Review Article Open Access
An Overview on Factors Underlying Gastric Cancer; Strategies for its Management With Particular Reference to Diet
Monica Gallo, Filomena Altieri, Chiara Stella Di Stadio, Giuseppina Miselli, Valentina Villano, Paolo Arcari*, Emilia Rippa*
Department of Molecular Medicine and Medical Biotechnology, University of Naples Federico II, via S. Pansini, 5 - 80131 Naples, Italy
- Corresponding Authors:
- Dr. Emilia Rippa
Department of Molecular Medicine and Medical Biotechnologies
University of Naples Federico II, Via S. Pansini 5, I-8031 Naples, Italy
Tel: +390817463119
Fax: +390817463653
E-mail: emilia.rippa@unina.it - Prof. Paolo Arcari
Department of Molecular Medicine and Medical Biotechnologies
University of Naples Federico II, Via S. Pansini 5, I-8031 Naples, Italy
Tel: +390817463120
Fax: +390817463653
E-mail: paolo.arcari@unina.it
Received Date: November 24, 2015, Accepted Date: February 15, 2016, Published Date: February 22, 2016
Citation: Gallo M, Altieri F, Stadio CSD, Miselli G, Villano V, et al. (2016) An Overview on Factors Underlying Gastric Cancer; Strategies for its Management With Particular Reference to Diet. J Gastrointest Dig Syst 6:399. doi:10.4172/2161-069X.1000399
Copyright: © 2016 Gallo M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
The incidence of stomach cancer and the number of victims of this disease have decreased dramatically over the last 60 years. However, gastric cancer still remains a very serious disease that requires further studies to enlarge knowledge on its causes and to prevention methods. To date, the causes of gastric cancer are still not yet well known but it is clear that some people are more prone than others to develop this disease. Gastric cancer affects mostly adults aged 55 and over and men in percentage double than women. Stomach ulcer apparently does not increase the risk of gastric cancer however, Helicobacter pylori, usually due to inflammation and gastric ulcers, may be an important risk factor for this disease. Moreover, patients who have undergone stomach surgery or suffering from pernicious anemia, achlorhydria or atrophic gastritis (that typically produce a reduction in the amount of acid) are subject to a higher risk of gastric cancer. Exposure in workplaces to certain agents such as dust or fumes is linked to a higher risk than average of developing stomach cancer. Smoking also contributes to increase this risk. Moreover, epidemiological studies and animal models, conducted for years, have shown that some eating habits can increase the risk of cancer. Other studies instead report that fresh foods (especially fruits and vegetables) play a protective function against gastric cancer. For this reason, this paper provides an overview of the possible causes of gastric cancer and the different therapeutic approaches, focusing in particular on the effects of diet.
Keywords
Gastric cancer; Helicobacter pylori ; Diet, Gastric biomarkers
Abbreviations
AICR: American institute for cancer research; APC: Adenomatous polyposis coli ; GC: Gastric cancer; EGC: Early gastric cancer; GKN1: Gastrokine 1; IARC: International agency for research on cancer; WCRF: World cancer research fund
Introduction
Gastric cancer (GC) is the most common cancer worldwide however, despite its recent decline, gastric cancer is the fourth most common cancer and the second leading cause of cancer-related death worldwide with 603,003 new cases among men and 330,290 new cases among women [1,2] with a poor prognosis and a survival rate at 5 years of about 20% [3-5] in most areas of the world, except in Japan where mass screening programs, staging systems, and treatment may contribute to superior 5-year survival rates of approximately 60% [6-10]. In all Europe, the incidence is about 104,620 and 69,394 among males and females, respectively [11], representing about 23% of all cancers. The ratio of males to females is 1.6:1 [12-14] and in six out of 10 cases the patient has more than 65 years. Stomach cancer still causes more than 10,000 deaths per year, reaching as global spread, just below the breast cancer and lung cancer [15]. The incidence is higher among lower social classes, although it is in a rapid decline (about 5% every five years) that is faster in women than in men. However, in developed countries, over the last three decades, the incidence of gastric cancer is declining. This phenomenon appears to be due, at least in part, to improved nutrition and to the decreased consumption of foods preserved in salt or smoked.
In this review, we tried to provide an overview of the possible causes at the origin and development of gastric cancer. Moreover, as for all types of cancer, since early detection is an important key aspect for prevention, possible strategies for a viable therapeutic management of the disease are reported, such as the search for possible early biomarkers. Finally, several data are considered regarding the potentially harmful foods that can increase the risk of cancer, as opposed to other foods that can reduce the risk.
Histological Types
The development of gastric cancer in most cases does not occur “de novo” from normal stomach but is rather a consequence of subsequent modifications of the gastric epithelium. There are two main types of stomach cancer based on histological classification. The intestinal type, that is well known and the diffuse type, still not well characterized. Intestinal type of stomach cancer is the most common and affects mostly men over the age of 50 years [16,17]. The development of the intestinal-type gastric cancer includes the transformation of normal mucosa into a mucosa similar intestinal epithelium (intestinal metaplasia). The presence of intestinal metaplasia increases the risk of gastric cancer in proportion to the extension of the area affected by metaplasia [18]. Intestinal metaplasia may progress to dysplasia and then to carcinoma. This tumor generally occurs as formations facing towards the interior of the cavity and with expansive growth. In contrast, the diffuse type gastric cancer presumably originates from changes in a single cell in the region of the collar mucous of the gastric glands. These cells then can proliferate and invade beyond the crypt to the own lamina [19,20]. The diffuse type of stomach cancer is less frequent and hits indifferently men and women aged on average 45 years.
If the cancer is diagnosed early i.e., it affects only the mucosa and sub mucosa (the first two layers of the tissues of the organ), it is called the early gastric cancer (EGC) and has the best chance of healing [21]. In about 50% of cases, the place where the cancer develops is at the level of the pylorus and the stomach cavity. In one-fifth cases, the location is at the level of the body of the stomach, while it is not usual that the tumor will establish itself at the level of the so-called small curvature. The time interval that elapses on average between the diagnosis of an EGC and cancer progression is approximately 37 months [22], while about 8 years are necessary for an EGC to progress in an advanced stage of disease [23]. The percentage of EGC in Japan (30-50%) is high than that occurring in Western countries [24]. The importance of a correct identification of EGC is represented by the excellent results achieved with surgical treatment and good prognosis of EGC patients after surgery. The spreading of GC can occur directly via lymphatic to the lymph nodes to the esophagus and the peritoneum, and to blood-borne bringing liver metastases, bone, lung, but rarely to the brain. To explain the emergence and development of stomach cancer, several factors can be considered and, among them, food plays a very important role in fact, a diet rich in starches, fats and smoked or salted foods (mostly containing nitrites and nitrates as precursors of carcinogenic nitrosamines) can promote the beginning of gastric cancer, as well as cigarette smoking and alcohol consumption, that can contribute to the neoplastic transformation of the gastric tissue.
Correa [25] proposed that the development of gastric carcinogenesis occurs through intermediate stage and is due to the combination of several process that involves many factors such as environmental irritants, acid secretion, bacterial growth and bacterial production of nitrites or N-nitroso compounds of the diet. These factors led to progressive spectrum of histological stages going from normal gastric epithelium to gastric adenocarcinoma of intestinal type [26].
Etiology and Risk Factors
The underlying cause of gastric cancer is still unclear however, several predisposing conditions have been identified, these include:
Genetic factors: Stomach cancer is particularly common in some families. Subjects owning type II Lynch syndrome, that developed a tumor of the colon without being affected by polyps, usually preceding the tumor, show a high risk of stomach cancer. The incidence of the disease in relatives of affected patients is 23 times higher than the general population. Also ethnic groups such as blacks Americans and people of Celtic origin, as part of the same nation, are more frequently affected than others. In addition, the incidence of stomach cancer among subjects belonging to blood group A is about 10-20% greater than that of the remaining population.s
Geographical factors: Gastric cancer tends to be more common in some countries and, within the same nations; the incidence may vary in different areas (higher in the north, where the climate is generally cold, than in the south). Moreover, it has been observed that immigrants from countries with high incidence of GC to countries with low incidence, showed a decrease in the frequency of the disease.
