 μmol/L), although the difference between both groups (abnormal imaging vs. normal imaging) was not significant. Odds ratios when observing TSB values with 342 μmol/L as cut-off, showed that participants with TSB ≥ 342 μmol/L were 12.4 times more likely of presenting with abnormal MR imaging (OR=12.4, 95% CI 2.191-70.672). In addition, a cross-tab comparison between MRI and milestones depicts MRI as having a 66.7% and 33.3% sensitivity and specificity rates for milestone attainment. The rate of milestone abnormality in participants with imaging changes was 6.0% (3 out of 50), with 2 out of the 3 participants having abnormal imaging and abnormal milestone attainment, while 1 had normal imaging but abnormal milestone attainment.
Conclusion: A significant relationship between high bilirubin levels and head imaging (MRI) was observed, but these changes in imaging could not significantly predict neurodevelopmental outcomes.
Keywords: Hyperbilirubinemia head imaging; Neurodevelopmental outcomes; Neonatal hyperbilirubinemia
Introduction
Neonatal Hyperbilirubinemia (NHB) is a common occurrence affecting about 60% of term and 80% of preterm infants [1,2]. In 1904, Schmorl described yellow staining of the basal ganglia in postmortem findings of infants with neonatal jaundice [3,4]. It refers to increased bilirubin levels seen in neonates with a variable clinical outcome from reversible Acute Bilirubin Encephalopathy (ABE) to irreversible kernicterus [5]. 10% of term and 25% of late preterm deliveries will undergo phototherapy to avoid acute and chronic bilirubin encephalopathy [6]. It is estimated that the cost of caring for a patient with kernicterus over a lifetime is estimated at around $USD 900000 while the estimated cost to prevent a single case of kernicterus with universal total serum bilirubin and/or transcutaneous bilirubin screening is between $USD 5.7 and 9.2 million [7], making neonatal jaundice a health concern.
Magnetic Resonance Imaging (MRI) is used as the imaging of choice for examining structural changes at the level of the basal ganglia and subthalamus, the two most affected cortical areas in encephalopathy [8]. The most widely accepted changes show abnormally increased signal intensity on T1-weighted imaging in these areas. Echo-planar Diffusion-Weighted Imaging (DWI) and Apparent Diffusion Coefficient (ADC) is another technique used to measure the diffusion of water molecules in brain tissue and helps to determine the architecture and integrity of the brain [9]. Few studies have been used to show the structural changes observed using DWI in neonatal hyperbilirubinemia [8,10].
Several studies have been done to show the effect of hyperbilirubinemia on the motor, cognitive, social/emotional and language in the subsequent growth of infants at variable degrees [11]. Can early detection of central nervous system damage in children with hyperbilirubinemia be detected to guide early intervention and reduce permanent damage and sequelae? Can Magnetic resonance imaging (MRI), as the preferred means of observing neuronal mitochondrial injury, be used to detect central nervous system damage early through MRI examination and as an indicator for predicting brain damage and late neurodevelopment? To answer these questions, we conducted retrospective cohort studies in 50 children with hyperbilirubinemia.
Patients and Methods
This retrospective cohort study initially comprised of 53 (23 males, 30 females) neonates with hyperbilirubinemia, with no known congenital abnormalities, delivered either through spontaneous vaginal delivery or cesarean section and admitted in the Neonatal department of the first affiliated hospital of Dalian Medical University from December 1st, 2019 to January 31st, 2021. Overall 50 participants (21 males, 29 females) were included in the study after follow-up and after their guardians gave their informed written consent to participate in the study.
For our study only neonates with hyperbilirubinemia were included. A gestational age (GA) of at least 35 weeks, a postnatal age less than 2 weeks, 5-10 minutes Apgar scores of at least 9 were required for inclusion. Other inclusion criteria were clinically observable jaundice on the first day of birth or on subsequent days, having laboratory data, head MRI and DWI imaging obtained from the neonatalogy department of the first affiliated hospital of Dalian Medical University, where they were admitted for observation and treatment. In addition, all infants were required to have a bilirubin induced neurologic dysfunction, BIND index score (assessing mental status, muscle tone and cry patterns) of 0 at admission. All participants were treated with phototherapy, given in accordance to the updated 2009 American Academy of Pediatrics guidelines for management of neonatal hyperbilirubinemia. Among 53 participants selected for the study, 3 neonates were lost to follow-up and therefore excluded from the study.
Exclusion criteria were (1) congenital anomaly, confirmed infections or metabolic derangement (2) intracranial hemorrhage or injury, (3) those who did not accept to do MRI, DWI imaging, (4) guardians who did not provide consent for the study, (5) guardians lost to follow-up and (6) absent audio brainstem response (ABR) imaging.
A standardized questionnaire was prepared using the age, gender, and contacts of the guardians of participants were obtained. A standard neurological and physical examination to exclude congenital deformities was done by an experienced Pediatrician, who equally used a modified Ballard score to estimate the gestational age of the participants upon admission. After exclusions the remaining 50 neonates were followed-up for a minimum period of one month to a maximum of 12 months and their neurodevelopmental growth accessed using a questionnaire for milestone attainment, adjusted for age. Informed consent was obtained from guardians.
Anthropometric measurements including gender, birth weight, head circumference of each participant was recorded. Birth weight was determined using a secca balance scale (Secca Corp, Columbia, MD, USA), with weight stratification defined by the American Association of Pediatrics as low birth weight LBW <2500 g, normal birth weight NBW 2500-4000 g and high birth weight HBW >4000 g. The head circumference of neonates was obtained using a measuring tape. In addition, the method of delivery (spontaneous vaginal or cesarean), gestational age at delivery, 5 and 10-minute APGAR scores, feeding methods (breastfeeding, formula feeding, or mixed feeding) were equally recorded. APGAR scores were graded on 10 with 10 being the maximum. The number of days from birth to observation of jaundice and/or icterus and the days from admission to head imaging (MRI, DWI) were equally obtained. Etiologies for hyperbilirubinemia were obtained if found after clinical, paraclinical and auxiliary examinations.
A questionnaire based on attainment of milestones at 1, 3, 6, 9 and 12 months respectively was designed for the purpose of this study, based on the Centre of Disease Control (CDC) [12] guidelines and the Denver II Developmental Screening Test (DDST) [13] and was used for follow-up to access development. It characterized development in terms of the following domains; social (emotional), language (communication), cognition, and motor (movement) skills with each criteria based on two questions for assessment and graded as a favorable response if either one or both questions were positively answered. This modified form can be seen in Table 1. These results were adjusted for chronological age in months of normal milestone attainment and graded out of 8 depending on the age in months, and was results were graded as normal if participants had at least a score of 6.
| Age | Social/ Emotional | Language/ Communication | Cognition (learn, think, solve) | Motor skills/ movement |
|---|---|---|---|---|
| 1 month | Starts smiling ( ) | Coos/gurgle ( ) | Follows object ( ) | Moves arms/legs ( ) |
| Focus on face ( ) | Reacts to sound ( ) | Act bored or fuzzy ( ) | Push up when lying on tummy ( ) | |
| 3 months | Spontaneous smile ( ) | Babbling ( ) | Follow side to side, 180 ( ) | Holds head ( ) |
| Copy facial expressions ( ) | Differing cries for pain, etc ( ) | Reaches for toy ( ) | Brings hand to mouth ( ) | |
| 6 months | Recognizes family ( ) | Responds to name ( ) | Brings object to mouth ( ) | Rolls over ( ) |
| Likes to play/ emotions ( ) | Imitates sounds ( ) | Looks around for things ( ) | Learns to sit up ( ) | |
| 9 months | Stranger fright ( ) | Point objects ( ) | Moves objects hand to hand ( ) | Crawling ( ) |
| Show goodbye/ wave ( ) | Understands “No,mama” ( ) | Peek-a-boo ( ) | Pull to stand ( ) | |
| 12 months | Stretches arm/leg when dressing ( ) | Tries to repeat words heard ( ) | Picks up toy when asked ( ) | Can walk more or less ( ) |
| Cries when dad/mom leaves ( ) | Responds to simple requests ( ) | Uses items correctly ( ) | Can stand alone ( ) |
Table 1: Milestone collection form.
