An Update on the Gut Microbiome and the Use of Probiotics for Disease Prevention in Preterm Infants
Received: 07-Jan-2018 / Accepted Date: 20-Feb-2018 / Published Date: 28-Feb-2018 DOI: 10.4172/2161-1165.1000339
Abstract
Very low birth weight infants (VLBWIs) are at high risk for inflammatory diseases including necrotizing enterocolitis (NEC) or neonatal sepsis, which are primary causes of neonatal mortality. The intestinal microbiota plays an essential role in maintaining local immune homeostasis and enhancing the intestinal barrier in preterm infants; however, appropriate intestinal colonization with normal flora after birth is interrupted by immature gastrointestinal tract, intestinal mucosal damage, insufficient nutrient transport, or formation of abnormal intestinal flora due to the use of antimicrobials in VLBWIs. Large randomized controlled trials and meta-analyses have highlighted the potential benefits of the clinical use of probiotics on NEC or neonatal sepsis for immunologically immature VLBWIs. However, standardized guidelines for the optimum strain, combination of strains, dosage, timing, and duration of probiotics are unknown for the routine application of probiotics in VLBWIs. Here, we review the results of previous studies on the effects of probiotics in preventing morbidity, NEC, or neonatal sepsis in VLBWIs with the administration of single-strain or multi-strain probiotics. Future clinical trials should address the safety of each probiotic strain and the potential efficacy of strain combinations for the routine use of probiotics in preterm infants. The key findings of the manuscript: This study reviewed the focus on the efficacy of probiotics for the prevention of sepsis and necrotizing enterocolitis in preterm infants weighing less than 1,500 g at birth according to single-strain probiotics or multi-strain probiotics.
Introduction
In recent years, the survival rate of very low birth weight infants (VLBWIs) has increased due to rapid developments in medical technology. Despite such improvements, resulting complications including necrotizing enterocolitis (NEC) and neonatal sepsis, which are prevalent diseases in the neonatal intensive care unit (NICU), are the primary cause of neonatal death and adverse long-term neurodevelopmental outcomes [1]. Because the gastrointestinal tract makes up a large portion of the body surface area, it is most often exposed to various antigens and microbes that can cause damage to the intestinal mucosal barrier. Infants with a weak immune system are often later diagnosed with neurodevelopmental deficits after surviving treatment for an infection [2]. The formation of an intestinal mucus membrane with normal flora in the intestine after birth is important for maintaining normal physiologic homeostasis. Typical growth of the intestine is stalled in VLBWIs, who require neonatal intensive care and are susceptible to intestinal mucosa damage from the formation of intestinal flora, insufficient nutrient transport due to an incomplete gastrointestinal (GI) tract, insertion of a nasogastric tube, or formation of abnormal intestinal flora due to the use of antimicrobials [3]. Furthermore, other necessary treatments can destroy the normal intestinal mucus membrane, allowing invasive infection to proceed.
Although minimizing invasive treatment is the optimum solution to reduce the possibility of complications in VLBWIs, preventing the abnormal establishment of intestinal pathogenic bacteria that can cause postnatal sepsis or NEC is also a method of reducing the complications of infection. Despite high morbidity and mortality rate in preterm infants due to frequent occurrence of NEC, effective treatment has not been suggested. Recently, growing evidence has indicated that the intestinal flora plays a pivotal role in brain development affecting future cognitive functioning and behavior through brain-gut communication [4]. Here, probiotics, which have a beneficial effect on health, have been suggested as a method to establish positive changes in the intestinal flora of the host [5,6]. It is increasingly clear that probiotics lower the incidence of NEC and the infant mortality rate [7-9]. This review aims to introduce formation and establishment of gut microbiome at birth as well as its role in preterm infants with NEC and sepsis by comparing effectiveness of single strain and multi-strain probiotics supplement.
Formation of the Intestinal Microbiota in Preterm Infants
The GI tract plays a pivotal role as an interface between the host and the environment. Three important factors for GI immunity include the intestinal microbiota, gastrointestinal surface protection, and local immune mechanisms such as gut-associated lymphoid tissue (GALT). M cells are specialized intestinal epithelial cells in lymphoid follicles that display a local immune mechanism as an important GALT. Intestinal microbiota activate the local immune response by interacting with the host to maintain local immune homeostasis and enhance the intestinal barrier. In vivo studies have suggested a critical role of the gut microbiota in secondary lymphoid tissues (Peyer’s patches and mesenteric lymph nodes) and tertiary lymphoid structures (isolated lymphoid follicle or cryptopatches) that are mediated by dendritic cells, T cells, and B cells [10,11].