Metabolic and nutrition factors: In high-risk countries, there are well-defined correlations between the consumption of certain foods and the incidence of stomach cancer; diet commonly used by the population of these countries is rich in carbohydrates and cereals, but low in fresh fruits and vegetables, fats and vitamins and in particular vitamin A. Of course, this dietary composition is not itself carcinogenic but it might be able to stimulate the action of some pathogens (bacteria or other) towards the gastric mucosa. Also some chemical compounds like nitrosamines (that contain nitrogen and oxygen), can lead to stomach cancer. Nitro compounds are formed in the stomach of humans when nitrates contained in certain foods are converted to nitrite; nitrites that in turn interact with amine to form nitrosamines that are carcinogenic. Bacteria colonizing the stomach that often could be already suffering from other diseases such as atrophic gastritis can catalyze the transformation of nitrates and nitrites into nitrosamines. Nitrates are present mainly in smoked foods since they have the function of preservatives; it is possible to find them, in small amounts, also in fish, meat, sausages, cheese and beer. Smoking also is also a factor that can increase the risk of stomach cancer [27].
Atrophic gastritis: Atrophic gastritis is a precancerous disease that precedes the development of stomach cancer. It is characterized by the progressive disappearance of the glands of the stomach and by the appearance in over 40% of cases of glands that are present in the intestine (intestinal metaplasia), thus atypical for gastric region. Atrophic gastritis is a very common disease in fact, about 20-25% of apparently healthy young men and 50-60% of the elderly suffers of this disease that has also been associated to 90% of stomach cancer cases.
Pernicious anemia: 6% of patients with this disease develop over time gastric cancer (the risk is about 23 times greater than in the general population); also in this case the predisposing condition is due to changes in intestinal metaplasia.
Gastric polyps: These benign tumors that represent 10% of all stomach cancers should be considered as true precancerous lesions: about 1.5% of all patients with gastric disorder suffer from polyps. The probability of malignant degeneration increases with the increase in the size of the polyp and in particular if the diameter exceeds 2 cm.
Gastric ulcer: Frequency of tumor degeneration of this disease is highly variable, in fact, it occurs on average in 10% of cases. Small ulcers degeneration seems to occur less frequently than larger ones; in fact, malignant degeneration of benign ulcer is an extremely rare event.
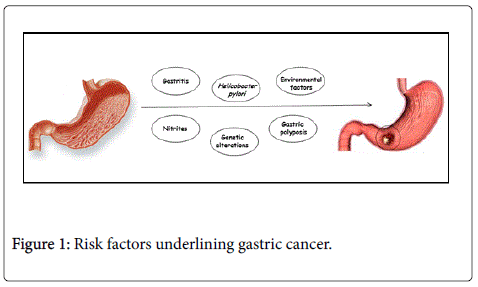
Helicobacter pylori : The most important etiological factors implicated in gastric carcinogpernicious anemiaenesis are diet and Helicobacter pylori (H. pylori ) infection. There is an association between H. pylori (H.pylori ) infection and stomach cancer (the risk is higher than 36 times). This association is not apparently related to the development of the ulcer, often occurring in these subjects, but is rather related to the onset of chronic atrophic gastritis induced by the microorganism. An international study on the population indicated that in countries with a high incidence of cancer of the stomach, there was a corresponding high prevalence of infection with H.pylori [28], a bacterium that is also responsible of gastric ulcer and duodenal ulcer. The presence in the stomach of H. pylori can alter in a longer time the delicate balances that exist at the level of organ. Gastric ulcers, atrophic gastritis and autoimmune gastritis associated with [29-31] are all conditions causing in gastric epithelium an excessive rate of cell proliferation. Increased production of oxidants and reactive nitrogen compounds including nitric oxide might lead to gastritis. Altered gastric acid secretion and increase gastric pH are generated by both gastritis and atrophy that also modify the bacterial flora and allow anaerobic bacteria to colonize the stomach. However, the different geographical risk of Gastric Cancer in Europe cannot explain a possible prevalence of infection since only a small percentage of people infected with H. pylori develop stomach cancer. Under this aspect, in Japan, the country in the world with the highest incidence of gastric cancer, the actual estimation is that out of 60 million people with Helicobacter infection, only 0.4% contracted a gastric cancer [32]. H. Pylori was isolated for the first time in 1982; in 1994 it was recognized as a human carcinogen by IARC, but its mechanism of action in the complex process of tumor growth has never been completely clarified [33]. Recent studies showed that the correlation between H. Pylori infection and GC represents a typical model of a multistep process characterized by some pre-neoplastic lesions with a high risk of progression (atrophic gastritis, intestinal metaplasia and dysplasia). Therefore, over the past decades, H. pylori infection has been clearly correlated with gastric carcinogenesis [34] (Figure 1).
Genetic Factors
The possibility to contract gastric cancer is about 2-3 times higher in first-degree relatives of patients with the disease [35-38]. A study performed on Scandinavian twin showed an increased risk of gastric cancer in the twin of a person with the disease [39]. To assess the contribution of hereditary and environmental factors, some models have been developed. These models have shown that to GC, inherited genes contributed 28%, environmental factors related to lifestyle for 10% and other environmental factors for the 62%. In addition, it has been also shown that there is a familial inclination that contributes to the beginning of the disease; for instance, some alterations of certain genes like p53 and Adenomatous polyposis coli (APC) [40-42] are caused to the commencement of tumors in different organs, including the stomach (called, in these cases, Lynch syndrome type II) [43,44].
Other possible causes that occur rarely are represented by gastric polyposis [45] i.e., by the appearance of small benign growths that if left untreated over time or treated by interventions with special surgical techniques used in the past, can degenerate and become malignant. In the latter case, it could be possible that after about 15 or 20 years form intervention, the point at which it was formed to the wound it starts to appear a tumor. Therefore, people operated to the stomach must be controlled periodically with gastroscopy.
Several recent studies have underlined the role that genetic alterations could play in the development and progression of gastric carcinoma [46]. Under this aspect, Molecular pathology should be of great help to understand the pathogenesis of this disease but also for providing useful prognostic molecular markers. For example, the overexpression of p53 by immunohistochemistry has been observed in 17-91% of invasive tumors [47] whereas, the incidence of p53 mutations in invasive carcinomas ranged from 0% to 77% [48-50]. These results however were often contradictory [51-56] probably because of the different immunohistochemical determination procedures utilized (i.e., different antibodies, techniques or methods of interpretation).
Other suggested biological prognostic factors were related to the expression level of p21 [57], to the expression of vascular endothelial growth factor (VEGF) [58], to the counts of microvessel density [59], to the over-expression of EGF-r [60] and of Cyclin D2 [61], to alterations of BAT-26 [62], uPA (urokinase type plasminogen activator) and PAI-1 (plasminogen activator inhibitor) [63,64], to serum levels of soluble receptor of IL-2 (SolIL-2R) [65,66] or some factors related to cell proliferation like the fraction of S phase, the Ki-67 or the nuclear antigen of cellular proliferation (PCNA) [67-73]. However, data on the correlation between molecular markers and response to chemotherapy are still controversial [74]. For instance, two studies on the immunohistochemical analysis of p53 made on endoscopic biopsy before treatment have shown a relationship between the expression of p53 and response to chemotherapy [75,76]. Also the expression of thymidylate synthase seems to correlate with response to chemotherapy [77,78]. Excision Repair Cross-Complementing (ERCC-1), a gene potentially involved in chemoresistance, it was demonstrated to be more highly expressed in gastric cancer patients not responding to chemotherapy [79]. However, it must be pointed out that all these data derive from backdated studies, thus there is a strong need for well-designed future studies in order to identify the role of molecular markers in predicting response and survival of patients with gastric cancer (Figure 1).