Maternal age, delivery method, blood group, and antepartum pathologies were equally collected.
MRI was performed with a 1.5 Tesla (T) clinical MR unit (Magnetom Sonata; Siemens, Erlangen, Germany), with a standard infant head coil after administration of 5% chloral hydrate 0.5 ml/kg per os for sedation. All examinations included axial sections of conventional spin-echo SE T1-weighted (repetition time TR=440 ms, echo time TE=8 ms) and SE T2-weighted (TR=4000 ms, TE=120 ms) sequences and sagittal T1-weighted and coronal T2-weighted sequences. Axial fluid attenuation inversion recovery (FLAIR) T1 (TR=1900 ms, TE=24 ms, inversion time TI=760 ms) while axial FLAIR T2 (TR=8000 ms, TE=124 ms, TI=2000 ms) were used. Lastly axial SE/Echo Planar Imaging (EPI) diffusion-weighted imaging (DWI) (TR=5000 ms, TE=75.4 ms, b-value B=1000 s/mm2).
Section thickness was 6 mm, with a 2-mm gap. Images were obtained by using a 256 × 256 displayed matrix and a 23 mm field of view FOV. Quantitative and qualitative analysis was done by an experienced neuroradiologist who was not aware of the study being conducted.
Universal screening for hearing loss or dysfunction was done in all participants who had their audition tested using Auditory Brainstem Response (ABR) imaging in both ears by an experienced nurse in the neonatal ward. ABR screening was done using CE-Chirp (MAICO Diagnostics GmbH, Berlin, Germany). Here characteristic peaks in the response arise from neural generators in the auditory nerve and brainstem fiber tracks and nuclei forming wave forms. Results of both ears were recorded as pass or refer for follow-up. Any abnormal results were further done by an otolaryngologist who was not aware of the study being conducted. Otoacoustic Emissions (OAE) were not conducted during our study.
Descriptive statistics was used to describe the socio-demographic and clinical characteristics of participants with hyperbilirubinemia. Continuous variables were presented as mean ± standard deviation, and categorical variables were expressed as percentages. Quantitative data (normally-distributed) were summarized as the mean and the standard deviation and compared using two independent sample t-test (student t-test). Mann-Whitey-U test and Kruskal-Wallis test were used for non-normally distributed quantitative data, while Chi-Squared (x2) was used for categorical data. In addition, binary logistic regression analysis was performed to separately access hyperbilirubinemia, changes in imaging, and their relationship with milestone attainment. The strength of association of data was assessed using Spearman correlation coefficient, rs. P-values ≤ 0.05 were considered statistically significant. Analysis was performed using the program IBM Statistical Package for the Social Sciences (SPSS, Inc. Version 25.0. Chicago, Illinois).
Results
Our study comprised of 50 neonates; 21 boys and 29 girls, admitted with a mean age of 3.3 ± 2.1 days and who presented in our department with a mean time of 2.3 ± 1.1 days from birth to clinical observation of jaundice from guardians. The mean weight of admission was 3360.5 ± 489.9 g with a mean gestational age of 38.2 ± 1.2 weeks, and a head circumference of 33.8 ± 1.4 cm. Partaking to nutrition, the participants received exclusive breastfeeding (44%), mixed feeding (52%), and exclusive formula (4%). The average peak TSB levels of those on breastfeeding, formula, and both breastfeeding and formula were 310.7 ± 59.3 μmol/L, 250.5 ± 77.8 μmol/L, and 300.4 ± 46.8 μmol/L. Average transcutaneous bilirubin, Tc Bil levels was 14.6 ± 3.5 mg/dL while serum peak total bilirubin level during hospital stay at 302.9 ± 53.8 μmol/L, accompanied by albumin levels at 34.2 ± 3.6 g/L. Most of the etiologies of hyperbilirubinemia could not be found hence 31 of the participants (62%) had idiopathic neonatal hyperbilirubinemia, followed by 9 suspected infections (abnormal white blood counts but sterile blood culture) at 18%, 7 with ABO incompatibility (14%), and 3 with polycythemia (6%). Exclusive phototherapy was initiated on 37 participants (74%), phototherapy and antibiotics on 7 (14%), phototherapy and albumin in 2 (4%), phototherapy and IVIG in 1 (2%), phototherapy combined with IVIG and albumin in 2 (4%), and phototherapy combined with mannitol and phenobarbital in 1 (2%). They respectively had mean peak TSB values of 296.3 ± 51.9 μmol/L, 307.6 ± 63.2 μmol/L, 388.1 ± 59.2 μmol/L,326.9 μmol/L, 314.8 ± 15.8 μmol/L, and 298.1 μmol/L. Maternally, the mothers of the participants had a mean age of 32.4 ± 3.4 years, who delivered through spontaneous vaginal; 28(56%) and cesarean; 22(44%) deliveries. 22 mothers (44%) were primpara while 28 mothers (56%) were multipara. They had the following blood groups A+ (26%), B+ (30%), AB+ (10%) and O+ (34%), and the following gestational pathologies; hypertension only (6%), diabetes only (10%), hypertension and diabetes (8%), thyroid disorder (4%), and others (HELLP, DIC, vaginitis all at 12%), while 60% had no known antepartum pathology. Baseline characteristics of participants were recorded in Table 2 below. In addition, baseline characteristics according to peak TSB values were recorded in Table 3.