Infants born via cesarean section without rupture of the amniotic membrane are at risk of infection from the amniotic fluid as bacteria begin to colonize the intestine quickly after birth. In fact, components of the maternal flora affect the passive transfer of neonatal microbiota to colonize the gut, supporting potential postnatal development of the immune system. Intestinal microbiota are transferred through the maternal vagina during delivery and also following exposure to the external environment, such as by breastfeeding or oral ingestion [12]. The settling period and composition of the intestinal flora are determined by gestational age, delivery method, feeding, antibiotic intake, probiotics, and additional factors of the surrounding environment including the NICU [13,14]. Human breast milk has attracted considerable attention as a source of intestinal colonization in normal gut microbial development resulting in bacterial diversity in the infant gut. Furthermore, maternal IgA hinders microbial attachment by binding nutritional antigens and controlling excessive immune activation [15]. Human milk oligosaccharide consumption by gut microbes indicates that human milk oligosaccharides contribute to the infant intestinal microbiota, which are important components of the intestines of breastfed infants [16,17]. Many factors in milk, including N-acetylglucosamine, glucose, lactoferrin, galactose, and fructose, select for Bifidobacterium species [18].
A previous study based on 16S ribosomal RNA pyrosequencing highlighted the diversity of stool microbiota in the meconium that depends on prenatal and postnatal factors in infants with a gestational age <32 weeks at birth [19]. The VLBWIs of a <30 week gestational age demonstrated a decreased number and decreased diversity of intestinal flora compared with infants with a >30 week gestational age. The impacts of intestinal flora were confirmed to differ with gestational age based on the sequences of causative organisms, such as Citrobacter , Enterococcus , and Klebsiella , which are reported to be causative organisms of both NEC and sepsis.
The Role of Intestinal Microbiota in Preterm Infants
The intestinal mucus contains intestinal commensal flora, and great numbers of bacteria are found in the intestines. Intestinal flora can be categorized into either primary flora [>109 colony-forming units (CFU)/g] or secondary flora [<106-109 CFU/g]. Although the intestines must distinguish between symbiotic microbiota and external pathogens, little is known about the mechanism of differentiation between the different species. Despite not understanding the mechanism, it is widely accepted that intestinal commensal flora is helpful in the host defense against external pathogens. The intestinal microbiota affects intestinal organ development by maintaining a symbiotic relationship with the host, activating intestinal cells, and controlling the structure of vessels in the intestinal villi, enhancing tight junctions between the cells, and increasing the secretion of mucus.
The dysbiosis of microbial colonization in VLBWIs tends to increase the risk of infections and inflammatory processes. NEC is a major threat that primarily affects preterm neonates and typically occurs in the first few weeks after birth [18]. Previous studies based on the analysis of microbiota from the feces of NEC patients and control patients have shown that unusual intestinal microbial species and an overall reduction in diversity of the microbiota are related with NEC [19,20]. Inappropriate early microbial colonization can be an injurycausing factor in VLBWIs with immature intestinal function, and the associated immune defense mechanism is susceptible to intestinal damage [21,22]. In short, VLBWIs displayed reduced levels of protective Bifidobacterium and a high prevalence of facultative anaerobic microorganisms such as Staphylococcus , Enterobacteriaceae , and Enterococcaceae [23,24].
Colonized intestinal flora can also protect against external pathogens. Crosstalk between the intestinal flora and epithelial cells regulates intestinal inflammation by interacting with the epithelium, endothelial cells, and lymphocytes across the mucus layer. Toll-like receptors (TLRs) are known to play a central role in this action. TLRs are a major focus of neonatal immunological research due to the wide range of basic science knowledge in this area [15]. Intestinal microflora that causes bacterial translocation in VLBWIs is associated with excessive TLR-4 signaling, which produces an inflammatory cascade and necrosis characteristic of NEC [25]. The direct activation of TLRs leads to the activation of M cells and dendritic cells that balance intestinal immunity, but this is skewed toward T helper type 2 cells via T helper type 1 cells, which also control other inflammatory responses [26].