Biological Markers
The initial stage of GC is troubled by the absence of specific symptoms before it spreading. Therefore, screening programs finalized to the detection of pre-cancerous lesions and early-stage GC might be the main tool for reducing GC-related mortality. The primary screening technique is upper endoscopy that represents the standard approach for confirmation of the diagnosis [80,81]. Unfortunately, endoscopy is a very invasive technique often characterized by serious uncommon side effects with the disadvantage of being high costly and endoscopic skill dependent. This state is further hampered by a lack of diagnostic biomarkers that can aid in the early detection and prognosis of gastric cancer and in the prediction of chemo-resistance. In addition, tissues from surgery are difficult to obtained and traditional gastric cancer biomarkers of are not successful. Early diagnosis allowing identify a disease before it manifests clinically, is an aspect of extreme importance in cancer in order to implement the most effective therapy.
Today, pepsinogen (PG) represents the only non-invasive test for GC detection. PGs are prevalently synthesized and secreted by the gastric chief cells. Their serum levels indirectly reflect their secretion in the stomach [82,83]. There are two PGs (PGI and PGII); PGI is produced only in the corpus mucosa while cardiac and pyloric glands, and proximal duodenal mucosa also secrete PGII [84]. Low levels of PGI and a low PGI/PGII ratio are indicative of atrophic changes in the gastric corpus. PG tests can detect gastric mucosal atrophy with a sensitivity of 66.7%-84.6% and a specificity of 73.5%-87.1% [85-87], whereas the sensitivity for GC detection ranges from 36.8%-62.3% [88-90]. These figures are not considered acceptable in populationbased screening settings. Therefore, PG test can be considered as primary screening test to identify subjects who are at high risk for GC. These subjects must then undergo to endoscopic examination followed by the histological analysis of gastric biopsy [91].
Generally, in the management of cancer, a selected protein might represent a useful and precious biomarker. Proteins as cancer biomarker can play an integral role in many approaches in cancer studies like diagnosis, detection, patient monitoring, treatment, and tumor classification [92,93]. Therefore, expensive and invasive cancer diagnostic tests can be switched to reasonable unambiguous biomarker test [94]. The use of biomarkers has begun to demonstrate its novel feature in the detection and management of patients with different malignancies [95,96] and there has been a continuous search of markers for possible use in the diagnosis of gastric cancer. Several proteomics studies have helped to highlight powerful diagnostic tools in the management of cancer. New proteomic technologies allow not only the screening of large number of samples but also the identification of pathologically significant proteins and quantify difference in protein expression under different conditions. Potential biomarkers for gastric cancer have been searched in several human specimens like blood, gastric fluid, tissues and H.pylori -infected samples [97]. Unfortunately, diagnostic biomarkers in gastric cancer, such as the carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), and carbohydrate antigen 72-4 (CA72-4), are not specific and sensitive enough and their sensitivity in the range of 18%-57% [98,99]. In fact, CEA and CA19.9 have increased in 30-40% of patients with gastric cancer, but significantly increased levels of these antigens were typically found in advanced disease, rather than in the early phase of the disease [100-104].
Recent research work reported the identification of gastrokine 1 (GKN1), a protein secreted in the stomach antrum that is highly expressed in normal tissues and down-regulated in H. pylori -positive patients [105,106]. GKN1 is down-regulated in primary tumors both at transcriptional and translational level. Therefore, based on these results, GKN1 could be used as a new molecular marker to help predict the early cell transformation and could be a potential new target for treatment of gastric cancer [107].
Human secreted proteins can be detected in body fluids, such as blood and urine. In fact, blood and urine represent the most and easily accessible body fluids compared to tissue and gastric fluid. Therefore, these fluids are potentially powerful sources for discovering highly effective biomarker for GC. Several potential blood biomarkers of GC have been found using proteomic strategies. For instance, ApoC-I and apoC-III [108], thrombin light chain A [109], and three protein masses with 1468, 3935, and 7560m/z [110] were highlighted with a high sensitivity (>89.9%) and high specificity (>71%) as potential blood biomarkers for GC. Also macrophage migration-inhibitory factor (MIF) [111] and a 2209-Da peptide [112] were identified as potential blood biomarkers for GC (sensitivity and specificity >28% and >83.3%, respectively). Moreover, C9 [113] (sensitivity: 90%; specificity: 74%) and IPO-38 [114] (sensitivity: 56.7%; specificity: 93.3%) were found to be biomarkers for different stages of gastric cancer or for comparing different cancers including gastric cancer, respectively. Endothelial lipase protein is instead a promising urinary gastric cancer biomarker and although it is not linked to tumor stage or grade, it could be useful for screenings with high diagnostic fidelity [115]. All these findings showed that proteomics can be efficaciously useful to discover blood/ serum biomarkers by using advanced diagnostic technologies and a sufficient amount of clinical sample [116]. Under this regard, also metabolomics is an emerging field that may offer practical solutions to the challenges mentioned above [117-120]. The potential up- and down-regulated biomarkers mentioned in these studies are reported in Table 1.
| Protein | Condition | Application | References |
|---|---|---|---|
| Blood | |||
| 2209-Da peptide High molecular weight (HMW) kininogen fragment |
Up-regulated | Early detection of gastric cancer | [141] |
| Apolipoproteins ApoC-I and ApoC-III | Dow-regulated | Diagnostic score for stomach cancer | [17] |
| Complementary Factor I precursor (CFI) | Dow-regulated | Diagnosis and indication of the advancement of gastric cancer | [79] |
| Macrophage migration-inhibitory factor (MIF) | Dow-regulated | Prognostic of cancer patients | [153] |
| Thrombin light chain A | Dow-regulated | Diagnosis of cancer patients | [33] |
| Complement component 9 (C9) | Up-regulated | Biomarker for gastric cancer detection | [16] |
| Fibrinopeptide a (FPA) | Up-regulated | Serum based tests for screening of high-risk individuals | [34] |
| Inter-alpha-trypsin inhibitor heavy chain 3 (ITIH3) | Up-regulated | Early detection of gastric cancer | [16] |
| IPO-38 | Up-regulated | Diagnosis and prognosis of gastric cancer | [48] |
| Peaks at 1468, 3935, and 7560 m/z | Up-regulated | Diagnosis of gastric cancer | [129] |
| Sialyl Lewis X epitopes (SLeX) | Up-regulated | Diagnosis and prognostic monitoring in stomach cancer | [8] |
| Urine | |||
| Endothelial lipase (EL) | Down-regulated | Large-scale screenings with high diagnostic fidelity |
[15] |
| Metabolite of prostaglandin E(2) (PGE-M) | Increased levels | Cancer biomarker and/or indicator of risk factors. |
[156] |
Table 1: Summarized markers specific to gastric cancer.