| Variables | Baseline value (n=50) | Mean ± SD | ||
|---|---|---|---|---|
| Gender: | Male Female | 21 (42%) | ||
| 29 (58%) | ||||
| Birth weight: | LBW (2(4%)2130.0 ± 14.1 | |||
| NBW (2500-4000 g) | 42 (84%) | 3297.0 ± 315.7 | ||
| HBW (>4000 g) | 6 (12%) | 4215.0 ± 141.7 | ||
| GA (weeks): | Preterm (<37) | 2 (4%) | 35.5 ± 0.7 | |
| Early term (37-38) | 25 (50%) | 37.5± 0.5 | ||
| Full-term (>38) | 23 (46%) | 39.3 ±0.5 | ||
| Peak TSB (μmol/L): | Low TSB (<256) | 10 (20%) | 225.6 ± 23.9 | |
| Moderate TSB (256-340) | 31 (62%) | 304.7 ± 21.2 | ||
| High TSB (>340) | 9 (18%) | 382.9 ± 26.2 | ||
| Nutrition: | Breastfeeding | 22 (44%) | ||
| Formula | 2 (4%) | |||
| Mixed | 26 (52%) | |||
| Delivery: | Vaginal | 28 (56%) | ||
| Cesarean | 22 (44%) | |||
| Therapy: | P only | 37 (74%) | ||
| P+A | 7 (14%) | |||
| P+Alb | 2 (4%) | |||
| P+Alb+IVIg | 1 (2%) | |||
| P+A+Alb+IVIG | 2 (4%) | |||
| P+M+Ph | 1 (2%) | |||
| Etiology: | Idiopathic | 31 (62%) | ||
| Infections | 9 (18%) | |||
| ABO incompatibility | 7 (14%) | |||
| Polycythemia | 3 (6%) | |||
| Age (days) | 3.3 ± 2.1 | |||
| HC (cm) | 33.8 ± 1.4 | |||
| Tc (mg/dL) | 14.6 ± 3.5 | |||
| Albumin (g/L) | 34.2 3.6 | |||
| Jaundice observation from birth (days): | 2.3 1.1 | |||
| Days from admission to imaging: | 3.4. ± 1.5 | |||
| Maternal age (years): | 32.4 3.4 | |||
| Gravida: | Primipara | 22 (44%) | ||
| Multipara | 28 (56%) | |||
| Antepartum pathology: | None | 30 (60%) | ||
| Hypertension only | 3 (6%) | |||
| Diabetes only | 5 (10%) | |||
| Hypertension and diabetes | 4 (8%) | |||
| Thyroid disorders | 2 (4%) | |||
| Others | 6 (12%) | |||
| Maternal blood group | A+ | 13 (26%) | ||
| B+ | 15 (30%) | |||
| AB+ | 5 (10%) | |||
| 0+ | 17 (34%) | |||
| ABR | Normal | 45 (90%) | ||
| Abnormal | 5 (10%) | |||
| MR imaging | Normal | 34 (68%) | ||
| Abnormal | 16 (32%) | |||
| Milestones | Normal | 47 (94%) | ||
| Abnormal | 3 (6%) | |||
Abbreviations: LBW: Low Birth Weight; NBW: Normal Birth Weight; HBW: High Birth Weight; GA: Gestational Age, HC: Head Circumference; Tc: Transcutaneous bilirubin; P: Phototherapy; P+A: Phototherapy and Antibiotics; P+Alb: Phototherapy and Albumin; P+Alb+IVIg: Phototherapy Albumin and Intravenous Immunoglobulins; P+M+Ph: Phototherapy, Mannitol and Phenobarbital; others include HELLP syndrome, APS, thyroiditis and vaginitis.
Table 2: Baseline characteristics of participants in our study.
| Factors | Mean peak TSB in μmol | P-value |
|---|---|---|
| Males | 325.8 ± 43.9 | |
| Females | 286.4 ± 54.8 | 0.009 |
| LBW | 258.9 ± 28.9 | |
| NBW | 305.4 ± 52.8 | |
| HBW | 300.2 ± 66.7 | 0.494 |
| Preterm | 282.0 ± 3.7 | |
| Early Term | 303.2 ± 55.7 | |
| Full-Term | 304.5 ± 54.9 | 0.856 |
| Breastfeeding | 310.7 ± 59.3 | |
| Mixed nutrition | 300.4 ± 46.8 | |
| Formula | 250.5 ± 77.8 | 0.304 |
| Etiology: Idiopathic | 306.1 ± 56.5 | |
| Probable infections | 307.3 ± 58.5 | |
| ABO hemolysis | 296.2 ± 54.4 | |
| Polycythemia | 276.0 ± 81.7 | |
| Intracranial hemorrhage | 294.4 ± 19.5 | 0.938 |
| Phototherapy only | 296.3 ± 51.8 | |
| Phototherapy and antibiotics | 307.6 ± 63.2 | |
| Phototherapy and albumin | 388.1 ± 59.2 | |
| Phototherapy, albumin and IVIG | 314.8 ± 15.8 | 0.32 |
| Maternal age: Less than 30 years | 332.3 ± 47.5 | |
| Maternal age: 30 years and above | 296.5 ± 53.4 | 0.07 |
| Primipara | 299.1 ± 39.9 | |
| Multipara | 306.0 ± 63.1 | 0.655 |
| Vaginal delivery | 317.9 ± 49.3 | |
| Cesarean delivery | 283.8 ± 54.2 | 0.024 |
| No antepartum pathology | 310.8 ± 58.1 | |
| Maternal hypertension | 305.3 ± 13.1 | |
| Maternal diabetes | 249.3 ± 59.1 | |
| Maternal hypertension and diabetes | 322.9 ± 48.5 | |
| Maternal thyroid disorders | 307.3 ± 2.5 | |
| Other antepartum pathologies | 292.4 ± 27.9 | 0.27 |
| A+ | 335.9 ± 38.9 | |
| B+ | 296.1 ± 54.1 | |
| AB+ | 312.0 ± 57.2 | |
| O+ | 281.0 ± 53.6 | 0.038 |
| Abnormal MRI | 342.5 ± 47.6 | |
| Normal MRI | 284.3 ± 46.3 | 0 |
| Abnormal DWI | 314.9 ± 19.5 | |
| Normal DWI | 302.2 ± 55.3 | 0.695 |
| Normal ABR | 302.9 ± 50.0 | |
| Abnormal ABR | 303.7 ± 88.9 | 0.975 |
| Normal Milestones | 304.1 ± 54.6 | |
| Abnormal Milestones | 284.8 ± 40.3 | 0.552 |
| Albumin289.3 ± 47.2 | ||
| Albumin ≥ 35 g/L | 320.3 ± 57.6 | 0.041 |
| Jaundice observation on day 1 | 293.9 ± 49.9 | |
| Jaundice observation >day 1 | 305.2 ± 55.0 | 0.561 |
| Radial artery | Ulnar artery | |
| Diameter (cm) | 0,23 ± 0,06 | 0,26 ± 0,02 |
| Flow (ml/min) | 24 ± 16 | 31 ± 22 |
| Flow rate (cm/sec) | 47 ± 16 | 56 ± 19 |
Table 3: Summary table of average peak TSB values with socio-demographic factors of participants.
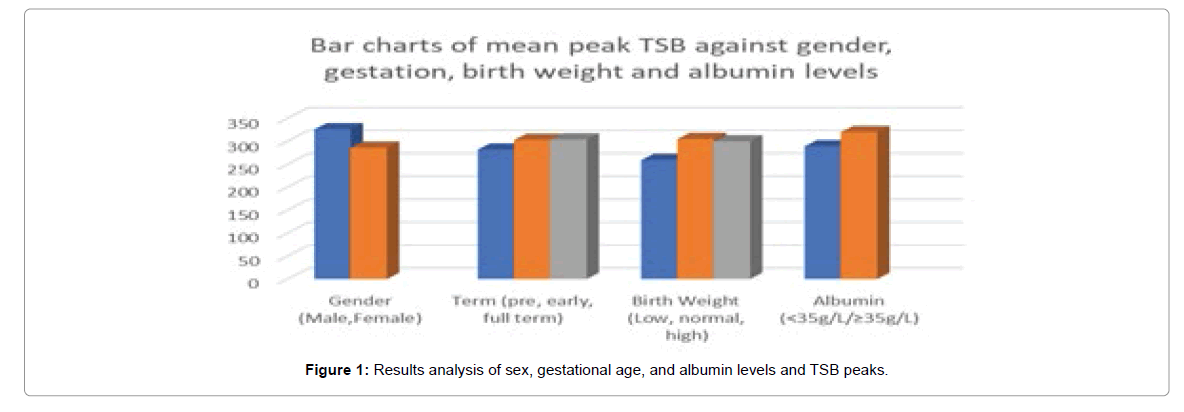
MRI and DWI modalities were done on average 3.4 ± 1.5 days after admission in our neonatal ward. All the participants were divided into two groups; the first group with MR and/or DWI imaging showing abnormalities (68%) and the second group without these changes (32%). Average peak TSB values of participants in the abnormal MRI group was 342.5 ± 47.6 μmol/L whereas average peak TSB values in the group with normal imaging was 284.3 ± 46.3 μmol/L and there was a significant difference between both groups (P=0.000), as seen in Table 3. In addition, peak TSB in the abnormal and normal DWI groups were 314.9 ± 19.5 μmol/L and 302.2 ± 55.3 μmol/L. Comparison of demographic and biochemical data in participants with hyperbilirubinemia with or without imaging changes were recorded in Table 4. The mean values of weights (LBW, HBW), gestational age (preterm and early term), and head circumference were lower in neonates with MRI changes compared to those without MRI changes. However, the total bilirubin levels (high and moderate risk TSB), transcutaneous bilirubin, albumin levels, age of admission, and days of observation of jaundice from birth were higher in neonates with MRI changes compared to those without. Moreover, the differences between both groups were not statistically significant (Figure 1).