Preterm infants encounter several challenges to intestinal microbiota formation after birth. Compared with normal infants, VLBWIs show downregulated variation in intestinal microbiota and attenuated TLR function [12]. This reduction in intestinal microbiota diversity allows pathogenic bacteria to develop into the primary flora at a decreased degree of intestinal maturity, increasing the risk and incidence of sepsis or NEC. VLBWIs are susceptible to pathogenic bacteria due to an incomplete innate immune response and a downregulated immune response. Furthermore, the use of histamine-2 blockers, steroids, or opioids can impact the formation of intestinal flora with such vulnerabilities in VLBWIs [15,27]. Therefore, emerging studies have focused on the use of probiotics to encourage the formation of healthy intestinal flora and to prevent inflammatory GI disorders in VLBWIs with vulnerable intestinal immunity [28,29].
Types and Roles of Probiotics
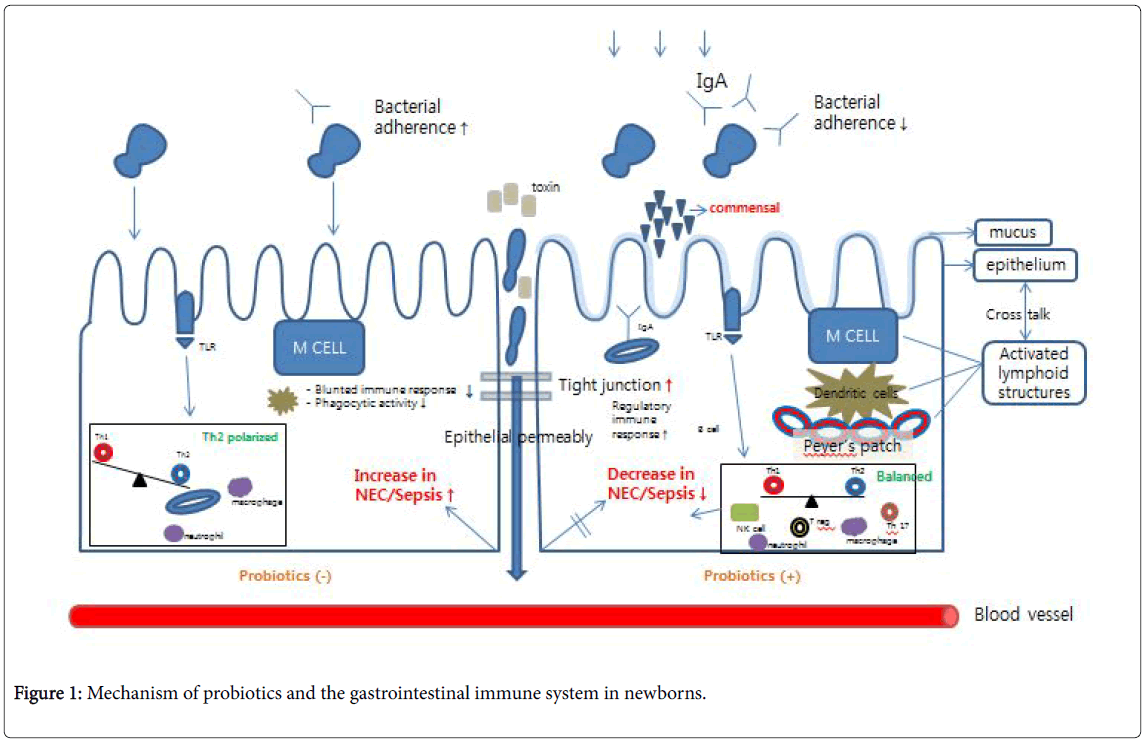
The World Health Organization (WHO) defines probiotics as live microorganisms that, when consumed in adequate amounts, confer a health benefit on the host by balancing the intestinal flora. The most important species are Lactobacillus and Bifidobacterium which are both present in dietary and fermented dairy products. In contrast, prebiotics are substances that cannot be digested and therefore improve the health of the host by influencing the growth and activity of the intestinal flora. Insulin, fructo-oligosaccharides, and galactooligosaccharides are examples of prebiotics. A vast number of studies previously identified the beneficial effects of the administration of specific probiotic strains, including enhancing the intestinal barrier, increasing the systemic immune response, and aiding in the formation of normal intestinal flora in preterm infants (Figure 1) [8,28,30]. Probiotics enhance intestinal epithelial cells and form a barrier that resists the invasion of pathogens and accelerates the secretion of mucin to impede the adherence and colonization of pathogens to epithelial cells. Probiotics are known to increase the mucus barrier by thickening the mucosa with induced mucin mRNA to prevent adhesion of pathogenic microbes, such as enteropathogenic Escherichia coli . They are also known to enhance the mucosal barrier by increasing the level of secretory IgA while also augmenting tight junctions and preventing hypoxic damage in vitro by decreasing vessel resistance through the production of nitric oxide [31]. Increasing immunity through a controlled immune response and induced cytoprotective responses is another benefit of probiotics [32,33]. The specific probiotics used in studies for preterm infants are usually Lactobacillus and Bifidobacterium , which secrete lactic acid, acetic acid, and butyric acid, inhibiting the growth of pathogenic microbes [34]. In addition, the microbiota plays a pivotal role in alleviating stress caused by invasive and/or antibiotic treatments along with the physical and emotional stress that result from separation from the mother. Probiotics also influence the long-term intestinal environment via the brain-gutmicrobiota signaling system [4].