Gastric Cancer and Diet
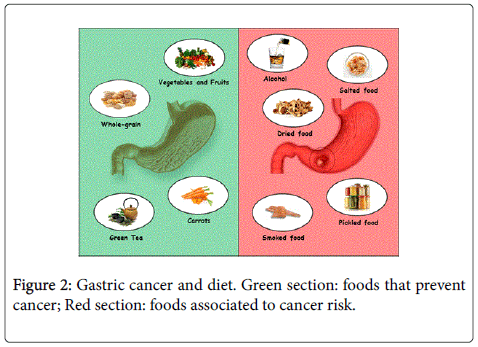
Epidemiological studies and animal models, conducted for years, have shown that some eating habits can increase the risk of cancer. However, is quite difficult to exactly quantify the risk associated with each type of food. This is due to the complexity of elements that are involved in etiopathogenesis of cancer and the considerable number of substances present in our diet. Nevertheless, there are numerous studies reporting the property of certain foods to reduce the risk of cancer. In particular, gastric cancer shows a higher incidence in some areas of the world, such as Japan, Korea, some countries of Eastern Europe and Latin America. Inhabitants of these geographical areas routinely consume dried, smoked, salted or pickled foods that, according to some studies, could play an important role in the development of stomach cancer. However, there is not yet a theory establishing that some types of diet and certain food components provide protection against the development of neoplastic disease. People moving from countries characterized by a high incidence of gastric cancer to countries with lower frequencies tend to maintain the high-risk of the population of origin. The subsequent generations of immigrants show a risk level that is close to that of the new host country [121]. All of these aspects help to suggest the close association of gastric cancer with changeable factors such as diet. Under this regard, several studies have shown that about 66-75% of the risk associated with gastric cancer could be reduced with increased consumption of fruits and vegetables in the diet and with a concomitant lower consumption of foods rich in salts [122]. In fact, diets high in fruits and vegetables, in combination or separately, reduce the risk of gastric cancer. Increased evidences have indicated that raw vegetables, citrus fruits and vegetables own a protective effect, whereas plants salted and pickled show a negative role. Several among all casecontrol studies have pointed out a positive association between gastric cancer and the consumption of highly salted foods such as salted fish, cured meat, and salted vegetables or the use of table salt. It must be noted that processed meat often contains chemical carcinogens like Nnitroso compounds as well as high amounts of salt [123,124]. Diets rich in salty foods, associated to high salt consumption, appears to increase the risk of stomach cancer, while diets rich in whole grains and green tea may decrease the risk of gastric cancer. Epidemiological studies have shown that whole-grain consumption displayed a protective effect against several cancers. Although is quite difficult to identify and isolate the protective properties of whole-grain from dietary fiber and other components, the protection property exerted by whole-grain far exceeds in eventual prospective epidemiological studies, the protection from the isolated nutrients and phytochemicals contained in whole-grain [125]. Despite that studies regarding the relationship between the use of whole-grain and the occurrence of gastric cancer are not so many, in most cases they are almost comparable [126].
It is well known that green tea contains polyphenols, commonly known as catechins. Antioxidant activities have been isolated from green tea and both in vitro and in vivo studies showed their ability to inhibit the nitrosation of polyphenols [127,128]. In addition, recent investigations proposed other possible mechanisms for cancer inhibitory effects of green tea, including modulation of signal transduction pathways leading to the inhibition of cell proliferation and transformation, induction of apoptosis and cell cycle arrest, inhibition of tumor invasion and angiogenesis [129-131]. However, while statistical significant studies have shown a reduction in the risk of gastric cancer with green tea drinking [132,133], other studies showed a contrary non-protective effect of green tea toward gastric cancer [134,135]. Hence, the consumption of green tea might be possibly associated with a decreased risk of gastric cancer however, further prospective studies with more detailed information are required to better clarify the role of green tea on gastric carcinogenesis. There is instead no apparent evidence that black tea or coffee consumption has any effect on the risk of gastric cancer. In this contest, the most important vitamins and derivatives contained in vegetables and fruit, like vitamin C and carotenoids seem to reduce the risk of gastric cancer whereas some doubt are still present regarding vitamin E [136-138].
The evidence on the effects of vitamin C comes from chemoprevention studies conducted with volunteers [139] in order to evaluate the role of vitamin C and beta-carotene on gastric cancer. The study indicated that vitamin C and beta-carotene show protective effect that consisted in an increase in the reduction of histologically proven diagnosis of intestinal metaplasia and/or atrophy (preneoplastic lesions). In another chemoprevention study conducted in Finland on male smokers [140], showed that administration of alphatocopherol and beta-carotene did not affected the risk of gastric cancer and/or prevalence of premalignant lesions [141], showed that the consumption of vitamin E reduced the risk of gastric cancer, however, in previous case control studies no statistically significant association between dietary vitamin E intake and gastric cancer [142-147] or inverse association was observed [148-153]. On the basis of the actual knowledge, a dietary high consumption of antioxidants (vitamin C, vitamin E, niacin), iron and potassium and low consumption of foods that are sources of sodium, decrease risk for gastric cancer, however, given the contradictory results, additional studies in a large number of subjects will be necessary to clarify the interaction between the various dietary micronutrients and risk of gastric cancer.
Finally, alcohol drinking and cigarette smoking may contribute to the transformation of the gastric tissue in tumor. Alcohol consumption may increase the risk of gastric cancer at the cardia [154,155] at the same level of the association between smoking and gastric cancer [156,157] (Figure 2).
Gastric Cancer and Mediterranean Diet
Mediterranean diet is characterized by a large intake of fruit, vegetables, cereals, legumes, nuts and seeds, fish and seafood, and by a relative use of alcohol such as red wine. In particular, olive oil represents the principal source of fats whereas a relatively low consumption of milk derivate like cheese and yoghurt and of red meat is registered [158-160]. Mediterranean diet is also correlated to a reduction of total mortality and in particular that associated to cancer. Mediterranean country like as France and Greece [86] are characterized by relatively low incidence of gastric cancer mortality whereas in southern Italy, where the Mediterranean diet is more diffused compared to the northern area of the country [28] gastric cancer mortality is even lower. Even though no scientific explanation is actually available, GC history of the last decade highlighted a worldwide decline of the disease most likely due to dietary improvements [66]. In a recent study regarding the relation between gastric cancer risk and the Mediterranean diet and conducted in Italy using data from two case-control studies between 1985 and 2007, convincing evidence of a beneficial role of the Mediterranean diet on gastric cancer were provided. The mechanism of action of the Mediterranean diet might be due to the synergic action of the nutrients, and not to a single nutrient. To partly explain the health benefits such as cardio-protective and anti-inflammatory effects, specific biochemical process, like decreases in oxidative stress, inflammation, plasma N-terminal pro-brain natriuretic peptide (NTproBNP) concentrations, and tumor necrosis factor α, were identified. In addition, intake of polyphenol from lignans, flavonols, and hydroxybenzoic acids present in Mediterranean food appear to be associated with decreased cardiovascular disease risks. Extra virgin olive oil, rich of phenolic compounds, vitamin E, and other lipid molecules, also possess a anti-atherogenic effects, improving endothelial function, lipid profiles, insulin sensitivity, glycemic control, and blood pressure levels. In definitive, these studies demonstrated that adherence to a Mediterranean diet positively affects health.
Conclusion
In this review, we have tried to highlight the numerous studies regarding the various environmental and genetic factors that are involved in the different stages of gastric cancer process. Therefore, under these aspects, a multifactorial and multistep model of gastric cancer is currently accepted. In particular, scientific studies have suggested that certain foods and/or food compounds may help prevent cancer whereas other kind of foods seems to play an opposite role. It still remains unclear how constituents present in fruit and vegetables play a significant role in gastric cancer prevention and why the effects of cancer prevention in the development of stomach cancer are only in some cases effective. Of coarse, the risk of gastric cancer also depends on several other factors, including smoking, alcohol consumption and obesity. All these findings and prospective studies suggest that a dietary modification directed toward a reduction of salt and salted food intake and to an increase intake of fruit, particularly vitamin C, as well as quitting smoking represents an effective way to prevent gastric cancer. Therefore, it is essential the commitment of basic medicine in the disclosure of risk factors of gastric cancer with a focus on food regulations. Such action, if done in a methodical, comprehensive and continuous, could help in the medium to long-term change in the incidence of this disease. To achieve this, it is also crucial that General Medicine provides specific training on these issues and collaborates in each geographical area with Health, patients' associations and citizens to counter the misleading buildup produced by other sources of "pseudo-information health" in terms of nutrition. Overall, this review wants to provide a set of data that confirm as a healthy lifestyle starting from feed can reduce the risk of cancer, but also of several chronic diseases.
