Research Article Open Access
An Ultra-Sensitive and Selective LC-UV Method for the Simultaneous Determination of Metformin, Pioglitazone, Glibenclamide and Glimepride in API, Pharmaceutical Formulations and Human Serum
Najma Sultana1 , Safila Naveed1,2* and Saeed Arayne M31Research Institute of Pharmaceutical Sciences, Department of Pharmaceutical Chemistry Faculty of Pharmacy University of Karachi, Pakistan
2Jinnah University for Women, Karachi, Pakistan
3Department of Chemistry, University of Karachi, Pakistan
- *Corresponding Author:
- Safila Naveed
Research Institute of Pharmaceutical Sciences
Department of Pharmaceutical Chemistry
Faculty of Pharmacy University of Karachi, Pakistan
Tel: 00921-36632471
E-mail: safila117@yahoo.com; safila117@gmail.com
Received date: October 31, 2013; Accepted date: December 16, 2013; Published date: December 18, 2013
Citation: Sultana N, Naveed S, Saeed Arayne M (2013) An Ultra-Sensitive and Selective LC-UV Method for the Simultaneous Determination of Metformin, Pioglitazone, Glibenclamide and Glimepride in API, Pharmaceutical Formulations and Human Serum. J Anal Bioanal Tech 5: 176. doi: 10.4172/2155-9872.1000176
Copyright: © 2013 Sultana N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
An effective and comprehensive method for the simultaneous quantification of 4 NIDDM drugs (metformin, glimepride, glibenclamide and pioglitazone) was achieved on a Purospher Start C18 (5 μm, 25×0.46 cm) and Supelco C18 column in 2, 3, 7, 9 min respectively. The optimized method involves a C18 column thermostated at 30°C, UV detection at 235 nm, at a flow rate of 1 mL min-1. Good separation of the analytes was achieved by gradient high performance liquid chromatography-UV/visible detector (HPLC-UV/visible) in API, pharmaceutical dosages and serum, mobile phase was a mixture of methanol: water (70:30v/v) the pH of which was adjusted to 3.0 by phosphoric acid.
The method exhibited consistent, high-quality recoveries of the four analytes which ranged from 93.8 ± 2.1 to 99.8 ± 1.5 (mean ± RSD) with a high precision for the drug and impurities. Linear regression analysis revealed an excellent correlation between peak responses and concentrations (R2 values of 0.9991–0.9999) for the drug and impurities. Validation under Food and Drug Administration (FDA) guideline of the analytical parameters include: linearity (r2>0.9996), LLODs (0.315, 2.3, 0.2,0.1 ng ml-1), LLOQs (0.95, 0.7, 0.59,0.32 ng-1), intra-day precision (0.001) and inter-day precision 0.9 expressed as relative standard deviation (R.S.D.) and robustness parameters (less than 1.98%) with accuracies between 98% and 102%. The plasma assay was validated for parameters such as specificity, accuracy and extraction recovery. This is the first simultaneous characterization and quantitative determination of multiple NIDDMS. Thus, this method provides a simple, sensitive, selective, accurate and precise assay for the determination of all compounds in active pharmaceutical preparations, dosage formulations and human serum with high percentage of recovery, good accuracy precision (no interference of excepients) and a short run time. The proposed method can be extended for routine analysis of anti-diabetics in pharmaceutical preparations, biological matrices, and clinical laboratories with standard equipment, drug interaction studies and forensic medicine, recoveries ranging from 94 to 99%.