Although Lactobacilli (L. acidophilus , L. casei , L. rhamnosus GG, L. reuteri , L. bulgaricus , L. plantarum ) and Bifidobacterium (B. bifidum , B. longum , B. infantis , B. lactis , B. breve ) are used as a primary strain of probiotics, Streptococcus thermophiles and Saccharomyces boulardii are also employed as strains. To be used as an effective probiotic, microbes should be non-pathogenic and must reach the intestine in a live form after direct ingestion. Commonly, combinations of [L. GG + B. longum ], [L. acidophilus + B. bifidum ], [L. acidophilus + B. infantis ], or [L. casei + B. breve ] are used. For two or more mixtures, [B. bifidum (± B. lactis ) + B. infantis + L. acidophilus ], [Lactobacillus (acidophilus + rhamnosus GG + casei + plantarum ) + B infantis + Streptococcus thermophilus ] or [B. infantis + B. lactis + Streptococcus thermophilus ] are commonly used.
The Effects of Probiotics and Their Prevention of NEC, Morbidity, and Sepsis in VLBWIs with the Administration of Single-strain or Multi-strain Probiotics
Recently, increasing numbers of studies have focused on the effects of probiotics, and meta-analyses have been performed to identify the clinical effects of probiotics in preterm infants [35-38]. Although there are differences among these analyses, a vast number of reports suggest that supplementation with probiotics prevents NEC and mortality in preterm infants. Mihatsch et al. [5] conducted a systematic review that identified the beneficial effects of some probiotics in preterm infants <37 weeks of gestational age with a significant decrease in the severity of NEC. Furthermore, a Cochrane Database review that included 37 randomized trials also reported a significant decrease in the risk of late-onset sepsis following administration of probiotics in preterm infants; however, these results were only seen when they excluded studies that had risk of bias [36]. A different Cochrane Database review of 24 randomized studies showed inconsistent results for nosocomial sepsis in preterm infants weighing less than 2,500 g at birth, but the small sample size was inadequate to prove significant benefit for sepsis [37]. Although the outcome suggested that the administration of probiotics was related to a decrease in NEC or mortality rate, there was no significant difference in the incidence of nosocomial sepsis. The most recent meta-analysis conducted in 2016 amongst 5,033 infants weighing less than 2,500 g and with a gestational age of 37 weeks also reported significant decrease in severe NEC and all-cause mortality in a group given a probiotic, but not for a cultureproven sepsis group [38]. The VLBWIs are predisposed to the development of dysbiosis of the gut microbiome and are a research priority group to study the effect of probiotics. “Dysbiosis” can also facilitate bacterial translocation through the intestinal mucosa barrier.