| Variables | Imaging changes (16) | No imaging changes (34) | P-value |
|---|---|---|---|
| Male (%) | 8(50.0) | 13(38.2) | |
| Female (%) | 8(50.0) | 21(61.8) | 0.543 |
| Gestational age (weeks) | 38.0 ± 1.4 | 38.4 ± 1.1 | 0.325 |
| Age (days) | 4.2 ± 2.2 | 2.9 ± 1.9 | 0.034 |
| Low peak TSB (μmol/) | 0.0 ± 0.0 | 225.6 ± 23.9 | |
| Moderate peak TSB (μmol/L) | 305.7 ± 15.9 | 304.3 ± 23.3 | |
| High peak TSB (μmol/L) | 389.9 ± 25.5 | 358.6 ± 5.6 | 0 |
| Preterm (weeks) | 35.0 ± 0.0 | 36.0 ± 0.0 | |
| Early term (weeks) | 37.3 ± 0.5 | 37.6 ± 0.5 | |
| Full term (weeks) | 39.3 ± 0.5 | 39.3 ± 0.5 | 0.708 |
| LBW (grams) | 2120 ± 0.0 | 2140 ± 0.0 | |
| NBW (grams) | 3306.4 ± 330.0 | 3292.3 ± 314.3 | |
| HBW (grams) | 4100.0 ± 0.0 | 4238.0 ± 145.3 | 0.332 |
| Transcutaneous bilirubin (mg/dL) | 15.7 ± 4.3 | 14.0 ± 3.0 | 0.11 |
| Albumin (g/L) | 34.9 ± 3.3 | 33.9 ± 3.6 | 0.329 |
| Head circumference (cm) | 33.4 ± 1.4 | 33.9 ± 1.4 | 0.394 |
| Jaundice observation on day 1 (%) | 2 (12.5%) | 8 (23.5%) | |
| Jaundice observation from day 2 (%) | 14 (87.5%) | 26 (76.5%) | 0.468 |
| Breastfeeding | 8 (50,0%) | 14 (41.2%) | |
| Mixed | 7 (43.8%) | 19 (55.9%) | |
| Formula | 1 (6.3%) | 1 (2.9%) | 0.489 |
| Abnormal milestones | 2 (12.5%) | 1 (2.9%) | |
| Normal milestones | 14 (87.5%) | 33 (97.1%) | 0.237 |
| Right basal ganglia signal intensity | 580.65 | 450.36 | 0.001 |
| Left basal ganglia signal intensity | 582.2 | 452.44 | 0.001 |
| Etiologies | 0.565 | ||
| Therapies | 0.087 |
Table 4: Comparison of demographic and biologic data of neonates with hyperbilirubinemia between those with normal and abnormal imaging.
Maternal factors such as age, gravida number, blood group, and antepartum pathologies were collected and compared between groups and recorded in Table 5. It can be seen that there is a statistically significant difference between antenatal pathologies in the group of infants presenting with MRI imaging compared to those without. Infants with mothers with both gestational hypertension (eclampsia, others) and gestational diabetes had higher bilirubin levels (322.9 ± 48.5 μmol/L) compared to those with other pathologies. In addition, the mean maternal age of infants presenting with changes on imaging is higher than those without changes. Moreover, no significant difference was found between imaging changes in infants who had primipara and multipara mothers to those without imaging changes. Lastly, maternal blood groups were similar in both groups, although mothers with the A+ blood group had infants with higher peak TSB values (335.9 ± 38.9 μmol/L) than infants of mothers from other blood groups. Peak TSB values in participants with mothers described above had these findings shown in Figures 2 and 3.
| Maternal variables | Imaging changes (16) | No imaging changes (34) | P-value |
|---|---|---|---|
| Maternal age (years): | 32.8 ± 3.8 | 32.2 ± 3.2 | 0.879 |
| Antepartum pathology (%): None | 6 (37.5) | 24 (70.6) | |
| Hypertension only | 2 (12.5) | 1 (2.9) | |
| Diabetes only | 1 (6.3) | 4 (11.8) | |
| Hypertension and diabetes | 3 (18.8) | 1 (2.9) | |
| Thyroid disorders | 1 (6.3) | 1 (2.9) | |
| Others | 3 (18.8) | 3 (8.8) | 0.047 |
| Maternal blood group: A+ | 7 (43.8) | 6 (17.6) | |
| B+ | 4 (25.0) | 11 (32.4) | |
| AB+ | 2 (12.5) | 3 (8.8) | |
| 0+ | 3 (18.8) | 14 (41.2) | 0.067 |
| Primipara (%): | 5 (31.3) | 17 (50.0) | |
| Multipara (%): | 11 (68.8) | 17 (50.0) | 0.24 |
| Vaginal delivery (%) | 8 (50.0) | 20 (58.8) | |
| Cesarean delivery (%) | 8 (50.0) | 14 (41.2) | 0.388 |
Table 5: Maternal factors and changes with imaging.
All neonates participating in the study were tested for audition after admission in the neonatology ward. The results of their audition testing went in line with understanding requests when milestone development was tested according to age. Mean peak TSB values for normal and abnormal milestone attainment were 304.1 ± 54.6 μmol/L and 284.8 ± 40.3 μmol/L respectively while peak mean TSB values for normal ABR and abnormal ABR were 302.9 ± 50.0 μmol/L and 303.7 ± 88.9 μmol/L. Additionally, there was no significant difference between MRI changes and normal MRI groups when evaluating milestones (p=0.237). Peak TSB values in participants were lower in those with abnormal milestone attainment compared to normal whereas participants with abnormal ABR had higher peak TSB values compared to those with normal ABR.