Keywords
Metformin; Quantification; Pioglitazone; Glibenclamide; Glimepride
Introduction
For many patients with type 2 diabetes, mono therapy with an oral antidiabetic agent is not sufficient and requires more than one antihyperglycemic drug to achieve optimal control. A fixed dose combination of metformin, pioglitazone, and glibenclamide showed significant efficacy in improving the glycemic control in type 2 diabetics. Thiazolidinedione class of drugs, rosiglitazone (ROS) and pioglitazone (PIO)) exert their glucose-lowering effect by binding to peroxisome proliferator-activated receptors gamma (PPARγ), thus increasing the receptor sensitivity to insulin [1-3]. Sulfonylurea drugs (glipizide (GLP), fliclazide (GLC), glibenclamide (GLB) and glimepiride act by increasing the secretion of insulin by the functioning β cells of the pancreas. This generation of hypoglycemic drugs is much more potent and is therefore effective at much lower dosages [4]. Combinations of metformin with glipizide, gliclazide or glibenclamide are available commercially as single dosage form. A combination tablet formulation is beneficial in terms of its convenience and patient’s compliance. Arayne et al. [5] quantified gliquidone and metformin [6-8] in bulk drug, pharmaceutical formulations and serum using LC and multivariate techniques respectively. Pioglitazone hydrochloride [9,10], glipizide and glimepride [11], pioglitazone and glimepiride [12] and six and eight antidiabetics drugs in combination [13,14] respectively have been determined in formulations and serum by RP-HPLC. Jain et al. [15] and Arayne et al. and Sultana et al. [16-19] reported simultaneous methods of NIDDM drugs with other co-adminstered drugs in formulations and human serum.
The present paper describes an isocratic reversed-phase high performance liquid chromatographic method with UV detector for the separation and quantification of four antidiabetic drugs i.e. metformin, pioglitazone, glibenclamide and glimepride in API, formulations and serum. The method would help in assay of drugs in a single run which reduces the time of analysis to less than 10 mins and does not require separate methods for each drug. The developed method was also validated successfully and applied to human plasma assay.
Experimental
The isocratic HPLC method uses a simple mobile phase with UV-detection at 230, 235 and 240 nm using Purospher STAR (5 μm, 25×0.46 cm) RP-and Supelco C18 (150×4.6 mm, 5 micron) columns. UV-detection is simple, rapid, selective and reproducible and sensitivity is adequate for routine use. Direct determination of the examined compounds in small volumes of human blood serum can be accomplished by protein precipitation using acetonitrile. The method was validated in terms of linearity, accuracy, precision, sensitivity, selectivity, and stability.
Wavelength selection
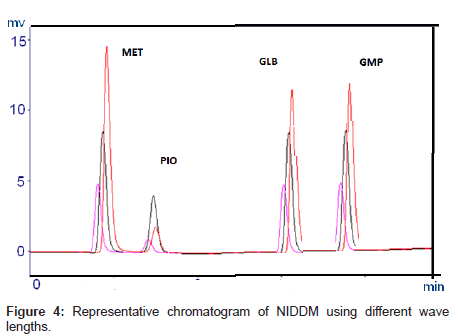
UV spectra of individual drugs were recorded in the wavelength range from 200 to 400 nm and overlap to each other. It was found that all the drugs exhibited high response at 235 nm.
Sample preparation
Standard stock solutions were prepared by dissolving 10 mg each of metformin, pioglitazone, glibenclamide and glimepride in the mobile phase and the volume was made up to 100 mL with the same solvent. For the calibration curves, six calibrators of drugs were prepared by making serial dilutions from stock solutions over the range of 2.5-25 μg mL-1.
Materials and reagents
Metformin and glimepride were gratis by Sanofi Aventis (Pakistan) Ltd, pioglitazone and glibenclamide from Ali Gohar Pharmaceuticals and Safe Pharmaceutical (Pvt) Ltd. Formulations of metformin (Neodipar 250 mg), glimepride (Amaryl 2 mg), pioglitazone (Poze 45 mg) and glibenclamide (Diazet 5 mg) were obtained from retail pharmacies. All reagents used were of HPLC grade. Methanols, acetonitrile of HPLC grade and orthophosphoric acid 85% used were of analytical grade from E. Merck, Germany. Deionized water was used to prepare mobile phase. Stock solutions and working solutions were prepared daily. All solutions were filtered through 0.45 μm filter and degassed using sonicator.
