An Unusual Cause of Central Diabetes Insipidus in a Young Female
Received: 26-Aug-2014 / Accepted Date: 24-Sep-2014 / Published Date: 27-Sep-2014 DOI: 10.4172/2161-0681.1000192
Abstract
Central Diabetes Insipidus (CDI) in adults is most commonly occurs as a result of hypothalamo- pituitary surgery, head injury or various inflammatory and infiltrative disorders. CDI with mass lesions in the sellar-suprasellar area occurs due to infiltrative disorders like lymphoma, Langerhan’s cell histiocytosis, and inflammatory disorders like hypophysitis, mass lesions like germinoma, craniopharyngioma and even metastases. Treatment and correct diagnosis depends on histopathology. Rosai-Dorfman Disease (RDD) is a rare disorder of unknown etiology characterized by abnormal proliferation of histiocytes. Extra nodal involvement is found in 40-50% of cases; with Central nervous system (CNS) involvement being uncommon hence in majority of instances intracranial disease is not suspected. Due to paucity of the reported cases, optimal treatment options are not known. We report a young female who presented with CDI, hyperprolactinemia and other features of hypopituitarism along with systemic manifestations including nodal and skeletal involvement. The patient was treated with combination of surgical debulking followed by oral glucocorticoid for 6 months with gratifying results.
Keywords: Rosai?dorfman disease, Sellar-suprasellar mass, Diabetes insipidus
310248Introduction
Central diabetes insipidus (CDI) is usually caused by damage to posterior pituitary gland, stalk or hypothalamus with most common causes in adults being pituitary surgery, traumatic brain injury, and infective, inflammatory or infiltrative diseases in the sellar region such as meningitis, tuberculosis, hypophysitis, sarcoidosis, lymphoma, langerhans cell histiocytosis. Benign histiocytic proliferation and infiltration of hypothalamo-pituitary area as an etiology of CDI is uncommon.
Rosai-Dorfman disease (RDD) or Sinus Histiocytosis with Massive Lymphadenopathy (SHML) was first described in 1969 by two pathologists Juan Rosai and Ronald Dorfman [1]. It is a benign, idiopathic histiocyte proliferative disorder with systemic symptoms such as fever, generalized weakness, fatigue, lymphadenopathy and pathognomonic histological and immuno-histochemical characteristics. RDD is equally prevalent in males and females [2] but central nervous system involvement though rare, occurs twice as common in males. RDD presenting as sellar-suprasellar mass is extremely rare with only few cases mentioned in the literature [2-8]. Here we report a case of RDD presenting as suprasellar mass causing CDI, hypopituitarism with hyperprolactinemia.
Case Report
A 32 year-old- female presented to our institute with secondary amenorrhoea, polyuria, polydipsia and weakness for 9 years, with galactorrhoea, headache, and irritability for the last four years. She had a significant weight gain of 40 kg in past 6 months. There was no family history of any lympho-proliferative disorder, malignancy or immunodeficiency syndrome. Before coming to us, a primary care physician prescribed her levothyroxine 100 mcg, cabergoline 0.5 mg once a week and combined estrogen and progesterone pills for 5 months, on basis of her clinical picture and hormonal profile. As her condition remained statusquo, she stopped all medicines 4 months before being admitted with us, except for levothyroxine which she restarted from last 15 days.
