Case Report Open Access
An Unusual Case of Clostridium difficile Infection in Trinidad and Tobago
Lemar Blake1, Patrick Eberechi Akpaka1*, Adash Ramsubahag2, Renea Ali2 and Asongna T. Folefoc3
1 Microbiology & Pathology Unit, Department of Paraclinical Sciences, Faculty of Medical Sciences, The University of the West Indies, St. Augustine, Trinidad and Tobago
2 Department of Life Sciences; Faculty of Science & Technology, The University of The West Indies; St. Augustine, Trinidad and Tobago
3 Department of Pathology and Laboratory Medicine, Faculty of Medicine, University of Calgary, Alberta, Canada
- *Corresponding Author:
- Patrick Eberechi Akpaka
Microbiology & Pathology Unit
Department of Paraclinical Sciences
The University of the West Indies
St. Augustine, Trinidad and Tobago
Tel: +1868-736-0440
Fax: +1868-663-3797
E-mail: peakpaka@yahoo.co.uk
Received Date: March 26, 2014; Accepted Date: April 28, 2014; Published Date: May 05, 2014
Citation: Blake L, Akpaka PE, Ramsubahag A, Ali R, Folefoc AT (2014) An Unusual Case of Clostridium difficile Infection in Trinidad and Tobago. Air Water Borne Diseases 3:115. doi: 10.4172/2167-7719.1000115
Copyright: © 2014 Blake L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Air & Water Borne Diseases
Abstract
Clostridium difficile infection (CDI) is the leading cause of healthcare-associated diarrhea. Psuedomembraneous colitis is a major complication of CDI and its outbreaks with increased severity and mortality has been reported. In this study we report the first fatal case of a toxin A and B gene positive strain of C. difficile associated with pseudomembraneous colitis in a patient from Trinidad and Tobago. Patients admitted to tertiary hospitals with history of diarrhea were investigated and reviewed. A fatal case of a 47-year old Trinidadian of African descent who presented with diarrheal stool at a public hospital in Trinidad and Tobago was reviewed and is being reported. The identified patient had his stool sample analyzed using conventional and molecular microbiology procedures to identify the C. difficile bacteria, toxins and genes. The antimicrobial susceptibility profile was also determined using agar dilutions according to CLSI guidelines.This patient’s stool sample was positive for Clostridium difficile toxin and the enzyme glutamate dehydrogenase which were demonstrated using the Quick Check Complete® kit. The antimicrobial susceptibility test produced an unusual profile - being resistant to meropenem, ampicillin, ceftriaxone, cefotaxime and ciprofloxacin. PCR analysis demonstrated that the strain produced a 1266bp and 204bp DNA fragment corresponding to the toxin A and B gene respectively, based on the primers used. This organism was noted to be responsible for the development of psuedomembranous colitis that led to the demise of this patient. Surveillance work need to be instituted as well as antibiotics stewardship so as to prevent any more case of inflammatory bowel diseases prevalence in our country.
Keywords
Clostridium difficile; Pseudomembraneous colitis; Polymerase Chain Reaction;Trinidad and Tobago
Introduction
Clostridium difficile is an anaerobic gram positive bacilli which is the major cause of psuedomembraneous colitis [1]. Infection with this organism may result in a range of presentations including asymptomatic carriage, mild diarrhea, psuedomembraneous colitis and toxic megacolon [2]. Various researches have shown that Clostridium difficile is present in approximately 10% of the normal healthy adult population and is implicated in 20-30% of nosocomial diarrhea.This organism is also the major role player implicated in antibiotic associated diarrhea [3-5].
Acquisition of Clostridium difficile infection often occurs in conjunction with disruption of the normal colonic microflora. Causes for such disruption may include antibiotic overexposure and underlying disease [6].Clostridium difficile spores and vegetative cells are brought into the body via ingestion. The vegetative cells are destroyed by the low pH of the stomach, however the spores continue on to the small intestine where they germinate upon exposure to bile acids [7]. The proliferating cells move into the colon where they attach themselves, being adequately protected by a polysaccharide layer. In a patient with good IgG response, this colonization leads to asymptomatic carriage; however in patients with poor IgG response toxin production may lead to manifestations of disease [6].