Instrumentation
Shimadzu HPLC system equipped with a LC-10 AT VP pump, an SPD-10 AV VP UV-VIS dual wave length detector, integrated via Shimadzu model CBM-102 Communication Bus Module to P-IV computer. Shimadzu CLASS-GC software (Version 5.03) was used for data acquisition and mathematical calculations and rheodyne manual injector fitted with a 20 μL loop. Chromatographic separation was carried out on a Purospher STAR RP-and and Supelco (150×4.6 mm, 5 micron) with a particle size of 10 μ and DGU-14 AM on-line degasser. UV visible 1601 Shimadzu double beam spectrophotometer was used to record the spectra.
Assay in formulation
Twenty tablets each of metformin, pioglitazone, glibenclamide and glimepride were weighed to obtain the average tablet weight and then powdered. 10 mg powder of each drug was weighed, dissolved in mobile phase and the volume was made up to 100 mL with the same mobile phase. The resulting solution was allowed to stand for 1 hour with intermittent sonication to ensure complete solubility of the drug (stock solution) which was then filtered and the filtrate diluted to the desired concentration for working solutions. All sample and standard solutions were filtered through 0.45 μm filter paper before injection into the system. A placebo tablet was also subjected to the same process as discussed above. The possibility of excepients interference in the analysis was studied.
Assay in serum
Plasma samples, obtained from healthy volunteers, were collected and stored. To 1.0 mL of plasma, 9.0 mL of acetonitrile was added; the mixture was vortexed for 1 min and then centrifuged for 10 mins at 10,000 rpm and the supernatant was filtered by 0.45 μ membrane filter. An aliquot of serum sample was fortified with metformin, pioglitazone, glibenclamide and glimepride to achieve final concentration.
Results and Discussion
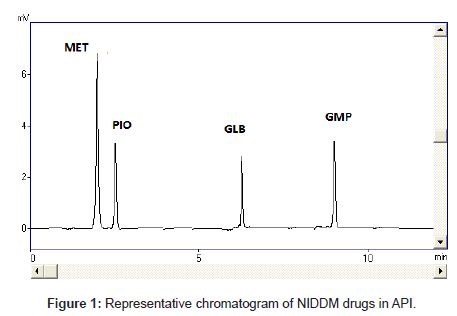
Diabetes mellitus is a heterogeneous group of disorders characterized by abnormalities in carbohydrate, protein and lipid metabolism [20]. An abnormality in insulin production or action or both is the central disturbance in diabetes mellitus, which results primarily in elevated fasting and postprandial blood glucose levels. In recent years, diabetes mellitus has become a common disease affecting human health seriously. All anti-diabetic drugs (Figure 1), chosen in this study are commonly used in clinics for type II diabetes mellitus patients
Method optimization and chromatographic conditions
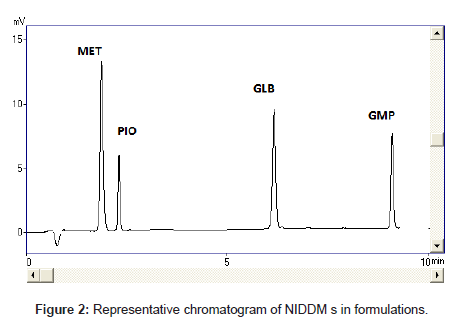
Optimization of the chromatographic conditions takes into account the various goals of method development and to weigh each goal (resolution, runtime, sensitivity, peak symmetry, etc.) accurately, according to the requirement of HPLC methods being used for the estimation of drugs in biological fluids. The drugs are not totally soluble in water whereas soluble in organic solvents like methanol and acetonitrile. The developed liquid chromatographic method for simultaneous determination of four NIDDMs was first optimized to choose the appropriate chromatographic conditions for efficient, accurate and economical