The patient was obese (BMI-40.8 kg/m2), normotensive with no palpable lymphadenopathy or hepatosplenomegaly. Her routine investigations were normal except for hypercholesterolemia (269 mg/ dl, 160-200) and hypertriglyceridemia (344 mg/dl, 100-150). Hormonal investigation revealed LH <0.1 mIU/ml ( 2.4-12.6), FSH 0.518 mIU/ ml (1.5-1.5), testosterone 0.095 nmol/L ( 0.2-2.9), estradiol 39.5 pg/ ml (12.5-166), ACTH 4.33 pg/ml (5-60), prolactin 64.8 ng/ml (4.79- 23.3), iPTH 23.22 pg/ml (15-65), morning cortisol 301 nmol/L ( 171- 536), T3 1.34 ng/ml (0.8-2.0), T4 12.28 μg/dl (4.8-12.7), TSH 0.018 μIU/ml (0.27-4.2). The baseline urine and serum osmolality were 59 mOsmol/kg (150-700) and 304 mOsmol/kg (275-290), respectively. With water deprivation, urine osmolality rose to 149.78 mOsmol/kg following administration of aqueous vasopressin (5 U s/c), suggestive of CDI. In the light of aforementioned investigations, she was started on oral desmopressin (100 mcg/day) and levothyroxine (100 mcg/day), following which she obtained relief.
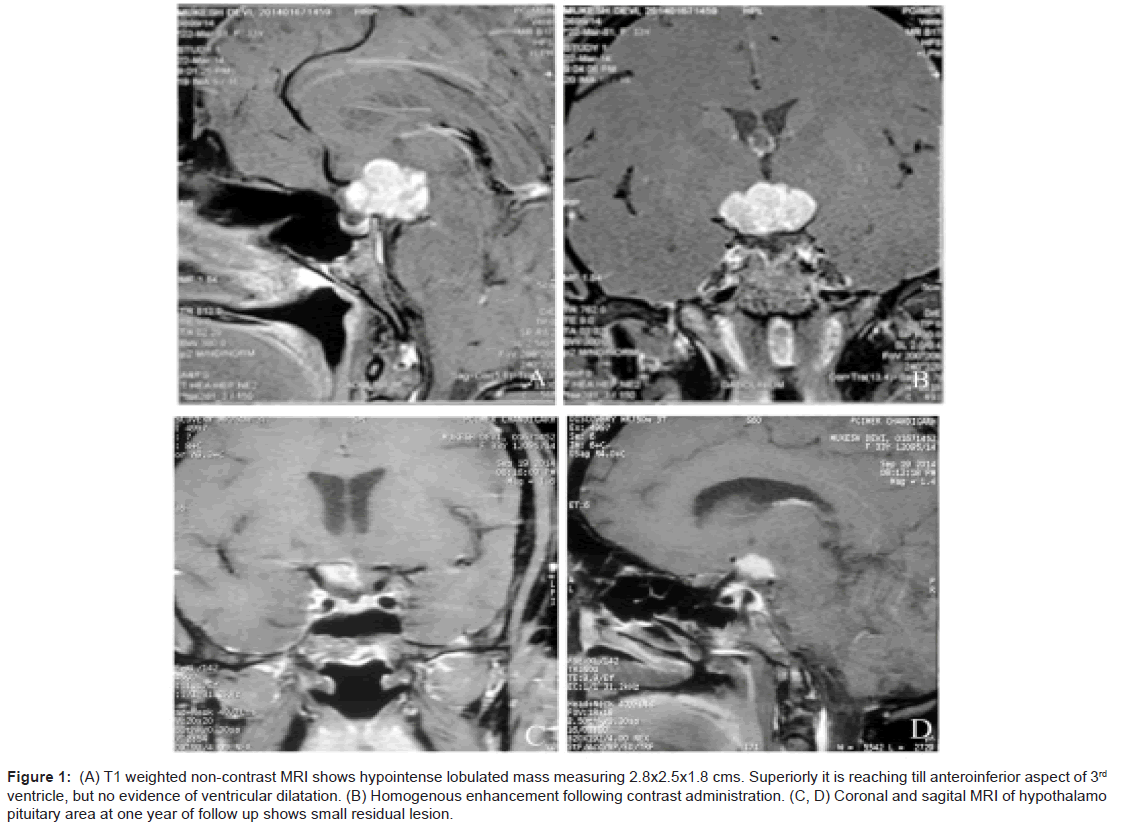
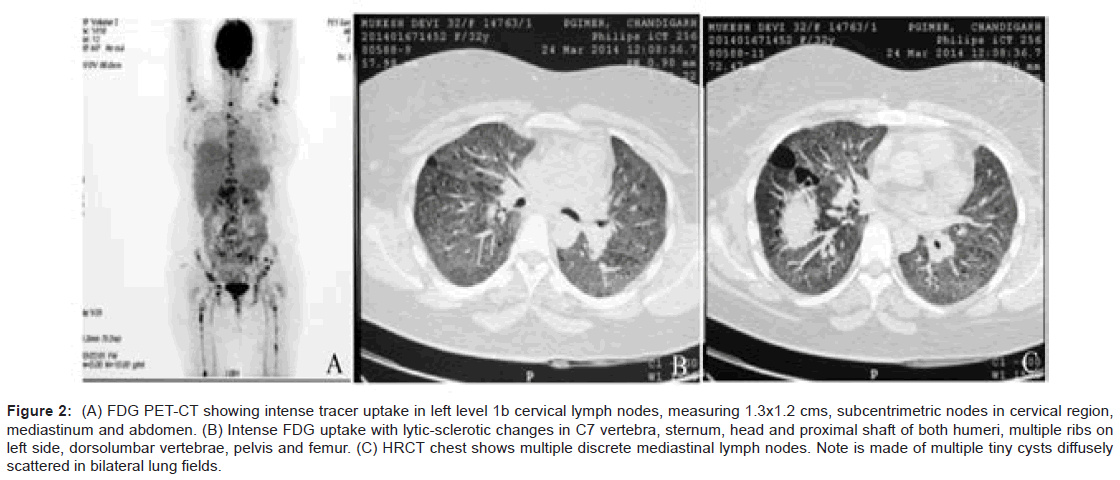
As a part of imaging studies, gadolinium enhanced MRI of brain showed a T1 hypointense, T2/FLAIR mildly hyperintense, lobulated mass in supasellar region, measuring 2.8×2.5×1.8 cms, with homogeneous enhancement post-contrast. The mass was abutting the optic chiasma and left cavernous sinus, with pituitary stalk not