Of single-strain studies, a multicenter study conducted in Taiwan compared the outcomes in 217 VLBWIs assigned to either a control group or intervention groups given B. bifidum and L. acidophilus along with probiotics [39]. In their investigation, the beneficial effects of probiotics were clearly noted along with a significant reduction in NEC (1.8% vs. 6.5%, respectively, p=0.02) and in the instance of NEC or the all-cause mortality rate (1.8% vs. 9.2%, respectively, p=0.02). In 2012, Wang et al. [40] reported that administration of probiotics decreased NEC (RR 0.33, 95% CI 0.24 to 0.46) and mortality rate (RR 0.56, 95% CI 0.43 to 0.73) in VLBWIs based on a published metaanalysis that used data from 20 other studies. In 2014, Oncel et al. [41] conducted a randomized controlled study to evaluate the effect of oral Lactobacillus reuteri on severity of NEC and on sepsis in preterm infants <32 weeks of gestational age. Although beneficial effect was not observed in incidence of NEC, significant reduction in sepsis was noted (6.5% vs. 12.5%). Similarly, investigation conducted by Demirel et al. [42] demonstrated that positive effects of probiotics were shown with decrease in clinical sepsis with slight difference (34.8% vs. 47.8%: control vs. probiotics), but were not observed in mortality from NEC using S. boulardii . In 2007, Stratiki et al. [43] reported that VLBWIs with B. lactis (2 × 10(7) cfu/g of dry milk) showed decreased intestinal permeability during a sugar absorption test and displayed increased head circumference despite no significant differences in NEC, sepsis, or mortality rate between the two groups compared to the control group. An Italian study reported low NEC and all-cause mortality in VLBWIs that received L. GG but did not show a statistically meaningful clinical difference in any subgroup [44]. The PiPS trial of probiotic efficacy did not support the routine administration of B. breve and found no evidence of benefit for prevention of NEC [45]. Studies conducted by Mihatsch et al. [35] using B. lactis and Sari et al. [46] with L. sporogenes both demonstrated a positive effect in improving the feeding tolerance with probiotics during breastfeeding, but there was no significant variation in NEC, sepsis, or mortality rate between the two groups (Table 1).
| Study | Inclusion Criteria | Number randomized in each group | Probiotic Species | Total Dose (cfu/day) | Duration | Decrease in NEC | Decrease in Sepsis | |
|---|---|---|---|---|---|---|---|---|
| Gestational age | Birth weight | |||||||
| Stratiki et al., 2007 [43] | 27-37 weeks | None | Probiotic: 41 | B. lactis | 0.2 billion/kg | Not stated | No effect | 31.7% vs. 69.4% |
| Control: 34 | ||||||||
| Mihatsch et al., 2010 [35] | Less than 30 weeks | Less than 1,500 g | Probiotic: 93 | B. lactis | 12 billion /kg | 28 days or more | No effect | No effect |
| Control: 90 | ||||||||
| Sari et al., 2011 [46] | Less than 33 weeks | Less than 1,500 g | Probiotic: 110 | L. sporogenes | 0.35 billion | 28 days or more | 5.5% vs. 9% (stage ≥ 2) | No effect |
| Control: 111 | ||||||||
| Demirel et al., 2013 [42] | Less than 32 weeks | 1,500 g or less | Probiotic: 135 | S. boulardii (Reflor®) | 5 billion | 28 days or more | No effect | 34.8% vs. 47.8% (clinical sepsis) |
| Control: 136 | ||||||||
| Oncel et al., 2014 [41] | 32 weeks or less | 1,500 g or less | Probiotic: 200 | L. reuteri | 0.1 billion | 28 days or more | No effect | 6.5% vs. 12.5% |
| Control: 200 | ||||||||
| Costeloe et al., 2016 [45] (the PiPS Trial) | 23-30 weeks | None | Probiotic: 650 | B. breve BBG | 0.2-0.53 billion | 28 days or more | No effect | No effect |
| Control: 660 | ||||||||
Table 1: Characteristics of studies with single-strain probiotics in very low birth weight infants.