Acknowledgements
This work was supported by funds from Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (2012CK5RPF_004), PON Ricerca e Competitività 2007-2013 (PON01_02782) and POR Campania FSE 2007-2013, Project CRÈME.
References
- Abad M, Ciudad J, Rincon MR, Silva I, Paz-Bouza JI, et al. (1998) DNA aneuploidy by flow cytometry is an independent prognostic factor in gastric cancer.Anal Cell Pathol 16: 223-231.
- Adachi Y, Mori M, Sugimachi K (1990) Persistence of mucosal gastric carcinomas for 8 and 6 years in two patients.Arch Pathol Lab Med 114: 1046-1048.
- Trichopoulou A, Lagiou P (1997) Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle.Nutr Rev 55: 383-389.
- Araya M, Terashima M, Takagane A, Abe K, Nishizuka S, et al. (1997) Microvessel count predicts metastasis and prognosis in patients with gastric cancer.J Surg Oncol 65: 232-236.
- Asaka M, Kudo M, Kato M, Sugiyama T, Takeda H (1998) Review article: Long-term Helicobacter pylori infection-from gastritis to gastric cancer. Aliment Pharmacol Ther 1: 9-15.
- Becker KF, Keller G, Hoefler H (2000) The use of molecular biology in diagnosis and prognosis of gastric cancer.Surg Oncol 9: 5-11.
- Benetou V, Trichopoulou A, Orfanos P, Naska A, Lagiou P, et al. (2008) Conformity to traditional Mediterranean diet and cancer incidence: the Greek EPIC cohort.Br J Cancer 99: 191-195.
- Bones J, Byrne JC, O'Donoghue N, McManus C, Scaife C, et al. (2011) Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J Proteome Res 10:1246-1265.
- Buckland G, Agudo A, Luján L, Jakszyn P, Bueno-de-Mesquita HB, et al. (2010) Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study.Am J Clin Nutr 91: 381-390.
- Carmack SW, Genta RM, Graham DY, Lauwers GY (2009) Management of gastric polyps: a pathology-based guide for gastroenterologists.Nat Rev Gastroenterol Hepatol 6: 331-341.
- Carnevale R, Pignatelli P, Nocella C, Loffredo L, Pastori D, et al. (2014) Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation.Atherosclerosis 235: 649-658.
- Cascinu S, Graziano F, Del Ferro E, Staccioli MP, Ligi M, et al. (1998) Expression of p53 protein and resistance to preoperative chemotherapy in locally advanced gastric carcinoma.Cancer 83: 1917-1922.
- Catalano V, Baldelli AM, Giordani P, Cascinu S (2001) Molecular markers predictive of response to chemotherapy in gastrointestinal tumors.Crit Rev Oncol Hematol 38: 93-104.
- Chen H, Tucker KL, Graubard BI, Heineman EF, Markin RS, et al. (2002) Nutrient intakes and adenocarcinoma of the esophagus and distal stomach, Nutr. Cancer 42: 33-40.
- Chong PK, Lee H, Loh MC, Choong LY, Lin Q, et al. (2010) Upregulation of plasma C9 protein in gastric cancer patients.Proteomics 10: 3210-3221.
- Chong PK, Lee H, Zhou J, Liu SC, Loh MC, et al. (2010) ITIH3 is a potential biomarker for early detection of gastric cancer.J Proteome Res 9: 3671-3679.
- Cohen M, Yossef R, Erez T, Kugel A, Welt M, et al. (2011) Serum apolipoproteins C-I and C-III are reduced in stomach cancer patients: results from MALDI-based peptidome and immuno-based clinical assays. PLoS One 6: e14540.
- Buckland G, Agudo A, Luján L, Jakszyn P, Bueno-de-Mesquita HB, et al. (2010) Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study.Am J Clin Nutr 91: 381-390.
- Coleman MP, Babb P, Daniecki P (199) Cancer survival trends in England and Wales, 1971-1995: deprivation and NHS region. Studies in medical and population subjects’ No. 61. London: The Stationary Office book.
- Conlin A, Smith G, Carey FA, Wolf CR, Steele RJ (2005) The prognostic significance of K-ras, p53, and APC mutations in colorectal carcinoma.Gut 54: 1283-1286.
- Conteduca V, Sansonno D, Lauletta G, Russi S, Ingravallo G, et al. (2013) H. pylori infection and gastric cancer: state of the art (review).Int J Oncol 42: 5-18.
- Correa P, Chen VW (1994) Gastric cancer.Cancer Surv 19-20: 55-76.
- Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, et al. (2000) Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy.J Natl Cancer Inst 92: 1881-1888.
- Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M (1975) A model for gastric cancer epidemiology.Lancet 2: 58-60.
- Correa P (1985) Clinical implications of recent developments in gastric cancer pathology and epidemiology.Semin Oncol 12: 2-10.
- Correa P (1992) Human gastric carcinogenesis: a multistep and multifactorial process-First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 52: 6735-6740.
- Cunningham SC, Kamangar F, Kim MP, Hammoud S, Haque R, et al. (2005) Survival after gastric adenocarcinoma resection: Eighteen-year experience at a single institution. J Gastrointest Surg 9:718-725.
- Decarli A, La Vecchia C, Cislaghi C, Mezzanotte G, Marubini E (1986) Descriptive epidemiology of gastric cancer in Italy.Cancer 58: 2560-2569.
- di Mario F, Cavallaro LG (2008) Non-invasive tests in gastric diseases.Dig Liver Dis 40: 523-530.
- Dinis-Ribeiro M, Yamaki G, Miki K, Costa-Pereira A, Matsukawa M, et al. (2004) Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening.J Med Screen 11: 141-147.
- Doménech M, Roman P, Lapetra J, García de la Corte FJ, Sala-Vila A, et al. (2014) Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: one-year randomized, clinical trial.Hypertension 64: 69-76.
- Duffy M, Lamerz R, Haglund C, Nicolini A, et al. (2014) Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer 134:2513-2522.
- Ebert MP, Lamer S, Meuer J, Malfertheiner P, Reymond M, et al. (2005) Identification of the thrombin light chain a as the single best mass for differentiation of gastric cancer patients from individuals with dyspepsia by proteome analysis. J Proteome Res 4: 586-590.
- Ebert MP, Niemeyer D, Deininger SO, Wex T, Knippig C,et al. (2006) Identification and confirmation of increased fibrinopeptide a serum protein levels in gastric cancer sera by magnet bead assisted MALDI-TOF mass spectrometry. J Proteome Res 5:2152-2158.
- Ebert MP, Röcken C (2006) Molecular screening of gastric cancer by proteome analysis.Eur J Gastroenterol Hepatol 18: 847-853.
- Ekström AM, Serafini M, Nyrén O, Hansson LE, Ye W, et al. (2000) Dietary antioxidant intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: a population-based case-control study in Sweden, Int. J. Cancer 87: 133-140.
- Fayçal J, Bessaguet C, Nousbaum JB, Cauvin JM, Cholet F, et al. (2005) Epidemiology and long term survival of gastric carcinoma in the French district of Finistere between 1984 and 1995.Gastroenterol Clin Biol 29: 23-32.
- Fenoglio-Preiser CM, Noffsinger AE, Belli J, Stemmermann GN (1996) Pathologic and phenotypic features of gastric cancer.Semin Oncol 23: 292-306.
- Fenoglio-Preiser CM, Wang J, Stemmermann GN, Noffsinger A (2003) TP53 and gastric carcinoma: a review.Hum Mutat 21: 258-270.