separately visible (Figure 1A and 1B). Fluoro deoxy glucose positron emission computed tomography (FDG PET-CT) (Figure 2A and 2B) was done that revealed FDG avid suprasellar soft tissue mass (standard uptake value max. 37.7), multiple cervical level II, peri-pancreatic, portocaval, precaval, paraortic and mesenteric lymph nodes along with lytic lesions in 7th cervical spine, bilateral humeri, scapulae, dorso-lumbar spine, sacrum, pelvic bones and faintly avid ground glass opacities (GGOs) in both lung fields (Figure 2C).
Figure 1: (A) T1 weighted non-contrast MRI shows hypointense lobulated mass measuring 2.8x2.5x1.8 cms. Superiorly it is reaching till anteroinferior aspect of 3rd ventricle, but no evidence of ventricular dilatation. (B) Homogenous enhancement following contrast administration. (C, D) Coronal and sagital MRI of hypothalamo pituitary area at one year of follow up shows small residual lesion.
Figure 2: (A) FDG PET-CT showing intense tracer uptake in left level 1b cervical lymph nodes, measuring 1.3x1.2 cms, subcentrimetric nodes in cervical region, mediastinum and abdomen. (B) Intense FDG uptake with lytic-sclerotic changes in C7 vertebra, sternum, head and proximal shaft of both humeri, multiple ribs on left side, dorsolumbar vertebrae, pelvis and femur. (C) HRCT chest shows multiple discrete mediastinal lymph nodes. Note is made of multiple tiny cysts diffusely scattered in bilateral lung fields.