analytical method. For stationary phase selection Supelco, Prospher Star C18 (5 μm, 25×0.46 cm) and Discovery C18 (5 μm, 25×0.46 cm) columns were tested with a variety of mobile phases and the best separation with less retention time was achieved with Prospher Star C18 (5 μm, 25×0.46 cm) column. During the development phase, mobile phases, methanol-water and acetonitrilewater in different ratios with variable (pH 2.5-4) were tried to fix on the best ratio for separation of components. Mobile phase containing acetonitrile:water resulted in asymmetric peaks with poor resolution, greater tailing factor (>2) and high run time. The successful use of mobile phase containing a mixture of methanol and water in the ratio of 70:30 (v/v) with pH adjusted to 3.0 exhibited good separation and high resolution with short analysis time. Methanol was selected because of its favorable UV transmittance, low viscosity, and better solubility. The flow rate of mobile phase greatly affected the analysis of the studied analytes. The analytes were monitored at 235 nm and the retention times were found to be 2, 3, 7 and 9 minutes for MET, PIO, GLB and GLM respectively (Figure 2). Although run time decreased significantly at higher flow rates, however resolution of peaks and sensitivity of the analytes decreased. The flow rate was therefore adjusted to 1.0 mL min-1, tailing was (<1.3) and resulted in good peak symmetry and resolution. Separation was best achieved at isobestic point of 235 nm whereas 230 and 240 nm wavelengths were also checked.
Validation of the developed method
The validation parameters studied according to ICH guidelines [21] were system suitability test, specificity and selectivity, linearity, accuracy, precision, detection and quantitation limits and robustness.
System suitability
The HPLC system was equilibrated with the initial mobile phase composition, followed by 6 injections of the same standard. These 6 consecutive injections were used to evaluate the system suitability on each day of method validation. Parameters of system suitability are peaks symmetry, theoretical plates of the column, mass distribution ratio (capacity factor), resoluteion and relative retention as summarized in Table 1.
| Metformin | Pioglitazone | Glibenclamide | Glimepride | |
|---|---|---|---|---|
| Retention time (Rt) | 2 | 3 | 7 | 9 |
| Capacity Factors (K’) | 2.3 | 2.4 | 2.4 | 2.3 |
| Theoretical plates (N) | 2763 | 2762 | 2763 | 2764 |
| Tailing factor (T) | 1. 2 | 1.2 | 1.3 | 1. 2 |
| Resolution (R) | 6.14 | 6.14 | 7.14 | 8.14 |
Table 1: System suitability parameters.
Linearity and sensitivity
Linearity was tested at the concentration range of 2.5-25 μg mL-1. Concentration of NIDDM versus peak area was subjected to least square linear regression analysis. A linear regression line in the concentration range of 2.5-25 μg mL-1 for MET, PIO, GLB and GMP respectively was obtained with correlation coefficient (r2) >0.998. Regression characteristics including slope, intercept, correlation coefficient values for each drug are given in Table 2. The standard curve, intercept and slope were determined by statistical software.