- Ferlay J, Bray F, Pisani P, Parkin DM (2001) Cancer incidence, mortality and prevalence worldwide, version 1.0. Lyon: IARC Press. IARC Cancer Base No. 5.
- Ferlay J, Bray F, Sankila R, Parkin DM (1999) Cancer incidence, mortality and prevalence in the European Union. Lyon: IARC Press.
- Fitó M, Estruch R, Salas-Salvadó J, Martínez-Gonzalez MA, Arós F, et al. (2014) Effect of the Mediterranean diet on heart failure biomarkers: a randomized sample from the PREDIMED trial.Eur J Heart Fail 16: 543-550.
- Fonseca L, Yonemura Y, De Aretxabala X, Yamaguchi A, Miwa K, et al. (1994) p53 detection as a prognostic factor in early gastric cancer.Oncology 51: 485-490.
- Gonzalez CA (2002) For the EPIC working group on gastric cancer. Vegetable, fruit and cereal consumption and gastric cancer risk. In: Riboli E, Lambert R, editors. Nutrition and lifestyle: opportunities. Lyon: IARC Press. IARC ScPubl No 156.
- Hakama M, Stenman UH, Knekt P, Järvisalo J, Leino A, et al. (1994) Tumour markers and screening for gastrointestinal cancer: a follow up study in Finland.J Med Screen 1: 60-64.
- Hao Y, Yu Y, Wang L, Yan M, Ji J, et al. (2008) IPO-38 is identified as a novel serum biomarker of gastric cancer based on clinical proteomics technology.J Proteome Res 7: 3668-3677.
- Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC (1997) The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case-control study in the U.S.Cancer 80: 1021-1028.
- Hirota T, Ming SC, Itabashhi M (1993) Pathology of early gastric cancer. In: Nishi M, Ichikawa H, Nakajima T, et al, editors. Gastric cancer. Tokyo: Springer Verlag 66-86.
- Hoshiyama Y, Kawaguchi T, Miura Y, Mizoue T, Tokui N, et al. (2002) A prospective study of stomach cancer death in relation to green tea consumption in Japan.Br J Cancer 87: 309-313.
- Hsing AW, Hansson LE, McLaughlin JK, Nyren O, Blot WJ, et al. (1993) Pernicious anemia and subsequent cancer. A population-based cohort study.Cancer 71: 745-750.
- Ichiyoshi Y, Oiwa H, Tomisaki S, Sakaguchi Y, Ohno S, et al. (1997) Overexpression of p53 is associated with growth pattern and prognosis in advanced gastric cancer.Hepatogastroenterology 44: 546-553.
- Jayavelu ND, Bar NS (2014) Metabolomic studies of human gastric cancer: review.World J Gastroenterol 20: 8092-8101.
- Ji BT, Chow WH, Yang G, McLaughlin JK, Gao RN, et al. (1996) The influence of cigarette smoking, alcohol, and green tea consumption on the risk of carcinoma of the cardia and distal stomach in Shanghai, China.Cancer 77: 2449-2457.
- Ji BT, Chow WH, Yang G, McLaughlin JK, Zheng W, et al. (1998) Dietary habits and stomach cancer in Shanghai, China.Int J Cancer 76: 659-664.
- Jonjic N, Kovac K, Krasevic M, Valkovic T, Ernjak N, et al. (1997) Epidermal growth factor-receptor expression correlates with tumor cell proliferation and prognosis in gastric cancer. Anticancer Res 17: 3883-3888.
- Kalnia Z, Meistere I, Kikuste I, Tolmanis I, Zayakin P, et al. (2015) Emerging blood-based biomarkers for detection of gastric cancer.World J Gastroenterol 21: 11636-11653.
- Kamangar F, Dores GM, Anderson WF (2006) Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. Journal Clin Oncol24:2137-2150.
- Kang JM, Kim N, Yoo JY, Park YS, Lee DH, et al. (2008) The role of serum pepsinogen and gastrin test for the detection of gastric cancer in Korea.Helicobacter 13: 146-156.
- Kelley JR, Duggan JM (2003) Gastric cancer epidemiology and risk factors.J Clin Epidemiol 56: 1-9.
- Kikuchi S, Kato M, Katsuyama T, Tominaga S, Asaka M (2006) Design and planned analyses of an ongoing randomized trial assessing the preventive effect of Helicobacter pylori eradication on occurrence of new gastric carcinomas after endoscopic resection. Helicobacter 11:147-151.
- Kikuyama S, Kubota T, Shimizu K, Miyakita M (1998) Ki-67 antigen expression in relation to clinicopathological variables and prognosis in gastric cancer.Oncol Rep 5: 867-870.
- Kim HJ, Lee SS, Choi BY, Kim MK (2007) Nitrate intake relative to antioxidant vitamin intake affects gastric cancer risk: a case-control study in Korea.Nutr Cancer 59: 185-191.
- Kimura H, Yonemura Y (1991) Flow cytometric analysis of nuclear DNA content in advanced gastric cancer and its relationship with prognosis.Cancer 67: 2588-2593.
- Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, et al. (1999) Accuracy of screening for gastric cancer using serum pepsinogen concentrations.Gut 44: 693-697.
- Kubicka S, Claas C, Staab S, Kuhnel F, Zender L, et al. (2002) p53 mutation pattern and expression of c-erbB2 and c-met in gastric cancer: relation to histological subtypes, Helicobacter pylori infection, and prognosis. Dig Dis Sci 47: 114-121.
- La Vecchia C, Franceschi S (2000) Nutrition and gastric cancer.Can J Gastroenterol 14: 51D-54D.
- La Vecchia C, Negri E, Franceschi S, Gentile A (1992) Family history and the risk of stomach and colorectal cancer.Cancer 70: 50-55.
- La Vecchia C (2009) Association between Mediterranean dietary patterns and cancer risk.Nutr Rev 67 Suppl 1: S126-129.
- Ladabaum U, Wang G, Terdiman J, Blanco A, Kuppermann M, et al. (2011) Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis.Ann Intern Med 155: 69-79.
- Larsson SC, Orsini N, Wolk A (2006) Processed meat consumption and stomach cancer risk: a meta-analysis.J Natl Cancer Inst 98: 1078-1087.
- Lazarevic K, Nagorni A, Bogdanovic D, Rancic N, Stosic L, et al. (2011) Dietary micronutrients and gastric cancer: hospital based study. Cent. Eur. J. Med 6: 783-787.
- Leja M, Kupcinskas L, Funka K, Sudraba A, Jonaitis L, et al. (2009) The validity of a biomarker method for indirect detection of gastric mucosal atrophy versus standard histopathology.Dig Dis Sci 54: 2377-2384.
- Leja M, You W, Camargo MC, Saito H (2014) Implementation of gastric cancer screening - the global experience.Best Pract Res Clin Gastroenterol 28: 1093-1106.
- Lenz HJ, Leichman CG, Danenberg KD, Danenberg PV, Groshen S, et al. (1996) Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumor response and overall survival.J Clin Oncol 14: 176-182.
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, et al. (2000) Environmental and heritable factors in the causation of cancer-analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343: 78-85.
- Lin LL, Huang HC, Juan HF (2012) Discovery of biomarkers for gastric cancer: a proteomics approach.J Proteomics 75: 3081-3097.
- Lissowska J, Groves FD, Sobin LH, Fraumeni JF Jr, Nasierowska-Guttmejer A, et al. (1999) Family history and risk of stomach cancer in Warsaw, Poland.Eur J Cancer Prev 8: 223-227.
- Lissowska J, Gail MH, Pee D, Groves FD, Sobin LH, et al. (2004) Diet and stomach cancer risk in Warsaw, Poland.Nutr Cancer 48: 149-159.
- Liu W, Liu B, Xin L, Zhang Y, Chen X, et al. (2007) Down-regulated expression of complement factor I: a potential suppressive protein for gastric cancer identified by serum proteome analysis. Clin Chim Acta 377:119-126.