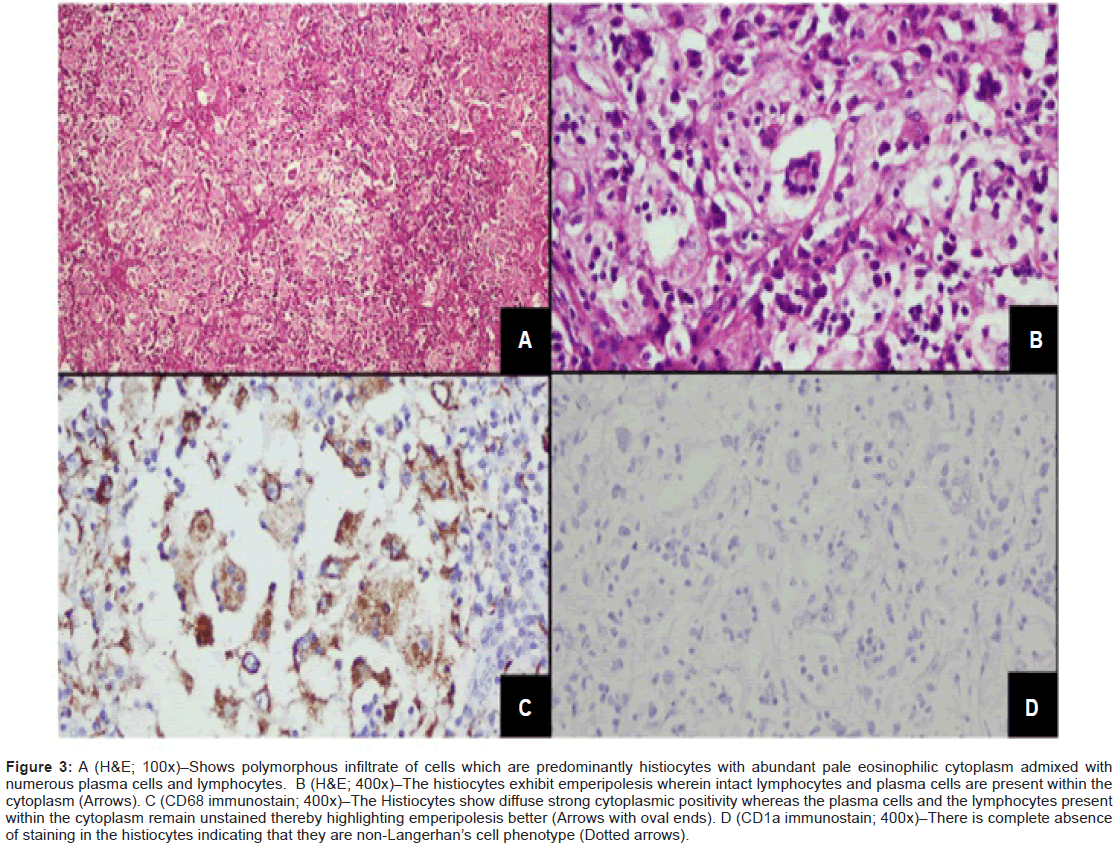
High Resolution Computed Tomography (HRCT) reconfirmed diffuse areas of GGOs and multiple tiny cysts in bilateral lung fields, with provisional diagnosis of lymphangioleiomyomatosis. In view of clinical symptomatology and a multisystem involvement, evaluation was done on lines of granulomatous etiology, but workup for tuberculosis and sarcoidosis was negative; hence first possibility of Langerhans cell histocytosis was kept. Alpha Fetoprotien (AFP) levels, βhCG levels, and malignant cytology of Cerebrospinal Fluid (CSF) were non-contributory. She underwent a stereotactic biopsy from suprasellar mass which was inconclusive, thus was subjected to right pterional craniotomy with attempted gross total excision of mass. Grossly the mass was greyish pink and adherent to adjacent structures. The histopathology report came as a surprise, revealing sheets of macrophages with interspersed lymphocytes and some macrophages showing emperipolesis (cell within cell), few multinucleated giant cells with surrounding areas of extensive necrosis. On immune staining, the macrophages showed strong positivity for CD68 and negativity for CD1a, which is highly suggestive of RDD (Figure 3A-D).