Detailed characteristics of multi-strain probiotics supplement in VLBWIs were also reviewed (Table 2). Recently, Jacobs et al. [47] conducted a randomized trial (ProPrems) in Australia and New Zealand that investigated VLBWIs at a gestational age <32 weeks using a preparation of three different strains (B. infantis (3 × 108) + Streptococcus thermophilus (3.5 × 108) + B. lactis (3.5 × 108)). There were no significant differences in sepsis (23% vs. 26%: probiotics vs. control) or mortality (4.9% vs. 5.1%: probiotics vs. control) rates between the two groups, but the infants who were given a compound strain of 109 until discharge showed a decreased rate of NEC (2% vs. 4.4%, p=0.03). However, the incidence of late-onset sepsis after administration of probiotics differed according to gestational age and showed a decrease in incidence in infants who were >28 weeks of gestational age. Previous studies showed that supplementation with probiotics reduced the incidence of feeding intolerance and sepsis in VLBWIs [41,42]. An investigation conducted in Mexico in 2013 analyzed the positive effects of probiotics in VLBWIs and showed statistically significant decrease in NEC and in the overall morbidity rate (9% vs. 25%, respectively, p=0.015) [48]. Studies were further conducted by Lin et al. [29,39] both using L. acidophilus and B. infantis in VLBWIs. While both study results demonstrated positive effects of multi-strain probiotics in decreasing NEC, trials tested at birth showed no effect in improving sepsis. However, probiotics groups administered at <34 weeks experienced alleviated severity in NEC (1.1% vs. 5.3%: probiotics vs. control). Results of administration of multiple strains were statistically significant in decreasing incidence of NEC, but the beneficial effects noted in NEC were not investigated in sepsis groups, showing either no effect or mild improvement. Of these results, studies conducted by Braga and Ren et al. [8,9] demonstrated meaningful data in NEC using multiple strains, but showed no effect in alleviating sepsis. Furthermore, Rapa et al. [21] reported that the compound administration of probiotics with L. acidophilus and B. infantis showed a meaningful alleviation in the incidence of NEC in breastfed VLBWIs, but did not demonstrate any difference in formulafed VLBW Is. The studies that utilize probiotic mixtures are still needed to prove their significant outcome in infants with birth weight less than 1,000 g (Table 2). Despite the controversy regarding a beneficial effect of probiotics on the incidence of NEC and sepsis, multi-strain probiotics seem to be a reasonable choice with their impact on reducing rates of NEC [44].
| Study | Inclusion Criteria | Number randomized in each group | Probiotic Species | Total Dose (cfu/day) | Duration | Decrease in NEC | Decrease in Sepsis | |
|---|---|---|---|---|---|---|---|---|
| Gestational age | Birth weight | |||||||
| Lin et al., 2005 [29] | None | Less than 1,500 g | Probiotic: 180 | L. acidophilus | 1 billion/kg | 28 days or more | 1.8% vs. 9.2% | No effect |
| Control: 187 | B. infantis | |||||||
| Lin et al., 2008 [39] | Less than 34 weeks | Less than 1,500 g | Probiotic: 222 | L. acidophilus | 1 billion/kg | 6 weeks | 1.1% vs. 5.3% | 12.2% vs. 19.3% |
| Control: 221 | B. infantis | |||||||
| Ren, 2010 [9] | 28-33 Weeks | 1,000–1,800 g | Probiotic: 80 | B. infantis | 0.016 billion | Up to 13 days | 3.7% vs. 7.1% | No effect |
| L. acidophilus | ||||||||
| Control: 70 | E. faecalis | |||||||
| Bacillus cereus (B. tetravaccine) | ||||||||
| Braga et al., 2011 [8] | None | 750-1,499 g | Probiotic: 119 | L. casei | 0.035 - 3.5 billion | 28 days or more | 0% vs. 3.6% (stage ≥ 2) | No effect |
| Control: 112 | B. breve | |||||||
| Jacobs, 2013 [47] (The ProPrems trial) | Less than 32 weeks | Less than 1,500 g | Probiotic: 548 | B. infantis | 1 billion | 28 days or more | 2.0% vs. 4.4% (stage ≥ 2) | 5.5% vs. 10.8% (subgroup analysis) |
| Control: 551 | B. bifidum | |||||||
| S. thermophilus | ||||||||
| Fernandez-Carrocera et al., 2013 [48] | “preterm” | Less than 1,500 g | Probiotic: 75 | L. acidophilus | 2.65 billion | 28 days or more | 9.3 vs. 25.3% (NEC or death) | No effect |
| L. rhamnosus | ||||||||
| L. casei | ||||||||
| Control: 75 | L. plantarum | |||||||
| B. infantis | ||||||||
| S. thermophillus | ||||||||
Table 2: Characteristics of studies with multi-strain probiotics in very low birth weight infants.