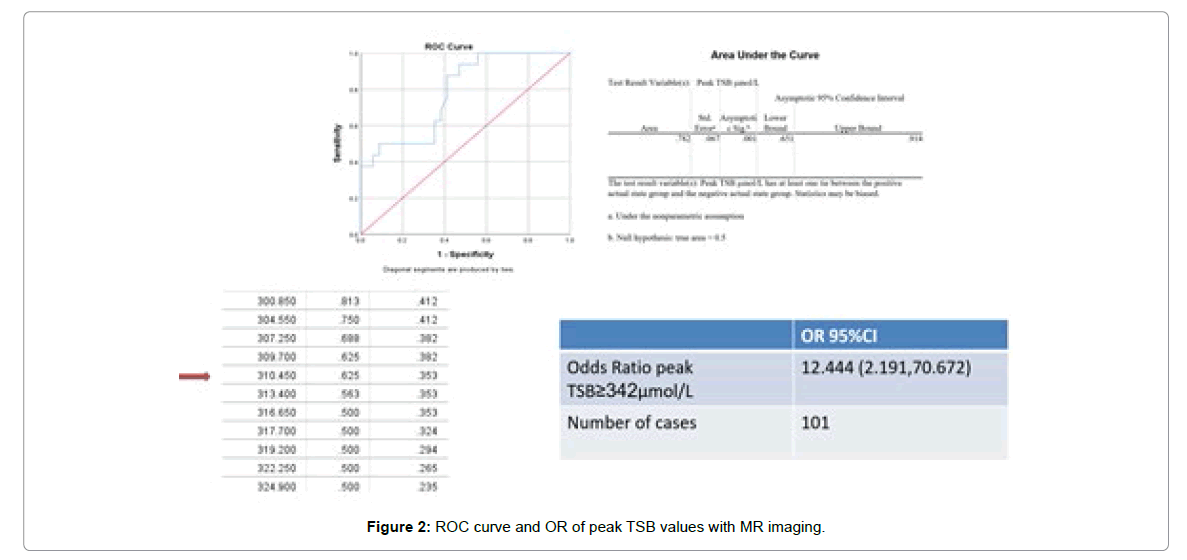
Axial T1 MRI signal intensity values of the left and right basal ganglia were measured and recorded in Table 6 below. These signal values were compared to peak TSB levels, ABR and milestone attainment and pearson correlation were obtained to evaluate the relationship between the variables. From Table 6 below, we can conclude that signal values of the left and right basal ganglia are significantly higher when compared to various levels of peak TSB (right P-value=0.005, left P-value=0.0040). Meanwhile it can be seen that signal values of abnormal ABR and milestones in participants are higher on average than normal ABR and milestones, although the difference is insignificant. Further extrapolation of cut-off values to determine the sensitivity and specificity of MR imaging using an Receiver operator characteristic, ROC curve shows a significant area=0.782, with a peak TSB value of 310.5 μmol/L having true and false positive values of 62.5% and 35.3% on MR imaging (Figure 2). This confirms MR imaging is a fairly good test for detecting changes as a result of hyperbilirubinemia as demonstrated by numerous earlier studies. In addition, a cross-tab comparison between MRI and milestones depicts MRI as having a 66.7% and 33.3% sensitivity and specificity rates for milestone attainment (Tables 7-9).
| Gender | Age | MRI | Criteria for abnormality of milestones |
|---|---|---|---|
| Male | 3 months | Abnormal | Can’t hold head, no spontaneous smile, doesn’t reach for toy, nor babble. |
| Male | 3 months | Normal | Doesn’t follow at 180, reach for toy, babble nor holds his head. |
| Male | 3 months | Abnormal | Indifferent cries, doesn’t bring hand to mouth constantly, nor reach for toys, can’t hold his head |
| Male | 6 months | Abnormal | Doesn’t try to sit, like to play, respond to name nor roll over. |
| Female | 6 months | Normal | Can’t roll over, try to sit up, recognize family, and absent response to name, |
| Female | 9 months | Abnormal | Can’t wave, crawl,point to objects, move objects from hand to hand nor has stranger fright. |
Table 6: Participants with abnormal milestones and criteria for abnormality.
| Variables | Right signal value | Left signal value | P-value | r |
|---|---|---|---|---|
| Milestones: Normal | 483.6 ± 134.9 | 487.0 ± 136.1 | 0.084a | 0.247a |
| Abnormal | 624.6 ±115.5 | 603.1 ±99.0 | 0.155b | 0.204b |
| ABR: Normal | 489.9 ±137.3 | 491.7 ±135.8 | 0.751a | 0.046a |
| Abnormal | 510.8 ±149.5 | 513.5±155.1 | 0.740b | 0.048a |
| Low TSB | 368.3 ±81.9 | 366.7 ±78.9 | ||
| Moderate TSB | 519.5 ±134.6 | 522.5 ±130.7 | 0.005a | 0.389a |
| High TSB | 535.2 ±124.4 | 537.1 ±131.0 | 0.004b | 0.400b |
Table 7: Comparison of ABR and milestones in neonates with hyperbilirubinemia with changes in MR imaging.
| MR imaging * Milestone attainment Crosstabulation | |||||
|---|---|---|---|---|---|
| MR imaging | Milestone attainment | Total | |||
| Normal | Abnormal | ||||
| Abnormal | Count | 14 | 2 | 16 | |
| % within Milestone attainment | 29.80% | 66.70% | 32.00% | ||
| Normal | Count | 33 | 1 | 34 | |
| % within Milestone attainment | 70.20% | 33.30% | 68.00% | ||
| Total | Count | 47 | 3 | 50 | |
| % within Milestone attainment | 100.00% | 100.00% | 100.00% | ||
Table 8: Sensitivity and specificity tests between MRI changes and Milestones.
| Variables | RR (95% CI) | p-value | R2 |
|---|---|---|---|
| Gender | 1.615 (0.487, 5.361) | 0.443 | 0.012 |
| Pregnancy number | 0.802 (0.455, 1.413) | 0.445 | 0.012 |
| Peak TSB | 0.971 (0.953 ,0.990) | 0.002 | 0.263 |
| Age of participant | 0.730 (0.532, 1.003) | 0.005 | 0.084 |
| Albumin | 0.916 (0.770, 1.090) | 0.323 | 0.02 |
| Delivery type | 0.700 (0.212, 2.311) | 0.558 | 0.007 |
| Feeding type | 1.246 (0.667, 2.292) | 0.48 | 0.01 |
| Maternal ABO | 1.635 (0.956, 2.794) | 0.072 | 0.068 |
| Transcutaneous bilirubin | 0.864 (0.721, 1.036) | 0.114 | 0.052 |
| Gestational pathology | 0.515 (0.317 ,0.836) | 0.007 | 0.074 |
| Gestational age | 1.301 (0.774, 2.187) | 0.32 | 0.02 |
| Birth weight | 1.001 (0.999, 1.002) | 0.435 | 0.012 |
| Jaundice observation from birth | 0.274 (0.103, 0.731) | 0.01 | 0.095 |
| Head circumference | 1.329 (0.842, 2.098) | 0.214 | 0.032 |
| Maternal age | 0.955 (0.799, 1.141) | 0.613 | 0.005 |
Table 9: Associated risk factors for neonates with hyperbilirubinemia on having abnormal MRI.