Figure 3: A (H&E; 100x)–Shows polymorphous infiltrate of cells which are predominantly histiocytes with abundant pale eosinophilic cytoplasm admixed with numerous plasma cells and lymphocytes. B (H&E; 400x)–The histiocytes exhibit emperipolesis wherein intact lymphocytes and plasma cells are present within the cytoplasm (Arrows). C (CD68 immunostain; 400x)–The Histiocytes show diffuse strong cytoplasmic positivity whereas the plasma cells and the lymphocytes present within the cytoplasm remain unstained thereby highlighting emperipolesis better (Arrows with oval ends). D (CD1a immunostain; 400x)–There is complete absence of staining in the histiocytes indicating that they are non-Langerhan’s cell phenotype (Dotted arrows).
After the diagnosis, patient was started on oral predinosolone at 60 mg/day followed by tapering, after 6 weeks of therapy. At present she is on 5 mg of maintenance dose of prednisolone. She is symptom free after one year of follow-up, though hormone supplementation continues and imaging is showing small residue (Figure 1D) for which gamma knife surgery is planned.
Discussion
RDD is a rare disorder involving the lympho-reticular system however, 40% of cases can have extra nodal involvement with or without nodal disease. The extra-nodal involvement can be diverse [9-11] including naso-pharynx, paranasal sinuses, thyroid, orbit, skin and gastro intestinal tract. After extensive review of literature we came across 126 cases of central nervous system (CNS) RDD with incidence of 5% in small case series [10,12-14]. RDD of CNS was misdiagnosed in the past as plasma cell granuloma or inflammatory pseudotumor, and out of the 10 cases of CNS RDD at our center in last 20 years, this is the only case involving sellar-suprasellar area, while remaining had dura based mass (5), parenchymal lesions (3) and meningo encephalitis like presentation (1).
RDD of CNS commonly presents as dura based lesion mimicking meningioma in more than 90% of cases reported. Two third of these cases have isolated intracranial disease [10,15-18] with involvement of sellar-suprasellar area being present only in 7 reported cases [Table 1]. Out of the 126 cases of RDD with CNS involvement in the literature, only 5 had CDI. Other features of CNS RDD can be due to hypothalamic involvement and mass effects of tumor leading to galactorrhoea, memory loss, amenorrhoea, headache, seizures and neurodeficits. Lymph node involvement in CNS RDD is present in less than one third of cases, which may not be clinically evident as in our index case there were no clinically palpable lymph nodes but F18FDG PET scan revealed generalized lymph node involvement. In the index case, the nodes were small in size, a rarity in RDD [19], in agreement with one of the two cases described by Wang et al with sellar involvement, but non-palpable lymph nodes [3].