Two major toxins-toxin A and toxin B are produced by Clostridium difficile vegetative cells [8]. Both toxins attach themselves to epithelial cells of the colon and enter the cells via endocytosis. The resulting vacuole has a pH which facilitates the glycosyltransferase portion of the toxin to be released into the cytoplasm. This glycosyltransferase moiety facilitates the transfer of a glucose molecule from uridine diphosphate to Rho, Rac or Cdc-42 molecules which leads to inactivation of these molecules [9]. These molecules are responsible for actin formation, regulation of apoptosis and transcriptional regulation [9]. The mechanism for both toxins is the same; however there are differences in the effect on intestinal cells. Toxin A is enterotoxic; and causes neutrophil infiltration, substance P production, chemokine production, disruption of tight junctions and apoptosis. Toxin B has more direct (cytotoxic) effects causing disruption of tight junctions and apoptosis. The overall effects are edema and an inflammatory response [9].
There are evidences of a third toxin known as a binary toxin that is produced by various Clostridium difficile strains; one of such is the NAP 1 strain [10]. This new strain is an actin-specific ADP-ribosyltransferase toxin, that has two dependent proteins; CDTa, which is the catalytic component and CDTb which is the binding component [11]. It is widely accepted that certain strains of Clostridium difficile have the propensity to cause outbreaks, including multiple-state outbreaks in healthcare facilities [12]. Even though toxigenic strains have been sensitive to the widely accepted cytotoxicity assay, its use has become limited and methods such as Enzyme Linked Immuno-Sorbent Assay (ELISA) testing are now routinely used [13].
Molecular analysis has now been added to the algorithm for Clostridium difficile detection. This includes toxin gene detection via simple PCR analysis [14,15]. This enables researchers and health-care workers to understand the genetic aspect of the organism and how it may be related to where the organism was found. The species-specific internal gene fragment (tpi) for Clostridium difficile, as well as the gene for toxin A and B can efficiently be tested for using specific primers; which in turn can show the pathogenic nature of Clostridium difficile [16].
In this study we we report the case of Clostridium difficile organism that produced toxin A and B, caused psuedomembranous colitis which resulted in the demise of a patient in Trinidad and Tobago. Surveillance work need to be instituted as well as antibiotics stewardship so as to prevent any more case of inflammatory bowel diseases prevalence in our country.
Materials and Methods
Presentation of case
A 47 year-old Trinidadian male of African descent was admitted to the Eric Williams Medical Sciences Centre (EWMSC) in Mount Hope, Trinidad and Tobago as a referral from his family doctor where he had been seeking medical attention for the past 5 months. He presented with complaints of fever, abdominal cramps, generalized swelling and moderate diarrhea. He had no history of travel abroad. However, he had a 6 month history of rheumatoid arthritis. (Rheumatoid Factor was positive at 720 IU/ml). While in the care of his family physician, he received steroids for persistent swelling of his upper and lower limbs. According to the patient, he visited his family physician because he was having diarrhea and fever after he and a relative consumed substantial amounts of seafood at a weekend social gathering. He stated that they both became sick with symptoms of diarrhea and malaise following the meal. Although his relative recovered, the patient remained ill and was then referred to the medical center by his family physician.
On admission to the EWMSC he was treated for arrhythmia with adenosine; and empirically with ceftriaxone for suspected septic arthritis. Steroid therapy was continued his symptoms dramatically improved and he was subsequently discharged. Four months later he was readmitted with similar complaints – diarrhea, fever, malaise. On physical examination the patient appeared very ill, pale and dehydrated. He was febrile, blood pressure was 115/81 mm/hg and pulse rate was 125 beats/min. He was in mild respiratory distress and had generalized abdominal tenderness and swelling. The right inguinal lymph node was soft, mobile and enlarged. Rectal examination showed the presence of soft stool mixed with bright red blood on the examining finger.