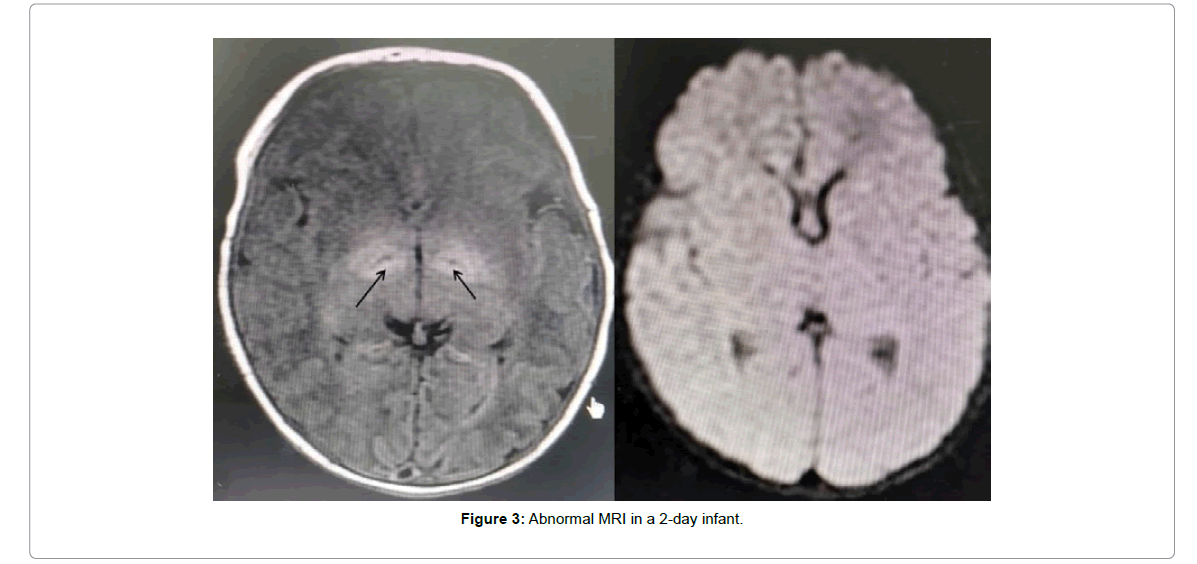
It is interesting to note that a 2-day old female infant who had MRI changes (bilateral symmetrical lentiform increased intensity) in Figure 3, had the second lowest albumin level among the participants (31.8 g/L), with moderate peak TSB levels (305.5 μmol/L), showed a left ear abnormal ABR and showed delayed milestone attainment at 9 months of age, whereas she showed normal attainment at 1, 3 and 6 months of age. Compared to her age group at 9 months, she scored 3 out of 8 from our questionnaire, whereby social skills were abnormal (cannot wave, no strangers fright), motor skills were abnormal (cannot crawl), abnormal communication skills (cannot point to expected objects), and abnormal cognition (cannot move objects from one hand to the other).
Direct logistic regression was employed to assess the impact of several demographic and clinical factors on the likelihood that infants with hyperbilirubinemia will present with abnormal changes on imaging. From our results obtained, peak TSB explained the greatest variance in abnormal MRI at 26.3%, followed by days in observing jaundice in participants (9.5%), age of participants (8.4%), and maternal gestational pathology (7.4%).
Odds ratios when observing TSB values with 342 μmol/L as cut-off compared to MR imaging was also statistically significant (p=0.003), as this cut-off showed that participants with TSB ≥ 340 μmol/L were 12.4 times more likely of presenting with abnormal MR imaging (OR=12.4, 95% CI 2.191-70.672).
Discussion
Neonatal jaundice is a chronic worldwide problem and a major cause of morbidity especially in developing countries [14-16]. Poor screening methods at birth or maternal follow-up, presence of hemoglobinopathies and blood cell defects, ill-equipped medical facilities, and high medical cost burden all cause a rise in morbidity due to hyperbilirubinemia compared to developed nations [15].Bilirubin encephalopathy is not uncommon in China, accounting for about 4.8% of neonatal hyperlirubinemia. Neonatal hyperbilirubinemia has been found to have an association with development outcomes as it is well known that chronic bilirubin encephalopathy (kernicterus) may lead to long-term neurological and physical deficits [17-19].
It is true the incidence of kernicterus has decreased in more developed nations partaking to meticulous screening and follow-up compounded with early phototherapy and/or exchange transfusion when required but cautious observation has been on the wane leading to an increase in cases seen in neonates with hyperbilirubinemia and Bilirubin Associated Neurological Dysfunction (BIND) [20].
Findings from our study showed that neonates with hyperbilirubinemia who have abnormal MR imaging had an association with their gestational ages, birth weights, albumin levels, peak TSB levels, maternal pathology which may have an effect on the neurodevelopment outcome of these neonates.
In the present retrospective cohort, it was observed that the mean gestational age of infants with hyperbilirubinemia which presented with high signal intensity on MR imaging was lower compared to those without changes in imaging. A lengthier change process in dynamic properties and mitochondrial changes has been observed in preterm infants, especially extremely premature ones and this may explain the abnormal image scans preceding the normal ones [21]. In addition, myelination may be a factor causing high signal intensity in preterm populations which may be seen falsely as abnormal imaging due to high bilirubin levels [22]. Our study also supports a higher risk of MRI changes in newborns with low gestational age.
Birth weight is another risk factor for hyperbilirubinemia. Birth weight and prematurity work in tandem as a low gestational age neonate will most likely have a low birth weight after delivery. A study by Woodward demonstrated that low-weight preterm infants with mild to moderate white matter abnormality on MRI had delays by 4 years of age [23]. These studies were done in preterm infants hence the delay in language development could not be ascertained to be due to prematurity, hyperbilirubinemia, or both. Another study showed an increased risk of cerebral palsy in low-birth-weight infants born preterm [24] However, Amin et al. found no difference in terms of audition and language after a 3-year follow-up of infants, whereby they found no difference in peak TSB levels between this group and control [25]. Wu after adjustment for socioeconomic factors and birth weight showed hyperbilirubinemia was not a risk factor for the development of autism [26]. Findings from our study showed that neonates who presented with jaundice had lower mean weights in the group with MRI changes compared to those without MRI changes. The difference though was not statistically significant (p=0.332). Contrary to their findings, our cohort showed a significant difference in peak TSB levels between groups, with the TSB levels of participants with MRI changes significantly higher than those without (p=0.000). Considering that it is related to the sample size, we will further expand the sample size to extend the follow-up time.
It is known that hyperbilirubinemia affects more boys than girls [27]. A study showed that male neonates with a TSB>340 μmol/L had an increased risk of having an IQ<85 on follow-up at 17 years with affected cognition and physical performance [28]. In addition, another study showed that male hyperbilirubinemic infants on phototherapy, followed during a 5-year period had an increased risk of developing epilepsy, but not females [29]. In our study, gender was shown to be statistically different in both groups in term of peak bilirubin levels and MRI changes (p=0.09). Although there were 42 males compared to 59 females, males had significantly higher TSB levels than females (325.8 ± 43.9 μmol/L vs 286.4 ± 54.8 μmol/L) respectively.
Unconjugated bilirubin is formed from senescent red blood cells and it binds to albumin to be transported to the liver for conjugation. Hence fluctuations in serum albumin levels can affect this association and result in higher bilirubin levels than normal [30]. In our study, the mean albumin levels in the group with MRI changes was higher but this group had higher mean peak bilirubin levels compared to the group with no MRI changes, who had lower mean albumin and lower peak bilirubin levels. Contrary to previous findings, our study showed higher peak TSB values in participants with higher albumin levels and vice-versa.
Auditory neural pathways are highly sensitive to bilirubin-induced toxicity especially in preterm infants who are at a higher risk of this toxicity [8,31]. Gunn rat models, used to study the effect of hyperbilirubinemia on the CNS show cell loss and gliosis due to hyperbilirubinemia are most prominent in the auditory nuclei of the brainstem [32]. The effect of hyperbilirubinemia on audition from audio-metric evidence for a predominantly high-frequency bilateral and symmetric hearing loss with recruitment and abnormal loudness growth functions correlates with pathologic lesions in the cochlear nuclei [33,34]. Infants born at ≤ 36 weeks gestational age with a mean peak TSB of 12 mg/dL was a significant predictor for hearing loss [35]. In our study, 6 out of 32 patients with abnormal MR imaging had an abnormal audio brainstem response (ABR). It is worth mentioning that among these 6 participants, only one went on to show a delayed attainment of milestones at 9 months of age after previously having normal milestones at 1, 3, and 6 months of age (Figure 3). Audiologic follow-up of patients showed normal ABR examinations. This concords with previous findings which show that auditory impairment due to hyperbilirubinemia is reversible. Improvement in ABR after exchange therapy suggested that previously thought auditory dysfunction as irreversible may be reversible [36].