| S.No. | Age /Gender | Reference | Sign & Symptoms | MRI Finding |
Endocrine Dysfunctions Dysfunctions |
DI | Treatment | Effect of treatment |
Out Comes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 32/ F | Dutta et.al.(present case) | CDI, Hypopituitarism, hyperprolactinemia, normal vision | Suprasellar mass of size 2.8×2.5×1.9 cm, T1-hypointense, T2/ flair-mildly hyperintense, homogenous contrast enhancement | TSH, ACTH, Gonadotropin, GH, ADH deficiency | Y | Surgrical debulking followed by tapering dose of oral prednisolone | Good response, mass remarkably decreased in size | Surviving at 1 year of follow up, hypopituitarism persisted |
| 2 | 44/F | Kutlubay et al.2014 | CDI, Xanthelasma like skin lesion, lymph adenopathy, | Not known | Not known | Y | Systemic methyl prednisolone | Cutaneous disease under control | Not known |
| 3 | 27 /M | Wang et al.2011 | CDI,vision loss, hypopituitarism | Sellar-supra sellar mass with anterior cranial extension, brightly enhancing on contrast | Y | Sub frontal –sphenoid surgery with gross total resection | Massive recurrence, CDI recurred after 3.7 years, received 50 Gy fractionated RT and intermittent prednisolone | Disease free at 5 years of follow up, hypopituitarism persisted | |
| 4 | 10/F | Wang et al. 2011 | CDI, Hypopituitarism, normal vision, no palpable lymphadenopathy | Sellar mass | TSH, GH, ACTH deficiency | Y | Transphenoidal resection followed by 20 Gy fractionated RT | Multiple intracranial recurrences | Prednisolone 5 mg twice daily for 5 years, CDI improved, no recurrence, hypopituitarism persisted |
| 5 | 63/F | Rotondo et al. 2010 | Ataxia, diarrhoea, weight loss, abdominal pain | Only documented dural thickening, GIT infiltration | Normal cortisol and TSH | N | NA | Death due to sepsis, Autopsy- Neurohypophysis involvement by RD, Pituitary gland showed only lactotroph hyperplasia |
Death |
| 6 | 43/M | Wan et al.2008 | Vision loss left eye, bitemporal hemianopia, headache, no lymphadenopathy | Not mentioned | Suprasellar solid mass 3×3×2.5 cm, encasing optic chiasma, isointense on T1 and T2, heterogenous contrast enhancement, pre-operative diagnosis-meningioma, | N | Transphenoidal surgery-right posterior clinoid mass | Vision improved at 3 months of follow up | Repeated MRI did not revealed recurrence |
| 6 | 15/F | Woodcok et al.1999 | Amenorrhea initially, headache, blurred vision, mild papilledema | Enlargement of pituitary infundibulum with extension of soft tissue into the suprasellar cistren | Hypothyrodism | N | Mild interval increase in size | Biopsy;steroids | 9 months alive |
| 7 | 45/F | Kelly et al.1999 | Fever, Headaches, collapse, diplopia, hypernatremia, polyuria, and thirst | Suprasellar lesion sized 2 cm in diameter, arising from the posterior pituitary. | Hypopituitrarism with low cortisol, thyroxine and gonadotrophins | Y | Diminished in size | Surgery; radiation; DDAPV, Chemotherapy; prednisolone |
3 years alive |
| 8 | 22/M | Ng. H.1995 | Polyuria and polydipsia, obese, lacked secondary sexual characteristics and libido | Posterior pituitary 1.2 cm in diameter, extending from the tuber cinerium to the posterior portion of the pituitary fossa | Mild hyperprolactinemia, depressed testosterone, subnormal GH and cortisol | Y | NA | Biopsy; DDAPV |
NA |
Table 1: Summary of cases with Rosai–dorfman disease involving sellar-suprasellar area.
Moreover, in our patient multiple generalized lytic bone lesions were detected by FDG-PET scan that were not seen on conventional radiography, thus FDG PET CT can be used as a modality to find out areas of sub clinical involvement and disease burden, prior to treatment [20]. Our case also had hyperprolactenemia, which was possibly due to stalk compression as shown on gadolinium enhanced MRI, where stalk was not separately visible from the suprasellar mass.
The differential diagnosis of CDI with sellar-suprasellar mass encompasses infectious, granulomatous as well as neoplastic causes like tuberculosis, sarcoidosis, histiocytosis X, germinoma, craniopharyngioma or even metastases. Before MRI imaging, even we kept a diagnosis of hypophysitis, with most probable causes being kept as tuberculosis or sarcoidosis as per our country’s scenario. Gadolinium enhanced MRI of brain showed a T1 hypointense, T2/FLAIR mildly hyperintense, lobulated mass in supasellar region, with homogeneous enhancement post-contrast, similar to previous reports of RDD [5,21,22]. In view of mass lesion, the patient was subjected to surgery for tissue diagnosis. The histopathology came as surprise to us and it completely changed the treatment.