Blood, stool and urine samples were taken for laboratory investigations. Stool was submitted specifically for identification of ova, cyst and parasite (OCP); as well as for culture. His hemoglobin was 11.1 g/dl and his white blood cell count was 25.6 x 109/dl. Blood and urine cultures came back negative; stool was also negative for OCP and culture with Cycloserine Cefoxitin Fructose Agar (CCFA) media. Flexible sigmoidoscopy revealed a pale mucus membrane and edema in the left transverse colon. There were no mucosal lesions visible. Computed Tomography (CT) of the abdomen/pelvis showed a hypodense mass in inguinal canal adjacent to the right iliopsoas muscle. Unfortunately, surgical intervention was not carried out to remove this mass as patient was too ill. The patient was treated with amantadine, levofloxacin, ibuprofen, prednisolone, Celebrex®, Panadol® and metronidazole 500 mg intravenously.
His diarrhea persisted, and twelve days later the white blood cell count was 30 x 109/l. A repeat CT scan showed a wedged shaped area in the periphery of the spleen, diffused thickening and mucosal enhancement of the distal ilium and the colon. Flexible sigmoidoscopy was repeated and demonstrated inflammation, jelly-like mucin, an enhanced, yellowish membrane and few mucosal lesions in the transverse colon. Biopsy of the region ruled out malignancy and a diagnosis of pseudomembranous colitis was made. Vancomycin was subsequently added to the treatment regimen; and was given orally at 500mg doses every 6 hours each day. The patient did not respond to treatment and eventually died before surgical procedures were carried out.
Laboratory Investigations
Prior to the patient’s death, a repeat stool samples were collected and tested for the organism using the C.diff Quick Check Complete® from Tech Labs. The stool sample was positive for the Clostridium difficile toxin however the ELISA kit used above did not indicate which toxin was present. The stool sample was cultured anaerobically using Cycloserine Cefoxitin Fructose Agar (CCFA) for 96 hours at 37°C. Culture revealed flat, ground- glass shaped greenish colonies which produced a horse scent; gram stain showed gram positive bacilli. The organism was confirmed as Clostridium difficile using Polymerase Chain Reaction to test for the organism’s house-keeping gene. No additional biochemical tests were carried out in Trinidad and Tobago. This isolate was further analyzed at Foothills laboratory of the University of Calgary, Calgary Alberta, Canada. Here, the isolate was re-cultured and incubated on a CCFA plate where positive growth was obtained (Figure 1). This isolate was also subjected to the Matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) test method which is a mass spectrometry technique that allows for DNA, peptides, proteins and sugars to be analyzed further confirmed the isolate as C.difficile. The ATCC 9686 control strains was analyzed with PCR and similar DNA fragments were obtained.
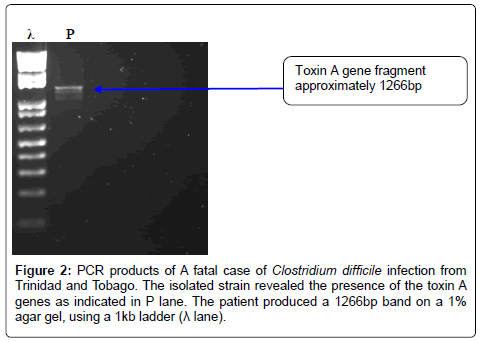
Figure 1: A fatal case of Clostridium difficile infection fromTrinidad and Tobago. Isolate was grown on a Cycloserine Cefoxitin Fructose Agar (CCFA) plate before being subjected to Matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) test and it confirmed the isolate as Clostridium difficile.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility of C. difficile was determined by minimum inhibitory concentration obtained by agar dilution method; as recommended by the Clinical Laboratory Standard Institute (CLSI) [16]. A 105 bacteria inocula was prepared by the direct suspension of the bacterial colonies, into 10 ml peptone water, equivalent to a 0.5 McFarlane standard (Biomerieux Vitek Inc, Hazel Hood Missouri). Fresh bacterial colony was taken from that which was grown for a 48-72 hour period on the CCFA agar. The suspended colonies were then inoculated 105 bacteria per spot on the Muller Hinton supplemented with 7% horse blood and a 2 fold increase in varying antibiotic concentration. Antibiotics used were: Metronidazole, Ampicillin, Pipercillin-Tazobactam, Meropenem, Penicillin G, Ceftriaxone, Cefotaxime and Ciprofloxacin. A total of 5 concentrations were used for each antibiotic according to the CLSI standard for anaerobes. Inoculated plates were incubated at 37°C for 48 hours.