| Systems | Drugs | Column | Conc. (µgmL-1) | r2 (b) | Standard error of estimate | Standard error | Intercept | Slope | LLOD (ngmL-1) |
LLOQ (ngmL-1) |
|---|---|---|---|---|---|---|---|---|---|---|
| LC 10 | MET | Supelco | 2.5-25 | 0.998 | 0.3266 | 0.1668 | -0.0454 | 14520 | 0.315 | 0.95 |
| Purospher | 2.5-25 | 0.999 | 0.3266 | 0.1668 | -0.0454 | 17505 | 0.315 | 0.96 | ||
| PIO | Supelco | 2.5-25 | 0.999 | 0.31038 | 0.1631 | -0.9252 | 12516 | 2.3 | 0.7 | |
| Purospher | 2.5-25 | 0.999 | 0.31038 | 0.1631 | -0.9252 | 13959 | 2.3 | 0.7 | ||
| GLB | Supelco | 2.5-25 | 0.998 | 0.3266 | 0.1668 | -0.0454 | 13520 | 0.2 | 0.59 | |
| Purospher | 2.5-25 | 0.999 | 0.3266 | 0.1668 | -0.0454 | 17505 | 0.2 | 0.58 | ||
| GMP | Supelco | 2.5-25 | 0.999 | 0.31038 | 0.1631 | -0.9252 | 11516 | 0.1 | 0.32 | |
| Purospher | 2.5-25 | 0.999 | 0.31038 | 0.1631 | -0.9252 | 13969 | 0.1 | 0.32 | ||
| LC 20 | MET | Supelco | 2.5-25 | 0.998 | 0.3266 | 0.1668 | -0.0454 | 13520 | 0.317 | 0.96 |
| Purospher | 2.5-25 | 0.999 | 0.3266 | 0.1668 | -0.0454 | 17505 | 0.317 | 0.96 | ||
| PIO | Supelco | 2.5-25 | 0.999 | 0.31038 | 0.1631 | -0.9252 | 11516 | 2.3 | 0.7 | |
| Purospher | 2.5-25 | 0.999 | 0.31038 | 0.1631 | -0.9252 | 13959 | 2.3 | 0.7 | ||
| GLB | Supelco | 2.5-25 | 0.998 | 0.3266 | 0.1668 | -0.0454 | 13520 | 0.19 | 0.58 | |
| Purospher | 2.5-25 | 0.999 | 0.3266 | 0.1668 | -0.0454 | 17505 | 0.19 | 0.58 | ||
| GMP | Supelco | 2.5-25 | 0.999 | 0.31038 | 0.1631 | -0.9252 | 11516 | 0.1 | 0.32 | |
| Purospher | 2.5-25 | 0.999 | 0.31038 | 0.1631 | -0.9252 | 13959 | 0.1 | 0.32 |
Table 2: Regression characteristics.
Accuracy and assay
Method accuracy was evaluated as the percentage of recovery of known amounts of metformin, pioglitazone, glibenclamide and glimepride to the pharmaceutical formulation and serum. It is performed at spike concentration that was 80, 100 and 120%. Each sample was injected five times and result range was 98-102%, compiled in Table 3, high recovery indicated that the method has a high degree of accuracy.
| Systems | LC 10 | LC 20 | ||||
|---|---|---|---|---|---|---|
| Parameters | Conc. (µgmL-1) | Conc. found | %Recovery | Conc. found | %Recovery | |
| Assay (spiking method) | MET | 8 | 8.06 | 100.8 | 8.06 | 100.7 |
| 10 | 9.919 | 99.19 | 9.99 | 99.99 | ||
| 12 | 11.92 | 99.34 | 11.92 | 99.33 | ||
| PIO | 8 | 7.96 | 99.52 | 8.21 | 102.67 | |
| 10 | 9.48 | 99.48 | 10.19 | 101.9 | ||
| 12 | 11.94 | 99.52 | 12.22 | 101.86 | ||
| GLB | 8 | 8.06 | 100.8 | 8.06 | 100.75 | |
| 10 | 9.919 | 99.19 | 9.99 | 99.99 | ||
| 12 | 11.92 | 99.34 | 11.92 | 99.33 | ||
| GMP | 8 | 7.96 | 99.52 | 8.21 | 102.67 | |
| 10 | 9.48 | 99.48 | 10.19 | 101.9 | ||
| 12 | 11.94 | 99.52 | 12.22 | 101.86 | ||
Table 3: Accuracy of metformin, pioglitazone, glibenclamide and glimepride.