Definitive diagnosis primarily relies on characteristic histopathology and immunohistochemistry of biopsy tissue. As for our patient, histopathology report taught us a lesson that proper biopsy should be decisive in planning further course of management. In the absence of signs of raised intracranial tension, the case could have been managed conservatively, as might have been the possibility with 7 previously described cases involving sellar-suprasellar area (6 were diagnosed on surgical specimen and one at autopsy). In terms of cytology, there were variable numbers of histiocytes intermixed with plasma cells, lymphocytes, and presence of emperipolesis (lymphocytophagocytosis), the characteristic finding in nodal disease, which is often less apparent in extra-nodal sites, however 6 out of 8 cases summarized by us showed this features, including our patient [2-8]. Immunostaining showed positivity for CD68, S100 and negativity for CD1a in all the cases including ours (Figure 3 A-D). Finding of polyclonal plasma cells, absence of acid fast bacilli and fungi, negativity for alpha-1 anti-chymotrypsin, alpha-1 anti-trypsin, CD15 and CD30 excluded other conditions [23,24].
Extensive review of all cases of RDD showed 70% had stable disease and 20% showed lasting remission without any treatment. Overall there were 21 deaths with 4 dying directly as a result of the disease. However, as CNS RDD is a potentially fatal immune dysregulation syndrome, more often than not it requires treatment [10], with surgery or steroids, chemotherapeutic agents, and radiation. Few case series has used only surgery as mode of treatment with good outcome [10,11,22,24].Our patient was started on steroid therapy (Prednisolone) 60 mg/day after excision of the suprasellar mass followed by tapering of steroids after six weeks. Consequently, the mass reduced in size, but CDI is still persistent requiring desmopressin. With multiple modalities of treatment, 7 out of 8 cases of sellar-suprasellar RDD had lasting remission, as we hope, should also be the case with our patient.
In conclusion, ours is the first case report, where extensive bone and lung involvement was found along with suprasellar mass presenting as hypopituitarism, hyperprolactinemia and CDI. This case illustrates the superiority of FDGPET over structural imaging to demonstrate precise extent of the disease, as well as the fact that all suprasellar masses might not need excision and can be managed conservatively. RDD should always be kept as a differential diagnosis of dura based, sellarsuprasellar mass with CDI with or without multi system involvement.
Acknowledgements
We would like to thank Dr. Viral N Shah for manuscript editing and constructive comments.
References
- Rosai J, Dorfman RF (1969) Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol 87: 63-70.
- Kutlubay Z, Bairamov O, Sevim A, Demirkesen C, Mat MC (2014) Rosai-Dorfman disease: a case report with nodal and cutaneous involvement and review of the literature. Am J Dermatopathol 36: 353-357.
- Wang F, Qiao G, Lou X, Song X, Chen W (2011) Intracranial recurrences of Rosai-Dorfman disease in the sellar region: two illustrative cases. ActaNeurochir (Wien) 153: 859-867.
- Rotondo F, Munoz DG, Hegele RG, Gray B, Khatun N, et al. (2010) Rosai-Dorfman disease involving the neurohypophysis. Pituitary 13: 256-259.
- Wan S, Teng X, Zhan R, Yu J, Gu J, et al. (2008) Isolated intracranial Rosai-Dorfman disease mimicking suprasellar meningioma: case report with review of the literature. J Int Med Res 36: 1134-1139.
- Woodcock RJ Jr, Mandell JW, Lipper MH (1999) Sinus histiocytosis (Rosai-Dorfman disease) of the suprasellar region: MR imaging findings--a case report. Radiology 213: 808-810.
- Kelly WF, Bradey N, Scoones D (1999) Rosai-Dorfman disease presenting as a pituitary tumour. ClinEndocrinol (Oxf) 50: 133-137.