PCR analysis
PCR analysis for the toxin A gene was carried out using the NK11 (5_TGATGCTAATAATGAATCTAAAATGGTAAC-3_) and NK9 (5_ CCACCAGCTGCAGCCATA_3). For toxin B gene NK104 (sequence- 5_-GTGTAGCAATGAAAGTCCAAGTTTACGC-3_) and NK105 (sequence-, 5-CACTTAGCTCTTTGATTGCTGCACCT-3_), were used as previously reported [17]. The house keeping gene (tpi) which is specific for Clostridium difficile was also assessed to further confirm the organism using the primer set tpi-F 5-AAAGAAGCTACTAAGGGTACAAA-3 and tpi-R 5-CATAATATTGGGTCTATTCCTAC-3 [14].
PCR conditions were as follows: the PCR mixtures were denatured (3 minutes at 95°C), and then a touchdown procedure was implemented, consisting of 30 seconds at 95°C, annealing for 30 seconds at temperatures decreasing from 65°C to 55°C during the first 11 cycles (with a °C decremental steps in cycles 1 to 11), and a final extension step of 72°C for 30 seconds. A total of 45 cycles were performed following methods previously described by [13]. PCR was performed using Promega GoTaq Green® master mix.
The ethics committee of the Faculty of Medical Sciences, the University of the West Indies, St. Augustine gave approval for this study.
Results
Patient stool C.difficile isolate produced positive PCR result for toxin A, B and the house-keeping gene. The antimicrobial susceptibility testing using agar dilution revealed that the isolate was resistant to ampicillin, ceftriaxone, meropenem, cefotaxime and ciprofloxacin. However it was sensitive to metronidazole, pipercillin-tazobactam, and penicillin G.
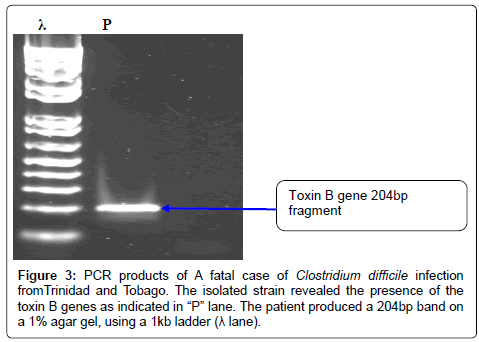
The Quick direct kit demonstrated the production of both toxins (A and B) and the glutamate dehydrogenase enzyme. All laboratory test procedures (isolation, ELISA and molecular) were repeated to confirm findings. However, no toxinotyping, ribotyping or cytotoxicity tests were carried out. The ATCC 9686 C. difficile strain reveals the presence of two major toxin genes A and B, (figure not shown) and was used as a positive control. The DNA fragment from the isolate is shown in lane P of figures 2 and 3, both toxin A and B gene fragments were shown respectively; a 1% agar gel and 1kb ladder was used for analysis. Sequencing of the genes revealed that the toxin A gene sequence is 99% similar to that of the lethal ZR81 strain and 98% similar to the ribotype 01 which has proved lethal in Germany.
Discussion
Clostridium difficile has two major virulent factors toxin A and toxin B [18]. Clostridium difficile is the main etiological agent linked to psuedomembranous colitis, and is responsible for over 90% of all cases [19]. Patients usually present with fever, abdominal pain, diarrhea, loss of appetite and fatigue [20]. Clostridium difficile-associated diarrhea seems to be on the increase and is implicated in 20-30% of all antibiotic associated diarrheal cases [21]. Fatal cases caused by toxin A and B producing Clostridium difficile strains, have been reported in countries such as, Canada, United States and Europe [7,11,14,22]. However not much data has been published on the extent to which psuedomembranous colitis caused by Clostridium difficile is associated with fatality. This is largely due to the fact that patients are normally treated before psuedomembranous colitis develops and case reports are also rarely published for isolated cases. Toxin A and B have enterotoxic and cytotoxic effects respectively; which have now been reported to show synergistic capabilities in their virulent processes [9]. These may lead to fluid infiltration, inflammation, disruption of tight junctions, psuedomembrane and mucus formation in the colon [5].