Precision
Precision of the proposed method was determined by repeatability (intra-day precision) and intermediate precision (inter-day precision). It was expressed as relative standard deviation (RSD). Five different concentrations of metformin, pioglitazone, glibenclamide and glimepride in the linear range were analyzed on the same day (intra-day precision) and two consecutive days (inter-day precision); every sample was injected five times. Both intra- and inter-day RSD values were in the range 0.06-1.5% confirming good precision (Table 4). The precision for all these analytes under investigation did not exceed 2% at any of the concentrations studied and well met the requirements of validation.
| MET | PIO | GLB | GMP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Systems | Conc. (µgmL-1) | Intra-day variation | Inter-day variation | Intra-day variation | Inter-day variation | Intra-day variation | Inter-day variation | Intra-day variation | Inter-day variation |
| LC 10 | 25 | 0.171 | 0.191 | 0.322 | 0.431 | 0.026 | 0.122 | 0.952 | 0.779 |
| 12.5 | 1.33 | 0.419 | 0.935 | 0.382 | 0.597 | 0.248 | 0.906 | 0.906 | |
| 6.25 | 0.525 | 0.189 | 0.377 | 0.425 | 0.62 | 0.107 | 0.62 | 0.737 | |
| 3.125 | 0.403 | 0.071 | 0.544 | 0.723 | 0.7 | 0.111 | 0.596 | 0.753 | |
| 1.5625 | 0.381 | 0.18 | 0.99 | 0.317 | 0.755 | 0.214 | 0.885 | 0.253 | |
| LC 20 | 25 | 0.175 | 0.124 | 0.174 | 0.269 | 0.193 | 0.131 | 0.529 | 0.357 |
| 12.5 | 0.291 | 0.397 | 0.17 | 0.866 | 0.367 | 0.208 | 0.301 | 0.307 | |
| 6.25 | 0.182 | 0.769 | 0.439 | 0.868 | 0.268 | 0.915 | 0.563 | 0.944 | |
| 3.125 | 0.516 | 0.854 | 1.05 | 0.086 | 0.382 | 0.355 | 0.013 | 0.205 | |
| 1.5625 | 0.258 | 0.759 | 0.963 | 0.447 | 0.926 | 0.649 | 0.144 | 0.282 | |
Table 4: Precision of metformin, pioglitazone, glibenclamide and glimepride at 235 nm.
Limit of detection and limit of quantification
LLOD=3.3o/S and LLOQ=10o/S; where o is the standard deviation of the lowest standard concentration and S is the slope of the standard curve. The LLOD were 0.315, 2.3, 0.2, 0.1 and LLOQ 0.95, 0.7, 0.59, 0.32 ng mL-1 for metformin, pioglitazone, glibenclamide and glimepride as given in Table 2.
Specificity and selectivity
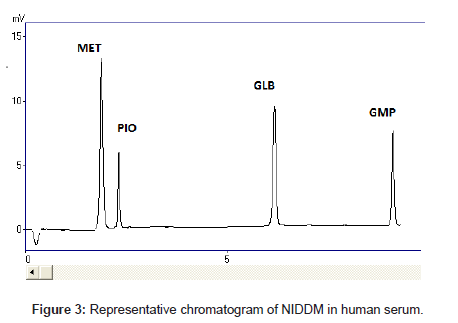
The selectivity and specificity of the method were performed. Chromatograms of blank mobile phase, excipients solution, pharmaceutical formulations and serum samples obtained from standard solutions were identical with those obtained from spiked solution containing an equivalent concentration. The representative chromatograms (Figures 1-4) showed no significant interference of unwanted excipients used in pharmaceutical formulations or endogenous components of serum at the retention time of MET, PIO, GLB and GMP. Accordingly, the proposed method can be considered selective. The method confirmed good resolutions equal to 6.14-8.41 (Table 1).
Robustness
Method robustness was performed by making minor changes while analyzing same sample at normal operating conditions and also by changing some operating analytical conditions such as wavelength, mobile phase composition pH and flow rate.
