An Overview of the Application of Hydrodinamic Cavitation for the Intensification of Wastewater Treatment Applications: A Review
Received: 24-May-2016 / Accepted Date: 03-Jun-2016 / Published Date: 10-Jun-2016
Abstract
Cavitation is a specific phenomenon in a liquid if any changes occur in the pressure field over time and distance. The effects of cavitation have become very useful in supporting chemical processes in the environmental protection technologies, especially in technologies related to the decomposition of substances particularly harmful to humans and his immediate surroundings. Cavitation can be realized by alterations in the flow and pressure (hydrodynamic cavitation). In hydrodynamic cavitation, flow geometry is changed in such a way that the kinetic energy increases at the expense of local liquid pressure, which can make cavities depending on the vapor pressure of the liquid. Traditional treatment methods do not always produce the expected results. There exists a certain group of organic non-biodegradable pollutants, having toxic, mutagenic, or carcinogenic properties. In the current literature there are many studies related to hydrodinamic cavitation and the use of associated processes in water and effluent treatment technologies.
Keywords: Hydrodinamic cavitation; Reactors; Wastewater treatment; Pollutant; Process intensification
18763Introduction
Wastewater discharge from industrial units containing newer and refractory chemicals is a significant problem for conventional treatment plants. The release of this toxic wastewater into the natural environment is not only hazardous to aquatic life but also creates significant environmental concerns. Conventional wastewater treatment methods like adsorption on activated carbon, extraction, and chemical oxidation have limitations such as limited applicability and lower efficiency [1,2].
Hydrodynamic cavitation seems to be a new, innovative technology for the decomposition of complex compounds and an alternative to ultrasound-induced cavitation [2,3]. A technology which utilises the cavitating liquid environment can be considered as a non-waste technology and environmentally friendly due to the possibility of degradation of low biodegradable, hazardous and carcinogenic organic compounds, which are resistant to conventional disposal methods [4].
Examples include pesticides, dyes, or high molecular organic compounds, which in the cavitating liquid environment become susceptible to biodegradation [5]. The examples which have been presented regarding hydrodynamic cavitation in environmental engineering suggest that this method is effective and less burdensome for the environment.
In hydrodynamic processes, cavitation occurs in a flowing liquid during a fall in the static pressure, caused by flow conditions or external influences [6]. It is commonly produced in constricted or curved channels and also as a result of motion of bodies in a liquid such as a ship’s propeller. Thus, this type of cavitation appears as a result of a local constriction to the flow path of the liquid or the detachment of the stream from the surface of streamlined bodies. Hydrodynamic cavitation can be innovated and prefers an energy efficient way of generating cavitation [7].
This work aims at highlighting essential aspects related to hydrodynamic cavitation, including the theoretical aspects for configuration and overview of applications relevant to wastewater processing based on the evaluation of the current literature.
Literature Review
Hydrodinamic cavitation process
The cavitation process is very complicated and many attempts have been made to theoretically explain the mechanism of its creation. The basic problem is the issue of the non-linear forced oscillation response of the bubbles, which they are subjected to during the cavitation process [7,8]
Cavitation forms and develops in a flowing liquid through zones, in which the pressure of the liquid falls below a critical value, normally close to the saturated vapour pressure at a given temperature for the liquid. The value for this pressure is dependent not only on the type of liquid, but also on the amount of pollutants such as micro-particles or macro-particles, and micro-bubbles containing incompletely dissolved gases [4]
Cavitation is a dynamic process, dependent on continuous changes over time to the volume and geometry of the bubbles and cavities. The timescale for this is in the order of milliseconds. After moving through the cavitating liquid into regions exceeding the critical pressure, the bubbles and cavities undergo sudden implosions in time periods significantly smaller than milliseconds, thus creating a local rise in pressure in different zones of the region. Locally, the pressures in the liquid can reach values of hundreds, and even thousands of megapascals. Characteristic effects which accompany the cavitation bubble implosions are hydrodynamic, mechanical, acoustic, chemical, thermal and even electrostatic [9]. If the cavitation occurs by pressure variation in the flowing liquid due to the presence of throttling devices such as venturi, orifice etc., it is called as hydrodynamic cavitation. Bernoulli’s Principle describes the hydrodynamic cavitation [10].
Pstatic+Pdynamic=Constant=Pstatic+½ ρν2
where Pstatic and Pdynamic are static and dynamic pressures, ρ and ν are the density and velocity of the liquid at the throat of the cavitating device. When the liquid passes through a cavitating device, the fluid velocity increases due to a decrease in the flow area and hence increases the dynamic pressure [11]. Since, the sum of dynamic and static pressure is constant according to the Bernoulli’s equation; increase in dynamic pressure subsequently reduces the static pressure. If the static pressure falls below the cavitation threshold of the solvent so many cavity formations are observed [12]. Recovery of static pressure in the downstream section of the cavitating device causes violent collapse of these cavities resulting into the formation of localized hot spots and highly reactive OH• and H• radicals [13].