Limitations of Probiotics in Preterm Infants and Future Directions
The perinatal and early postnatal periods are often called a “window of vulnerability” for microbiota establishment because of their putative role in producing an immune-modulator with potentially life-long consequences. In contrast, infants with poor immunity were found to be susceptible to infections caused by probiotics. Though such infections caused by administered probiotics were more frequently found in adults, late-onset sepsis due to infection from identical strain (L. rhamnosus strain GG) was noticed in VLBWIs with underlying diseases and incomplete immunity [49,50]. Probiotics are live microorganisms that may impact patients with incomplete immunity and intestinal integrity with diverse effects due to the characteristics. Careful attention is needed, especially clear identification of relation between probiotics and sepsis is yet to be established in cases of VLBWIs with severe disease where intestinal integrity is threatened. A larger trial is needed to examine the long-term effects of probiotics, especially since preterm infants are exposed to risk of infection due to having an incomplete immune system. Several studies have identified the benefits of probiotics in reducing the rates of NEC and morbidity in VLBW preterm infants; however, the clinical use of probiotics remains unclear without standard guidelines. Therefore, multilateral approaches including the type, amount, and period of administration will be needed to safely and efficiently use probiotics in preterm infants. Currently, data regarding long-term follow-up on probiotic administration is sparse. In order to establish standardized guidelines, appropriate strain should be selected from VLBWIs and breastfeeding should be encouraged to develop normal intestinal flora and barrier. Recent studies support that multiple strains probiotics is the most promising therapy to prevent NEC and mortality in VLBWIs, but identical effectiveness was relatively found less in sepsis. Beneficial effects on NEC discovered in multi-strain probiotics were marginal in single strain probiotic. Further studies on the optimal combination of species, influence of probiotics on neurodevelopment, long-term immunity and sepsis are needed to decrease the incidence of NEC and promote intestinal integrity in VLBWIs as a preventive strategy.
Competing Interests
The authors declare that they have no competing interest.
Funding
This study was supported by the research fund of Hanyang University (HY-2015)
References
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, et al. (2008) Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 153: 170-175.
- Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, et al. (2005) Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 115: 696-703.
- Neu J, Bernstein H (2002) Update on host defense and immunonutrients. Clin Perinatol 29: 41-64.
- Sherman MP, Zaghouani H, Niklas V (2015) Gut microbiota, the immune system, and diet influence the neonatal gut-brain axis. Pediatr Res 77: 127-135.
- Mihatsch WA, Braegger CP, Decsi T, Kolacek S, Lanzinger H, et al. (2012) Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clin Nutr 31: 6-15.
- Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T (2011) Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 16: CD005496
- Deshpande G, Rao S, Patole S, Bulsara M (2010) Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 125: 921-930.
- Braga TD, da Silva GA, de Lira PI, de Carvalho Lima M (2011) Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 93: 81-86.
- Ren YF, Wang LL. [Effects of probiotics on intestinal bacterial colonization in premature infants]. Zhongguo Dang Dai Er Ke Za Zhi 12: 192-194.
- Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, et al. (2002) Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol 168: 57-64.
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL (2005) An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122: 107-118.
- Murguia-Peniche T, Mihatsch WA, Zegarra J, Supapannachart S, Ding ZY, et al. (2013) Intestinal mucosal defense system, Part 2. Probiotics and prebiotics. J Pediatr 162: S64-71.
- Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, et al. (2000) Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 30: 61-67.
- Berrington JE, Stewart CJ, Embleton ND, Cummings SP (2013) Gut microbiota in preterm infants: assessment and relevance to health and disease. Arch Dis Child Fetal Neonatal Ed 98: F286-290.
- Bourlioux P, Koletzko B, Guarner F, Braesco V (2003) The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium "The Intelligent Intestine," held in Paris, June 14, 2002. Am J Clin Nutr 78: 675-683
- Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, et al. (2010) Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem 58: 5334-5340.
- Marcobal A, Sonnenburg JL (2012) Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect 18: 12-15.
- Claud EC, Walker WA (2001) Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J 15: 1398-1403.
- Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, et al. (2010) Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr 156: 20-25.
- Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, et al. (2009) 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 3: 944-954.