When a parameter was intentionally changed ± 2% (in mobile phase), ± 0.1% (in flow rate) and ± 0.05% (pH 3) from its optimum condition, the shifting in retention time of ± 0.1% was observed. The relative standard deviation in each case was found not more than ± 2%. The data in Table 4 or 5 shows that the results are within the acceptable criteria, this indicated better robustness of the developed method and hence found suitable for analysis of drugs.
| Level | K’ | T | (Rs) | |
|---|---|---|---|---|
| A: pH of mobile phase | ||||
| 2.8 | -0.2 | 4.8 | 1.43 | 2.4 |
| 3 | 0 | 4.5 | 1.43 | 2.4 |
| 3.2 | 0.2 | 4.5 | 1.45 | 2.2 |
| Mean ± S.D (n=6) | 4.5 ± 0.3 | 1.43 ± 0.020 | 2.3 ± 0.1 | |
| B: Flow rate (mLmin-1) | ||||
| 0.8 | -0.2 | 4.1 | 1.45 | 2.2 |
| 1 | 0 | 4.3 | 1.44 | 2.6 |
| 1.2 | 0.2 | 4.4 | 1.44 | 2.7 |
| Mean ± S.D (n=6) | 4.3 ± 0.212 | 1.44 ± 0.015 | 2.36 ± 0.026 | |
| C: Percentage of water in mobile phase (v/v) | ||||
| 25 | -5 | 4.6 | 1.42 | 2.38 |
| 30 | 0 | 4.3 | 1.43 | 2.36 |
| 35 | 5 | 4.5 | 1.46 | 2.33 |
| Mean ± S.D (n=6) | 4.36 ± 0.070 | 2.36 ± 0.025 | 2.36 ± 0.025 | |
| C: Wavelength (nm) | ||||
| 225 | -5 | 4.5 | 1.42 | 2.38 |
| 235 | 0 | 4.3 | 1.43 | 2.36 |
| 240 | 5 | 4.4 | 1.45 | 2.32 |
| Mean ± S.D (n=6) | 4.3 ± 0.070 | 1.43 ± 0.015 | 2.36 ± 0.030 | |
K’=Capacity factors, N=Theoretical plates, T=Tailing factor, Rs=Resolution
Table 5: Robustness of the method (n=6).
Ruggedness
Ruggedness of our method was determined in two different labs. Lab 1 was at Research Institute of Pharmaceutical Sciences, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Karachi while other lab was in Department of Chemistry, University of Karachi. Two different instruments one was LC 20 and other was LC 10 and two different columns Purospher Start C18 and Supelco C18 were used. The developed method did not show any remarkable difference in calculated results from acceptable limits.
Recovery of drugs from human plasma
The recovery of four anti-diabetic drugs from human plasma was determined by standards freshly comparing peak areas of spiked plasma extracts with those neatly prerpared in methanol. Plasma samples (n=6) spiked with the analytes at their respective LLOQ, low, middle and high QC levels were analyzed. The area ratios of the targeted drugs were compared with those obtained from blank extracts spiked with the 4 target drugs after extraction (taken as 100% recovery of the drug from that particular matrix). Recoveries of the drugs are summarized in Table 3. The method was found to be suitable for therapeutic purposes.
Conclusion
A simple, specific, selective and precise method was developed for the simultaneous determination of four anti-diabetic drugs metformin, pioglitazone, glibenclamide, and glimiperide. The mobile phase is economical and simple to prepare with little or no variations with a short run time of less than 10 mins. The sample recoveries in human plasma were in good agreement and no interference of endogenous materials was found, as well as no lengthy extraction procedures were required in the estimation. Hence, this method can be easily and conveniently used for the routine analysis of the drugs in plasma samples for pharmacokinetic studies, forensic materials, clinical laboratories and to study drug interactions.
References
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, et al. (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 270: 12953-12956.
- Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, et al. (1996) The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem 39: 665-668.
- Young PW, Buckle DR, Cantello BC, Chapman H, Clapham JC, et al. (1998) Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator-activated receptor gamma. J Pharmacol Exp Ther 284: 751-759.
- Satoskar RS, Bhanbarkar D, Nirmala N (2001) Pharmacology & Pharmacotherapeutics. 17th edition, Popular Prakashan, India.
- Arayne MS, Sultana N, Mirza AZ, Siddiqui FA (2010) Validated RP-HPLCmethod for the quantitation of gliquidone in pharmaceutical formulation and human serum. J Chil Chem Soc 55: 156-158.