Maternal factors such as delivery methods, age, and antepartum pathology can be risk factors for neonatal hyperbilirubinemia. In our study, it was shown that mothers with hypertension and diabetes with other factors had infants with higher mean bilirubin levels and had significant changes on imaging compared to mothers of infants without MRI changes (p=0.047). Our study showed there were more mothers of A+ blood groups in the MRI change group compared to the group without change. Multipara deliveries were associated with infants having higher TSB levels than primipara deliveries although the difference was not significant (p=0.638).
Cesarean delivery is a well-known protective factor in infants with neonatal jaundice and decreased chances of readmission because of hyperbilirubinemia due to prolonged hospital stay, hence improved monitoring, early formula supplementation, less placental transfusion and stress prior to delivery prompting induction of conjugating enzymes or metabolism of bilirubin as an oxidant. In addition, cesarean sections serve as a protective factor against neonatal jaundice possibly by production of more conjugating enzymes in stressed infants accompanied by less transplacental circulation after such delivery [37]. In our study, 45 of 101 participants had cesarean deliveries, with 16 (50.0%) in the MRI changes group while 29 (42.0%) in the normal MRI group, although the difference between both groups was not statistically significant. Interestingly, infants born through cesarean sections had mean peak values of 283.8 ± 54.2 μmol/L compared to 317.9 ± 49.3 μmol/L born by spontaneous vaginal delivery and the difference between peak TSB in both groups was statistically significant (p=0.024). This confirmed cesarean delivery as a protective factor of hyperbilirubinemia as previously discussed. In addition, 12 participants born by spontaneous vaginal delivery had peak TSB values >342 μmol/L compared to those born by cesarean section with 6 participants in that range.
Koziol established a set of hypotheses of sub-cortical mechanisms of cognitive and behavioral dysfunction due to hyperbilirubinemia [38]. Lesions confirmed to more rostral and dorsal-medial regions of the globus pallidus produce the greatest cognitive defect in patients [39]. Bilirubin-related impairment within the dorsal regions of the globus pallidus affects working memory functions, critical for a child’s success [40]. Hence some of the cognitive deficits observed in infants as they grow up may partake to the deposition of toxic unconjugated bilirubin on the aforementioned brain structures which may be observed on MR imaging and may help us gain a better insight in understanding the origin of some observed cognitive, motor, auditory and social deficits. Typical magnetic resonance imaging (MRI) shows a transition of symmetrical hyper intensity in the global pallidus and thalamus earlier on T1WI (T1 Weighted Imaging) to this symmetrical hyper intensity on T2WI (T2 Weighted Imaging) indicating a poorer prognosis in acute bilirubin encephalopathy [8,18,41,42]. The debate persists if lower levels of bilirubin can cause brain damage in term neonates and if different degrees of bilirubin levels correlate with different patterns of brain injury, thereby leading to a change in imaging observed [43]. There was a significant difference between participants with MRI changes, who had a mean bilirubin level of 342.5 ± 47.6 μmol/L to 284.3 ± 46.3 μmol/L in those without changes (p=0.000), with a pearson coefficient of -0.510. There was a significant correlation between serum bilirubin levels and signal strength on magnetic resonance imaging, i.e. increased serum bilirubin and increased MRI signal intensity. ROC curve showed that bilirubin levels of 310.5 μmol had true and false positive rates of 62.5% and 35.3% on MR imaging. Odds ratios when observing TSB values with 342 μmol/L as cut-off showed that participants with TSB ≥ 342 μmol/L were 12.4 times more likely of presenting with abnormal MR imaging (OR=12.4, 95%CI 2.191-70.672). Therefore, we believe that: TSB ≥ 342 μmol/L can be used as a reference standard for testing head MRIs in children with hyperbilirubinemia.
The high symmetric signal in the globus pallidus of four 5-to-21-day old neonates with acute kernicterus had disappeared during follow-up but there was no correlation with prognosis of the patient 44. Our study designed a form based on CDC guidelines for milestone attainment and the Denver II Developmental Screening Test (DDST) scale for infant development. Among all the participants involved, 6 participants had abnormal scores during the study, with 1 participant having a delay at 9 months during follow-up. Moreover comparison of signal intensity values against milestones revealed no difference (p=0.084, 0.155 for right and left basal ganglia respectively). These results suggest that abnormal MRIs are not accurate in scoring CDC developmental milestones for follow-up.
Among all the participants involved, 6 participants had abnormal scores during the study, with 1 participant having a delay at 9 months during follow-up. Johnson observed cognitive deficits in neonates as they develop regardless of the cause of neonatal jaundice [44,45]. Some aspects of nervous system functioning and cognition in infants and children may not be apparent until much later than 3 years [46].Therefore, the effect of imaging changes in MRI on the long-term prognosis of children with hyperbilirubinemia may require longer follow-up times and increased sample size for further study.
Limitations of our retrospective cohort involve a small sample size and loss of participants during follow-up for various reasons. Another limitation was the reliability of the follow-up of some participants on their guardians who had to recall from memory if the participants had indeed attained their milestones when required. The difficulty of patients coming personally for rechecks and examinations was accentuated by the current pandemic which required the population from coming to hospitals unless when absolutely necessary. Lastly but not the least was the short time for follow-up as most neurological deficits are observed at around 2 years of age after maturation of frontal cortices and neuropsychiatric symptoms are observed much later as infants grow and the multiple confounding factors which could not be controlled as participants remained at home in different environment and conditions. This was not possible in our study as follow-up time was shorter. We hope that our study may serve as a blueprint for future research with a greater number of participants and ample time for the follow-up of such patients to observe their neurodevelopmental outcomes as they mature.
Conclusion
In children with hyperbilirubinemia, with the increase of total serum bilirubin levels, the probability of abnormalities in the head magnetic resonance imaging (MRI) increased significantly ≥ 342 μmol/L. Although early MRI imaging changes cannot independently predict the outcome of early neurodevelopment in infants with hyperbilirubinemia, it is necessary to extend the follow-up regarding growth and development in children with MRI abnormalities.
References
- Stevenson DK, Dennery PA, Hintz SR. (2001) Understanding newborn jaundice.J Perinatol. 21: 21-39.
- Vinod BK, Stark AR, Lazzeroni LC, Poland R, Gourley GR, et al. (2013) Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr 162: 477-482.
- Shapiro SM (2003) Bilirubin toxicity in the developing nervous system. Pediatr Neurol. 29: 410-421.
- Cashore WJ (1990) The neurotoxicity of bilirubin. Clin Perinatol. 17:437-47.
- Juretschke LJ (2005) Kernicterus: Still a concern. Neonatal Netw. 24:7-19.
- Sarici SU (2010) Incidence and etiology of neonatal hyperbilirubinemia. J Trop Pediatr. 56:128-9.
- Suresh GK, Clark RE (2004) Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 114: 917-24.
- Shapiro SM (2010) Chronic bilirubin encephalopathy: Diagnosis and outcome. Semin Fetal Neonatal Med. 15:157-63.
- Cece H, Abuhandan M, Cakmak A, Yildiz S, Calik M, et al. (2013) Diffusion-weighted imaging of patients with neonatal bilirubin encephalopathy. Jpn J Radiol. 31:179-85.