- Repa A, Thanhaeuser M, Endress D, Weber M, Kreissl A, et al. (2015) Probiotics (Lactobacillus acidophilus and Bifidobacterium infantis) prevent NEC in VLBW infants fed breast milk but not formula [corrected]. Pediatr Res 77: 381-388.
- Torrazza RM, Neu J (2013) The altered gut microbiome and necrotizing enterocolitis. Clin Perinatol 40: 93-108.
- Arboleya S, Binetti A, Salazar N, Fernandez N, Solis G, et al. (2012) Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol 79: 763-772.
- Arboleya S, Solis G, Fernandez N, de los Reyes-Gavilan CG, Gueimonde M (2012) Facultative to strict anaerobes ratio in the preterm infant microbiota: a target for intervention?. Gut Microbes 3: 583-588.
- Hackam DJ, Good M, Sodhi CP (2013) Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin Pediatr Surg 22: 76-82.
- Neu J, Mihatsch WA, Zegarra J, Supapannachart S, Ding ZY, et al. (2013) Intestinal mucosal defense system, Part 1. Consensus recommendations for immunonutrients. J Pediatr 162: S56-63.
- Neu J, Bradley CL, Ding ZY, Tucker HN, Berseth CL (2013) Feeding the preterm infant: opportunities and challenges of bringing science to the bedside. J Pediatr 162: S101-106.
- Claud EC, Walker WA (2008) Bacterial colonization, probiotics, and necrotizing enterocolitis. J Clin Gastroenterol 42: S46-52.
- Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, et al. (2005) Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 115: 1-4.
- Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, et al. (2009) Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA 302: 1421-1428.
- Abdel-Latif ME, Bajuk B, Ward M, Oei JL, Badawi N, et al. (2013) Neurodevelopmental outcomes of extremely premature infants conceived after assisted conception: a population based cohort study. Arch Dis Child Fetal Neonatal Ed 98: F205-211.
- Husebye E, Hellstrom PM, Midtvedt T (1994) Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig Dis Sci 39: 946-956.
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, et al. (2001) Molecular analysis of commensal host-microbial relationships in the intestine. Science 291: 881-884.
- Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J, Pohlandt F (2010) Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology 98: 156-163.
- Rao SC, Athalye-Jape GK, Deshpande GC, Simmer KN, Patole SK (2016) Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-analysis. Pediatrics 137: e20153684.
- AlFaleh K, Anabrees J (2014) Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 10: CD005496.
- Sawh SC, Deshpande S, Jansen S, Reynaert CJ, Jones PM (2016) Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. Peer J 4: e2429.
- Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, et al. (2008) Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 122: 693-700.
- Wang Q, Dong J, Zhu Y (2012) Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg 47:241-248.
- Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, et al. (2014) Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 99: F110-115.
- Demirel G, Erdeve O, Celik IH, Dilmen U (2013) Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatr 102: e560-565.
- Stratiki Z, Costalos C, Sevastiadou S, Kastanidou O, Skouroliakou M, et al. (2007) The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 83: 575-579.
- Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF (2002) Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 82: 103-108.
- Costeloe K, Bowler U, Brocklehurst P, Hardy P, Heal P, et al. (2016) A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial. Health Technol Assess 20: 1-194.
- Sari FN, Dizdar EA, Oguz S, Erdeve O, Uras N, et al. (2011) Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr 65: 434-439.
- Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, et al. (2013) Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 132: 1055-1062.
- Fernandez-Carrocera LA, Solis-Herrera A, Cabanillas-Ayon M, Gallardo-Sarmiento RB, Garcia-Perez CS, et al. (2013) Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 98: F5-9.
- Rautio M, Jousimies-Somer H, Kauma H, Pietarinen I, Saxelin M, et al. (1999) Liver abscess due to a Lactobacillus rhamnosus strain indistinguishable from L. rhamnosus strain GG. Clin Infect Dis 28: 1159-1160.
- Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, et al. (2005) Lactobacillus sepsis associated with probiotic therapy. Pediatrics 115: 178-181.
Citation: Lee HJ (2018) An Update on the Gut Microbiome and the Use of Probiotics for Disease Prevention in Preterm Infants. Epidemiology (Sunnyvale) 8: 339. DOI: 10.4172/2161-1165.1000339
Copyright: © 2018 Lee HJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5381
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 4453
- PDF downloads: 928