- López-Carrillo L, López-Cervantes M, Ward MH, Bravo-Alvarado J, Ramírez-Espitia A (1999) Nutrient intake and gastric cancer in Mexico.Int J Cancer 83: 601-605.
- Ludwig JA, Weinstein JN (2005) Biomarkers in cancer staging, prognosis and treatment selection.Nat Rev Cancer 5: 845-856.
- Lunet N, Valbuena C, Carneiro F, Lopes C, Barros H (2006) Antioxidant vitamins and risk of gastric cancer: a case-control study in Portugal.Nutr Cancer 55: 71-77.
- Maeda K, Kang SM, Onoda N, Ogawa M, Sawada T, et al. (1998) Expression of p53 and vascular endothelial growth factor associated with tumor angiogenesis and prognosis in gastric cancer.Oncology 55: 594-599.
- Maeta M, Saito H, Katano K, Kondo A, Tsujitani S, et al. (1998) A progressive postoperative increase in the serum level of soluble receptors for interleukin-2 is an indicator of a poor prognosis in patients with gastric cancer. Int J Mol Med 1: 113-116.
- Malvezzi M, Bonifazi M, Bertuccio P, Levi F, La Vecchia C, et al. (2010) An age-period-cohort analysis of gastric cancer mortality from 1950 to 2007 in Europe.Ann Epidemiol 20: 898-905.
- Markowitz SD, Bertagnolli MM (2009) Molecular origins of cancer: Molecular basis of colorectal cancer.N Engl J Med 361: 2449-2460.
- Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, et al. (2001) Nutrient intake and risk of subtypes of esophageal and gastric cancer.Cancer Epidemiol Biomarkers Prev 10: 1055-1062.
- Metzger R, Leichman CG, Danenberg KD, Danenberg PV, Lenz HJ, et al. (1998)ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol 16: 309-316.
- Micheli A, Mugno E, Krogh V, Quinn MJ, Coleman M, et al. (2002) Cancer prevalence in European registry areas.Ann Oncol 13: 840-865.
- Mitrou PN, Kipnis V, Thiebaut AC, Reedy J, Subar AF, et al. (2007) Mediterranean dietary pattern and prediction of allcause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med 167:2461-2468.
- Mizuno S, Kobayashi M, Tomita S, Miki I, Masuda A, et al. (2009) Validation of the pepsinogen test method for gastric cancer screening using a follow-up study.Gastric Cancer 12: 158-163.
- Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, et al. (2012) Identification of Lynch syndrome among patients with colorectal cancer.JAMA 308: 1555-1565.
- Moyers SB, Kumar NB (2004) Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials.Nutr Rev 62: 204-211.
- Mukhtar H, Ahmad N (2000) Tea polyphenols: prevention of cancer and optimizing health.Am J Clin Nutr 71: 1698S-702S.
- Nagano J, Kono S, Preston DL, Mabuchi K (2001) A prospective study of green tea consumption and cancer incidence, Hiroshima and Nagasaki (Japan).Cancer Causes Control 12: 501-508.
- Nakane Y, Okamura S, Akehira K, Boku T, Okusa T, et al. (1994) Correlation of preoperative carcinoembryonic antigen levels and prognosis of gastric cancer patients.Cancer 73: 2703-2708.
- Nakata B, Chung KH, Ogawa M, Ogawa Y, Yanagawa K, et al. (1998) p53 protein overexpression as a predictor of the response to chemotherapy in gastric cancer.Surg Today 28: 595-598.
- Nardone G, Martin G, Rocco A, Rippa E, La Monica G, Caruso F, Arcari P, et al. (2008) Molecular expression of gastrokine 1 in normal mucosa and in Helicobacter pylori-related preneoplastic and neoplastic lesions. Cancer Biol Ther 7:1890-1895.
- Nardone G, Rippa E, Martin G, Rocco A, Siciliano RA, et al. (2007) Gastrokine 1 expression in patients with and without Helicobacter pylori infection.Dig Liver Dis 39: 122-129.
- Nekarda H, Schmitt M, Ulm K, Wenninger A, Vogelsang H, et al. Prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in completely resected gastric cancer. Cancer Res 54: 2900-2907.
- Nomura AM, Hankin JH, Kolonel LN, Wilkens LR, Goodman MT, et al. (2003) Case-control study of diet and other risk factors for gastric cancer in Hawaii (United States).Cancer Causes Control 14: 547-558.
- Nouraie M, Pietinen P, Kamangar F, Dawsey SM, Abnet CC, et al. (2005) Fruits, vegetables, and antioxidants and risk of gastric cancer among male smokers.Cancer Epidemiol Biomarkers Prev 14: 2087-2092.
- Okusa Y, Ichikura T, Mochizuki H (1999) Prognostic impact of stromal cell-derived urokinase-type plasminogen activator in gastric carcinoma.Cancer 85: 1033-1038.
- Palli D, Galli M, Caporaso NE, Cipriani F, Decarli A, et al. (1994) Family history and risk of stomach cancer in Italy.Cancer Epidemiol Biomarkers Prev 3: 15-18.
- Park JY, von Karsa L, Herrero R (2014) Prevention strategies for gastric cancer: a global perspective.Clin Endosc 47: 478-489.
- Parkin DM, Bray FI, Devesa SS (2001) Cancer burden in the year 2000. The global picture. Eur J Cancer 37: S4-66.
- Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB (2002) Cancer incidence in five continents, Vol VIII. Lyon, France, IARC Scientific Publications No. 155.
- Parkin DM (2004) International variation.Oncogene 23: 6329-6340.
- Pavone LM, Del Vecchio P, Mallardo P, Altieri F, De Pasquale V, et al. (2013) Structural characterization and biological properties of human gastrokine 1.Mol Biosyst 9: 412-421.
- Pectasides D, Mylonakis A, Kostopoulou M, Papadopoulou M, Triantafillis D, et al. (1997) CEA, CA 19-9, and CA-50 in monitoring gastric carcinoma.Am J Clin Oncol 20: 348-353.
- Pelucchi C, Tramacere I, Bertuccio P, Tavani A, Negri E, et al. (2009) Dietary intake of selected micronutrients and gastric cancer risk: an Italian case-control study.Ann Oncol 20: 160-165.
- Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, et al. (2001) Phases of biomarker development for early detection of cancer.J Natl Cancer Inst 93: 1054-1061.
- Pooladi M, Rezaei-Tavirani M, Hashemi M, Hesami-Tackallou S, Khaghani-Razi-Abad S, et al. (2014) The study of “dihydropyrimidinase related proteins (DRPs)” expression changes influence in malignant astrocytoma brain tumor. Iran J Cancer Prev 7:130-133.
- Posner MR, Mayer RJ (1994) The use of serologic tumor markers in gastrointestinal malignancies.Hematol Oncol Clin North Am 8: 533-553.
- Qiu JL, Chen K, Zheng JN, Wang JY, Zhang LJ, et al. (2005) Nutritional factors and gastric cancer in Zhoushan Islands, China.World J Gastroenterol 11: 4311-4316.
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, et al. (2002) SEER Cancer Statistics Review, 1975-2002, National Cancer Institute. Bethesda, MD
- Roukos DH, Kappas AM (2005) Perspectives in the treatment of gastric cancer.Nat Clin Pract Oncol 2: 98-107.
- Rugge M, Sonego F, Panozzo M, Baffa R, Rubio J Jr, et al. (1994) Pathology and ploidy in the prognosis of gastric cancer with no extranodal metastasis.Cancer 73: 1127-1133.
- Saito H, Tsujitani S, Ikeguchi M, Maeta M, Kaibara N (1999) Serum level of a soluble receptor for interleukin-2 as a prognostic factor in patients with gastric cancer.Oncology 56: 253-258.