Psuedomembranous colitis is a mucosal disease, recognizable by numerous yellow plaques which are 0.2 - 2.0 cm in diameter; these attach themselves to varying lengths of the colon [22]. Patients with psuedomembranous colitis are normally treated based on the stage of the disease. Metronidazole and vancomycin are the two major drugs used; and in mild to moderate cases, supportive therapy along with the withdrawal of the offending drug is the first step to recovery [23].
For patients with severe psuedomembranous colitis, vancomycin is the preferred treatment and is given at 125 mg qds for 10-14 days [24]. A colectomy is carried out for patients who have megacolon consisting of a diameter >10 cm [25].
In this case the patient proved to be positive on culture which is indicated by the presence of the organism in an amount greater than 2000 of 10 x 1010 bacteria present per gram of wet feces. The isolated organism produced the enzyme glutamate dehydrogenase and toxins, both of which were detected using the C.diff Quick Check Complete Kit® (Tech Labs). However this test was unable to differentiate if the toxin produced was A, B or both. The test is described to be 87.8% sensitive and 99.4% specific [26].
The DNA from the organism was positive for the confirmatory housekeeping tpi gene (not shown). Previous studies have shown strains of similar gene fragment lengths [10]. Non-toxigenic Clostridium difficile strains have been shown to adhere far less frequently to intestinal mucosa than toxigenic strains [19]. It has also been shown that the adhesions produced by toxigenic strains binds to gut, mucosa and cells more frequently than non-toxigenic strains [19]. This ability of the cell may cause increased colonization and an enhanced ability to carry out its toxigenic effects.
As stated in the case presentation, the patient clearly stated during interview that he had consumed substantial amounts of seafood at a weekend social gathering. This indicates that the patient may have been infected in a community setting after ingesting contaminated seafood. Re-infection may have occurred later at a similar event since seafood is a popular dish in Trinidad and Tobago. Consideration should also be given to the patient’s past medical history which includes a diagnosis of rheumatoid arthritis. It is known that increased risk factors for the acquisition of C.difficile associated diarrhea include the severe underlying diseases and a faulty response to the toxins [27]. This has important implications for this patient because of the production of abnormal immunoglobulin G and formation of immune complexes in rheumatoid arthritis. Patients at greatest risk for severe disease are those who have had surgical intervention and or have been treated with immunosuppressive therapy since such patients cannot mount an adequate response [27]. This patient, while having not received surgical intervention, had been maintained on steroid therapy for rheumatoid arthritis. The antimicrobial susceptibility produced a very unusual result. Normally, Clostridium dificille is usually susceptible to cephalosporins and fuoroquinolones but this isolate was resistant to them. This could only be possible becausse of the prior exposure to these antibiotics for which resistance may have developed.
Conclusion
This case demonstrates that both the toxins A and B plays major roles in the formation of psuedomembranous colitis and that such resistant strains of Clostridium difficile are present in the population. Further research is needed on similarly detected strains, to better understand their significance in inflammatory bowel diseases.
Acknowledgements
Nursing staff of the EWMSC and technical staff of the microbiology department for their support. Funding for this study was partly provided by the University of the West Indies, St. Augustine Campus.
References
- Bauer MP, Kuijper EJ, van Dissel JT (2009) European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficileinfection (CDI). ClinMicrobiol Infect 15: 1067-1079.
- Cleary RK (1998) Clostridium difficile-associated diarrhea and colitis: clinical manifestations, diagnosis, and treatment. Dis Colon Rectum 41: 1435-1449.
- Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, et al. (2011) Host and pathogen factors for Clostridium difficileinfection and colonization. N Engl J Med 365: 1693-1703.
- McFarland LV, Mulligan ME, Kwok RY, Stamm WE (1989) Nosocomial acquisition of Clostridium difficileinfection. N Engl J Med 320: 204-210.
- Price AB, Davies DR (1977) Pseudomembranous colitis. J ClinPathol 30: 1-12.
- Kelly CP, Pothoulakis C, LaMont JT (1994) Clostridium difficilecolitis. N Engl J Med 330: 257-262.