- Arayne MS, Sultana N, Zuberi MH, Siddiqui FA (2009) Spectrophotometric Quantitation of Metformin in Bulk Drug and Pharmaceutical Formulations using Multivariate Technique. Indian J Pharm Sci 71: 331-335.
- Porta V, Schramm SG, Kano EK, Koono EE, Armando YP, et al. (2008) HPLC-UV determination of metformin in human plasma for application in pharmacokinetics and bioequivalence studies. J Pharm Biomed Anal 46: 143-147.
- Kar M, Choudhury PK (2009) HPLC method for estimation of metformin hydrochloride in formulated microspheres and tablet dosage form. Indian J Pharm Sci 71: 318-320.
- Mehta RS, Patel DM, Bhatt KK, Shankar MB (2005) UV and visible spectrophotometric analysis of pioglitazone hydrochloride in bulk and tablets. Indian Journal of Pharmaceutical Sciences 67: 487-489.
- Mahadik SP, Senthilkumar GP (2012) Method development & validation of pioglitazone in bulk and pharmaceutical dosage forms by using spectrophotometric Method. Asian Journal of Biochemical and Pharmaceutical Research 2: 159-165.
- Sultana N, Arayne MS, Ali SN, Zuberi MH (2012) Simultaneous determination of glipizide and glimepride by RP-HPLC in dosage formulations and in human serum. Med Chem Res 21: 2443-2448.
- A K, G S, C MR, Bhat K, A R, et al. (2008) Simultaneous determination of pioglitazone and glimepiride in bulk drug and pharmaceutical dosage form by RP-HPLC method. Pak J Pharm Sci 21: 421-425.
- Venkatesh P, Harisudhan T, Choudhury H, Mullangi R, Srinivas NR (2006) Simultaneous estimation of six anti-diabetic drugs--glibenclamide, gliclazide, glipizide, pioglitazone, repaglinide and rosiglitazone: development of a novel HPLC method for use in the analysis of pharmaceutical formulations and its application to human plasma assay. Biomed Chromatogr 20: 1043-1048.
- Lakshmi KS, Rajesh T (2011) Separation and quantification of eight antidiabetic drugs on a high-performance liquid chromatography: its application to human plasma assay. ISRN Pharm 2011: 521353.
- Jain D, Jain S, Jain D, Amin M (2008) Simultaneous estimation of metformin hydrochloride, pioglitazone hydrochloride, and glimepiride by RP-HPLC in tablet formulation. J Chromatogr Sci 46: 501-504.
- Arayne MS, Sultana N, Zuberi MH, Siddiqui FA (2013) Simultaneous determination of metformin, captopril, lisinopril and enalapril by RP-HPLC: Its applications in dosage formulations and in human plasma. Med Chem Res 22: 5717-5722.
- Arayne MS, Sultana N, Mirza AZ (2011) Simultaneous determination of gliquidone, pioglitazone hydrochloride, and verapamil in formulation and human serum by RP-HPLC. J Chromatogr Sci 49: 114-117.
- Sultana N, Safila Naveed, Arayne MS (2013) Monitoring of in vitro interaction studies of enalapril with hypoglycemic agents by LC-UV. Research and Reports in Medicinal Chemistry 3: 1-7.
- Sultana N, Naveed S and Arayne MS (2013) Development and validation of a simple and efficient RP-LC method for analysis of captopril, metformin, pioglitazone and glibenclamide in API, formulations and human serum. Pharm Anal Acta 4: 257.
- Craig CR, Stitzel RE (2004) Modern Pharmacology with Clinical Applications. Lippincott Williams & Wilkins, Philadelphia, USA.
- ICH Harmonised Tripartite Guideline (1996) Validation of Analytical Procedures: Text and Methodology Q2(R 1). International Conference on Hormonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, London.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14948
- [From(publication date):
February-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10307
- PDF downloads : 4641