- Sari S, Yavuz A, Batur A, Bora A, Caksen H. (2015) Brain magnetic resonance imaging and magnetic resonance spectroscopy findings of children with kernicterus. Pol J Radiol. 11;80:72-80.
- Wusthoff CJ, Loe IM (2015) Impact of bilirubin-induced neurologic dysfunction on neurodevelopmental outcomes. Semin Fetal Neonatal Med. 20:52-57.
- Bayley N, Aylward GP (2019) Bayley Scales of Infant and Toddler Development. Fourth Edition.
- Smitherman H, Stark AR, Bhutani VK (2006) Early recognition of neonatal hyperbilirubinemia and its emergent management. Semin Fetal Neonatal Med. 11: 214-24.
- Slusher TM, Zamora TG, Appiah D, Stanke JU, Strand MA, et al. (2017) Burden of severe neonatal jaundice: A systematic review and meta-analysis. BMJ Paediatr Open. 25:e000105.
- Bhutani VK, Zipursky A, Blencowe H, Khanna R, Sgro M, et al. (2013) Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res. 74:86-100.
- Abbey P, Kandasamy D, Naranje P (2019) Neonatal Jaundice. Indian J Pediatr. 86: 830-841.
- Gkoltsiou K, Tzoufi M, Counsell S, Rutherford M, Cowan F (2008) Serial brain MRI and ultrasound findings: relation to gestational age, bilirubin level, neonatal neurologic status and neurodevelopmental outcome in infants at risk of kernicterus. Early Hum Dev. 84: 829-38.
- Gazzin S, Tiribelli C (2011) Bilirubin-induced neurological damage. J Matern Fetal Neonatal Med. 24 154-5.
- Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM (2009) Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). J Perinatol. 29:S25-45.
- Rodrigues CM, Solá S, Castro RE, Laires PA, Brites D, et al. (2002) Perturbation of membrane dynamics in nerve cells as an early event during bilirubin-induced apoptosis. J Lipid Res. 2002 43: 885-94.
- Taoka T, Aida N, Ochi T, Takahashi Y, Akashi T, et al. (2011) Transient hyperintensity in the subthalamic nucleus and globus pallidus of newborns on T1-weighted images. AJNR Am J Neuroradiol. 32(6):1130-7.
- Woodward LJ, Clark CA, Pritchard VE, Anderson PJ, Inder TE (2011) Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev Neuropsychol. 36: 22-41.
- Wu YW, Kuzniewicz MW, Wickremasinghe AC, Walsh EM, Wi S, et al. (2015) Risk for cerebral palsy in infants with total serum bilirubin levels at or above the exchange transfusion threshold: a population-based study. JAMA Pediatr. 169: 239-46.
- Amin SB, Prinzing D, Myers G (2009) Hyperbilirubinemia and language delay in premature infants. Pediatrics. 123: 327-31.
- Wu YW, Kuzniewicz MW, Croen L, Walsh EM, McCulloch CE, et al. (2016) Risk of Autism Associated with Hyperbilirubinemia and Phototherapy. Pediatrics. 138: e20161813.
- Burke BL, Robbins JM, Bird TM, Hobbs CA, Nesmith C, et al. (2009) Trends in hospitalizations for neonatal jaundice and kernicterus in the United States, 1988-2005. Pediatrics. 123: 524-32.
- Seidman DS, Paz I, Stevenson DK, Laor A, Danon YL, et al. (1991) Neonatal hyperbilirubinemia and physical and cognitive performance at 17 years of age. Pediatrics. 88: 828-33.
- Maimburg RD, Olsen J, Sun Y (2016) Neonatal hyperbilirubinemia and the risk of febrile seizures and childhood epilepsy. Epilepsy Res. 124:67-72.
- Wei CC, Chang CH, Lin CL, Chang SN, Li TC, et al. (2015) Neonatal jaundice and increased risk of attention-deficit hyperactivity disorder: a population-based cohort study. J Child Psychol Psychiatry. 56: 460-7.
- Amin SB (2004) Clinical assessment of bilirubin-induced neurotoxicity in premature infants. Semin Perinatol. 28: 340-7.
- Schutta HS, Johnson L (1867) Bilirubin encephalopathy in the Gunn rat: A fine structure study of the cerebellar cortex. J Neuropathol Exp Neurol. 26: 377-96.
- Keaster J, Hyman CB, Harris I (1969) Hearing problems subsequent to neonatal hemolytic disease or hyperbilirubinemia. Am J Dis Child. 117: 406-10.
- Shapiro SM, Nakamura H (2001) Bilirubin and the auditory system. J Perinatol. 21: S52-5
- Bergman I, Hirsch RP, Fria TJ, Shapiro SM, Holzman I, et al. (1985) Cause of hearing loss in the high-risk premature infant. J Pediatr. 106: 95-101.
- Wennberg RP, Ahlfors CE, Bickers R, McMurtry CA, Shetter JL (1982) Abnormal auditory brainstem response in a newborn infant with hyperbilirubinemia: Improvement with exchange transfusion. J Pediatr. 100: 624-6.
- Golmirzaei J, Namazi S, Amiri S, Zare S, Rastikerdar N, et al. (2013) Evaluation of attention-deficit hyperactivity disorder risk factors. Int J Pediatr. 2013:953103
- Koziol LF, Budding DE, Chidekel D (2013) Hyperbilirubinemia: Subcortical mechanisms of cognitive and behavioral dysfunction. Pediatr Neurol. 48: 3-13.
- Wisnowski JL, Panigrahy A, Painter MJ, Watchko JF (2014) Magnetic resonance imaging of bilirubin encephalopathy: Current limitations and future promise. Semin Perinatol. 38: 422-8.
- Yan R, Han D, Ren J, Zhai Z, Zhou F, et al. (2018) Diagnostic value of conventional MRI combined with DTI for neonatal hyperbilirubinemia. Pediatr Neonatol. 59:161-167.
- Assefa Neknek G, Woldemichael K, Moges A, Zewdneh Solomon D (2018) MRI of bilirubin encephalopathy (kernicterus): A case series of 4 patients from Sub-Saharan Africa, May 2017. Radiol Case Rep. 16;13:676-679.
- Shroff MM, Soares-Fernandes JP, Whyte H, Raybaud C (2010) MR imaging for diagnostic evaluation of encephalopathy in the newborn. Radiographics. 30: 763-80.
- Soorani-Lunsing I, Woltil HA, Hadders-Algra M (2001) Are moderate degrees of hyperbilirubinemia in healthy term neonates really safe for the brain? Pediatr Res. 50: 701-5.
- Harris MC, Bernbaum JC, Polin JR, Zimmerman R, Polin RA (2001) Developmental follow-up of breastfed term and near-term infants with marked hyperbilirubinemia. Pediatrics. 107:1075-80.
- Johnson L, Bhutani VK (2011) The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Perinatol. 35: 101-13.
- Grimmer I, Berger-Jones K, Bührer C, Brandl U, Obladen M (1999) Late neurological sequelae of non-hemolytic hyperbilirubinemia of healthy term neonates. Acta Paediatr. 88: 661-3
Citation: Takama R, Shuang M, Tianc C, Hong J (2021) Analysis of Factors Related to Head MRI Changes in Neonatal Hyperbilirubinemia and Effect on Neurodevelopmental Outcomes. Neonat Pediatr Med.7:213. DOI: 10.4172/2572-4983.1000213
Copyright: © 2021 Takama R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2664
- [From(publication date): 0-2021 - Nov 28, 2025]
- Breakdown by view type
- HTML page views: 1824
- PDF downloads: 840