- Poutanen SM, Simor AE (2004) Clostridium difficile-associated diarrhea in adults. CMAJ 171: 51-58.
- Samie A, Obi CL, Franasiak J, Archbald-Pannone L, Bessong PO, et al. (2008) PCR detection of Clostridium difficiletriose phosphate isomerase (tpi), toxin A (tcdA), toxin B (tcdB), binary toxin (cdtA, cdtB), and tcdC genes in Vhembe District, South Africa. Am J Trop Med Hyg 78: 577-585.
- Voth DE, Ballard JD (2005) Clostridium difficiletoxins: mechanism of action and role in disease. ClinMicrobiol Rev 18: 247-263.
- McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, et al. (2005) An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 353: 2433-2441.
- Alonso R, Martín A, Peláez T, Marín M, Rodríguez-Creixéms M, et al. (2005) Toxigenic status of Clostridium difficilein a large Spanish teaching hospital. J Med Microbiol 54: 159-162.
- Johnson S, Samore MH, Farrow KA, Killgore GE, Tenover FC, et al. (1999) Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficilein four hospitals. N Engl J Med 341: 1645-1651.
- Lemee L, Dhalluin A, Testelin S, Mattrat MA, Maillard K, et al. (2004) Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (Toxin A), and tcdB (Toxin B) genes for toxigenic culture of Clostridium difficile. J ClinMicrobiol 42: 5710-5714.
- Higazi TB, Mohannad AS, Burkett M, Pusok R (2011) PCR detection of Clostridium difficileand its Toxigenic Strains in Public Places in Southeast Ohio. Intl. J. Microbiol. Res., 2 :105-111.
- Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73: 5261-5267.
- Clinical and Laboratory Standard Institute (CLSI) (2012) Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Information Supplement; M100-S22; 31
- Kato H, Kato N, Watanabe K, Iwai N, Nakamura H, et al. (1998) Identification of toxin A-negative, toxin B-positive Clostridium difficileby PCR. J ClinMicrobiol 36: 2178-2182.
- Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB (1978) Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med 298: 531-534.
- Borriello SP (1998) Pathogenesis of Clostridium difficileinfection. J AntimicrobChemother 41 Suppl C: 13-19.
- Gebhard RL, Gerding DN, Olson MM, Peterson LR, McClain CJ, et al (1985) Clinical and endoscopic findings in patients early in the course of Clostridium difficile-associated pseudomembranous colitis. American J Med 78: 45-48.
- Figueroa I, Johnson S, Sambol SP, Goldstein EJ, Citron DM et al (2012) Relapse versus reinfection: Recurrent Clostridium difficileinfection following treatment with fidaxomicin or vancomycin. Clin Infect Dis; 55(suppl 2), S104-S10
- Persson S, Torpdahl M, Olsen KE (2008) New multiplex PCR method for the detection of Clostridium difficiletoxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. ClinMicrobiol Infect 14: 1057-1064.
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, et al (2010) Clinical practice guidelines for Clostridium difficileinfection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control HospEpidemiol; 31: 431-455.
- Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, et al. (2013) Guidelines for diagnosis, treatment, and prevention of Clostridium difficileinfections. Am J Gastroenterol 108: 478-498.
- Koss K, Clark MA, Sanders DS, Morton D, Keighley MR, et al. (2006) The outcome of surgery in fulminant Clostridium difficilecolitis. Colorectal Dis 8: 149-154.
- Sharp SE, Ruden LO, Pohl JC, Hatcher PA, Jayne LM, et al (2010) Evaluation of the C. Diff QuikChek Complete Assay, a new glutamate dehydrogenase and A/B toxin combination lateral flow assay for use in rapid, simple diagnosis of Clostridium difficiledisease. J ClinMicrobiol 48: 2082-2086.
- Hassett J, Meyers S, McFarland L, Mulligan ME (1995) Recurrent Clostridium difficileinfection in a patient with selective IgG1 deficiency treated with intravenous immune globulin and Saccharomyces boulardii. Clin Infect Dis 20 Suppl 2: S266-268.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16051
- [From(publication date):
May-2014 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 11417
- PDF downloads : 4634