- Sasazuki S, Sasaki S, Tsubono Y, Okubo S, Hayashi M, et al. (2003) The effect of 5-year vitamin C supplementation on serum pepsinogen level and Helicobacter pylori infection.Cancer Sci 94: 378-382.
- Schatzkin A, Park Y, Leitzmann MF, Hollenbeck AR, Cross AJ (2008) Prospective study of dietary fiber, whole grain foods, and small intestinal cancer.Gastroenterology 135: 1163-1167.
- Sendler A, Gilbertz KP, Becker I, Mueller J, Berger U, et al. (2001) Proliferation kinetics and prognosis in gastric cancer after resection.Eur J Cancer 37: 1635-1641.
- Setiawan VW, Zhang ZF, Yu GP, Lu QY, Li YL, et al. (2001) Protective effect of green tea on the risks of chronic gastritis and stomach cancer.Int J Cancer 92: 600-604.
- Slavin J (2004) Whole grains and human health.Nutr Res Rev 17: 99-110.
- Sofi F, Abbate R, Gensini GF, Casini A (2010) Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis.Am J Clin Nutr 92: 1189-1196.
- Sofi F, Cesari F, Abbate R, Gensini GF, Casini A (2008) Adherence to Mediterranean diet and health status: meta-analysis.BMJ 337: a1344.
- Stemmermann GN (1994) Intestinal metaplasia of the stomach. A status report.Cancer 74: 556-564.
- Stewart BW, Kleihus P (2003) World Cancer Report. Lyon: IARC Press.
- Su Y, Shen J, Qian H, Ma H, Ji J, et al. (2007) Diagnosis of gastric cancer using decision tree classification of mass spectral data.Cancer Sci 98: 37-43.
- Tahara E, Semba S, Tahara H (1996) Molecular biological observations in gastric cancer.Semin Oncol 23: 307-315.
- Takano Y, Kato Y, van Diest PJ, Masuda M, Mitomi H, et al. (2000) Cyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer cases.Am J Pathol 156: 585-594.
- Takezaki T, Gao CM, Wu JZ, Ding JH, Liu YT, et al. (2001) Dietary protective and risk factors for esophageal and stomach cancers in a low-epidemic area for stomach cancer in Jiangsu Province, China: comparison with those in a high-epidemic area.Jpn J Cancer Res 92: 1157-1165.
- Tresserra-Rimbau A, Rimm EB, Medina-Remon A (2014) Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr Metab Cardiovasc Dis 24:639-647.
- Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, et al. (1995) Diet and overall survival in elderly people.BMJ 311: 1457-1460.
- Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D (2000) Cancer and Mediterranean dietary traditions.Cancer Epidemiol Biomarkers Prev 9: 869-873.
- Tsubono Y, Hisamichi S (2000) Screening for gas- tric cancer in Japan. Gastric Cancer 3:9-18.
- Tsubono Y, Nishino Y, Komatsu S, Hsieh CC, Kanemura S, et al. (2001) Green tea and the risk of gastric cancer in Japan.N Engl J Med 344: 632-636.
- Tsukuma H, Mishima T, Oshima A (1983) Prospective study of "early" gastric cancer.Int J Cancer 31: 421-426.
- Turati F, Edefonti V, Bosetti C, Ferraroni M, Malvezzi M, et al. (2013) Family history of cancer and the risk of cancer: a network of case-control studies.Ann Oncol 24: 2651-2656.
- Umemura H, Togawa A, Sogawa K, Satoh M, Mogushi K, et al. (2011) Identification of a high molecular weight kininogen fragment as a marker for early gastric cancer by serum proteome analysis.J Gastroenterol 46: 577-585.
- Urpi-Sarda M, Casas R, Chiva-Blanch G (2012) The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J Nutr 142:1019-1025.
- Van den Brandt PA, Botterweck AA, Goldbohm RA (2003) Salt intake, cured meat consumption, refrigerator use and stomach cancer incidence: a prospective cohort study (Netherlands). Cancer Causes Control 14:427-438.
- Varis K, Taylor PR, Sipponen P, Samloff IM, Heinonen OP, et al. (1998) Gastric cancer and premalignant lesions in atrophic gastritis: a controlled trial on the effect of supplementation with alpha-tocopherol and beta-carotene. The Helsinki Gastritis Study Group. Scand J Gastroenterol 33: 294-300.
- Verberne L, Bach-Faig A, Buckland G, Serra-Majem L (2010) Association between the Mediterranean diet and cancer risk: a review of observational studies.Nutr Cancer 62: 860-870.
- Victorzon M, Nordling S, Haglund C, Lundin J, Roberts PJ (1996) Expression of p53 protein as a prognostic factor in patients with gastric cancer.Eur J Cancer 32A: 215-220.
- Wang D, DuBois RN (2013) Urinary PGE-M: a promising cancer biomarker.Cancer Prev Res (Phila) 6: 507-510.
- Wang ZY, Hong JY, Huang MT, Reuhl KR, Conney AH, et al. (1992) Inhibition of N-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mice by green tea and black tea.Cancer Res 52: 1943-1947.
- (1997) WCRF-AICR World Cancer Research Fund and American Institute for Cancer Research, Food, Nutrition and Prevention of Cancer: a Global Perspective. American Institute of Cancer Research, Washington.
- Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, et al. (1995) Mediterranean diet pyramid: a cultural model for healthy eating.Am J Clin Nutr 61: 1402S-1406S.
- Willett WC (2006) The Mediterranean diet: science and practice.Public Health Nutr 9: 105-110.
- Wu MS, Lee CW, Sheu JC, Shun CT, Wang HP, et al. (2002) Alterations of BAT-26 identify a subset of gastric cancer with distinct clinicopathologic features and better postoperative prognosis. Hepatogastroenterology 49: 285-289.
- Xia HH, Yang Y, Chu KM, Gu Q, Zhang YY, et al. (2009) Serum macrophage migration-inhibitory factor as a diagnostic and prognostic biomarker for gastric cancer.Cancer 115: 5441-5449.
- Xiangming C, Hokita S, Natsugoe S, Tanabe G, Baba M, et al. (2000) p21 expression is a prognostic factor in patients with p53-negative gastric cancer.Cancer Lett 148: 181-188.
- Xu Y, Ho CT, Amin SG, Han C, Chung FL (1992) Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants.Cancer Res 52: 3875-3879.
- Dong X, Wang G, Zhang G, Ni Z, Suo J, et al. (2013) The endothelial lipase protein is promising urinary biomarker for diagnosis of gastric cancer.Diagn Pathol 8: 45.
- Yamada Y, Yoshida T, Hayashi K, Sekiya T, Yokota J, et al. (1991) p53 gene mutations in gastric cancer metastases and in gastric cancer cell lines derived from metastases.Cancer Res 51: 5800-5805.
- Yanaoka K, Oka M, Mukoubayashi C, Yoshimura N, Enomoto S, et al. (2008) Cancer highrisk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol Biomarkers Prev 17: 838-845.
- Yang CS, Maliakal P, Meng X (2002) Inhibition of carcinogenesis by tea.Annu Rev Pharmacol Toxicol 42: 25-54.
- Yeh KH, Shun CT, Chen CL, Lin JT, Lee WJ, et al. (1998) High expression of thymidylate synthase is associated with the drug resistance of gastric carcinoma to high dose 5-fluorouracil-based systemic chemotherapy.Cancer 82: 1626-1631.
- Yonemura Y, Fonseca L, Tsugawa K, Ninomiya I, Matsumoto H, et al. (1994) Prediction of lymph node metastasis and prognosis from the assay of the expression of proliferating cell nuclear antigen and DNA ploidy in gastric cancer. Oncology 51: 251-257.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 13874
- [From(publication date):
April-2016 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 12938
- PDF downloads : 936