- Ng HK, Poon WS (1995) Sinus histiocytosis with massive lymphadenopathy localized to the sella. Br J Neurosurg 9: 551-555.
- Cooper SL, Jenrette JM (2012) Rosai-Dorfman disease: management of CNS and systemic involvement. ClinAdvHematolOncol 10: 199-202.
- Adeleye AO, Amir G, Fraifeld S, Shoshan Y, Umansky F, et al. (2010) Diagnosis and management of Rosai-Dorfman disease involving the central nervous system. Neurol Res 32: 572-578.
- Wu M, Anderson AE, Kahn LB (2001) A report of intracranial Rosai-Dorfman disease with literature review. Ann DiagnPathol 5: 96-102.
- Foucar E, Rosai J, Dorfman R (1990) Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. SeminDiagnPathol 7: 19-73.
- Foucar E, Rosai J, Dorfman RF, Brynes RK (1982) The neurologic manifestations of sinus histiocytosis with massive lymphadenopathy. Neurology 32: 365-372.
- Warrier R, Chauhan A, Jewan Y, Bansal S, Craver R (2012) Rosai-Dorfman disease with central nervous system involvement. ClinAdvHematolOncol 10: 196-198.
- Kattner KA, Stroink AR, Roth TC, Lee JM (2000) Rosai-Dorfman disease mimicking parasagittal meningioma: case presentation and review of literature. SurgNeurol 53: 452-457.
- Gupta DK, Suri A, Mahapatra AK, Mehta VS, Garg A, et al. (2006) Intracranial Rosai–Dorfman disease in a child mimicking bilateral giant petroclivalmeningiomas: A case report and review of literature. Childs NervSyst 22: 1194–1200
- Sharma MS, Padua MD, Jha AN (2005) Rosai-Dorfman disease mimicking a sphenoid wing meningioma. Neurol India 53: 110-111.
- Purav P, Ganapathy K, Mallikarjuna VS, Annapurneswari S, Kalyanaraman S, et al. (2005) Rosai-Dorfman disease of the central nervous system. J ClinNeurosci 12: 656-659.
- Sachdev R, Setia N, Jain S (2007) Sinus histiocytosis with massive lymphadenopathy. Is the lymph node enlargement always massive? Med Oral Patol Oral Cir Bucal 12: E198-200.
- Deshayes E, Le Berre JP, Jouanneau E, Vasiljevic A, Raverot G, et al. (2013) 18F-FDG PET/CT findings in a patient with isolated intracranial Rosai-Dorfman disease. ClinNucl Med 38: e50-52.
- Konishi E, Ibayashi N, Yamamoto S, Scheithauer BW (2003) Isolated intracranial Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy). AJNR Am J Neuroradiol 24: 515-518.
- Andriko JA, Morrison A, Colegial CH, Davis BJ, Jones RV (2001) Rosai-Dorfman disease isolated to the central nervous system: a report of 11 cases. Mod Pathol 14: 172-178.
- Warnke RA, Weiss LM, Chan JK, Cleary ML, Dorfman RF (1995) Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). In: Rosai J, eds. Atlas of Tumor Pathology, 3rd Series: Tumors of the Lymph Nodes and Spleen. Washington: Armed Forces Institute of Pathology :349–360
- Deodhare SS, Ang LC, Bilbao JM (1998) Isolated intracranial involvement in Rosai-Dorfman disease: a report of two cases and review of the literature. Arch Pathol Lab Med 122: 161-165.
Citation: Jarial KDS, Hajela A, Rastogi A, Gupta K, Gasper LB, et al. (2014) An Unusual Cause of Central Diabetes Insipidus in a Young Female. J Clin Exp Pathol 4:192. DOI: 10.4172/2161-0681.1000192
Copyright: © 2014 Jarial KDS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16688
- [From(publication date): 11-2014 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 12106
- PDF downloads: 4582