Factors affecting the formation of cavitation
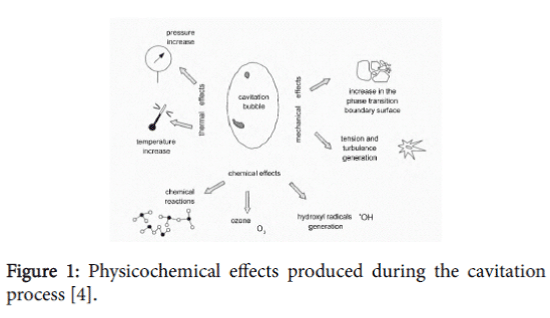
Cavitation is caused by a number of factors which encompass not only the physical properties of the liquid, described by the appropriate physical properties [14,15], the thermal state of the liquid, but also the gaseous impurities dissolved in the liquid or other liquid impurities [16] and also impurities in the form of submerged bodies. Cavitation drives many important physicochemical effects, which can be utilized to degrade and/or oxidize pollutants found in water and sewage. Cavitation bubbles produced by pressure pulsations fulfill the function of “micro reactors” that reach extreme temperatures and pressures and produce powerful oxidants like hydroxyl radicals and excellent initiators of chain reactions in a short time [17]. The best-known indication of cavitation is cavitation noise produced over a frequency range from 100 Hz to 100 kHz, the result of bubble implosions. Cavitation favours energy dissipation leading to a temperature rise in the vicinity of the collapsing bubbles and cavities. The implosions also produce a series of other physicochemical and mechanical effects (Figure 1).
Figure 1: Physicochemical effects produced during the cavitation process [4].
The formation of the cavitation bubbles and their implosions is characterised by very high energy densities in the order of 1018 kW/ml. The formation and disappearance of cavities can occur over millions of locations in the reactor producing local conditions of high temperatures and pressures in close proximity to where physicochemical processes are taking place [4].
Hydrodynamic cavitation reactor
The intensity of the technological processes in hydrodynamic cavitation devices is associated with a range of physicochemical and mechanical effects (shock waves, cumulative microstreams, self-excited oscillations, turbulence), caused by the implosion of cavitation bubbles. In turn, this leads to a concentration of bubbles and an increase in their energies, located near the centre of the cloud. Under such conditions, during an implosion, the pressure rises to almost an order of magnitude greater than during the implosion of a single bubble [14,18]. Intensive shock waves in the system lead to a pressure increase at the centre of the bubble. If a significant increase in the surface area of the phase transition boundary repeats, the chemical composition of the system changes [19]. These effects, due to the large concentration of cavitation bubbles, lead to favourable conditions for the initiation of physicochemical processes, which under normal conditions are complex or difficult to conduct.
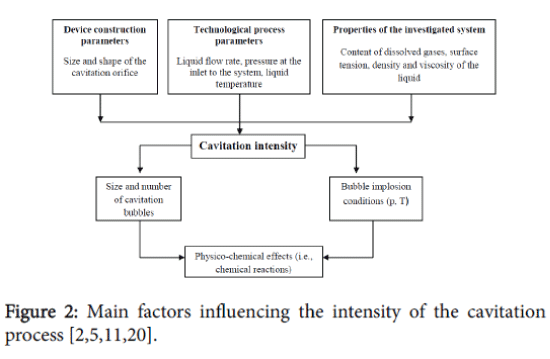
There are three main factors that specify the formation of the hydrodynamic cavitation field and the effectiveness of the cavitation process (Figure 2).
The first group consists of parameters such as the size and shape of the cavitation inducer and the flow chamber which determine the structural characteristics of the reactor. The second group consists of parameters which characterize the liquid medium in general: viscosity, density, surface tension and the dissolved gas contents. The third group includes the technological process parameters; the “processing” time (the number of times which the medium passes through the cavitation region) and the interdependence between temperature and pressure of the process. [11].
The technological effectiveness of the cavitation process depends on the cumulative effect of the above mentioned parameters. The range of the cavitation process parameters particularly the number of cavitation bubbles and their implosion conditions (i.e. pressure and temperature) is quite extensive [11]. The magnitudes of the pressures and temperatures during bubble collapse, as well as the number of free radicals at the end of cavitation, are strongly dependent on the operating conditions and configuration of the hydrodynamic cavitation reactors [12].
The most important parameters which affect the cavitation process intensity are shown in Table 1.
| Item | Property | Favorable conditions |
|---|---|---|
| 1 | Inlet pressure/Rotor speed of the equipment | -Use increased pressures/ rotor speed -Operate below an optimum value to avoid super-cavitation |
| 2 | Diameter of the constriction e.g., hole diameter on the orifice plate [11,21] |
-Carry out an optimisation for the application -Select higher diameters for applications which require intense cavitation -Select lower diameters with a large number of holes for applications with reduced intensity |
| 3 | Percentage of the free area for the flowi.e., the cross-sectional area of holes on the orifice to the total cross-sectional area of the pipe [11,21-24] | -Use smaller free areas to produce high intensive cavitation [11,21-24]. |
Table 1: Optimum operating conditions of hydrodynamic cavitation reactors [11,16].
Making suggestions to select a specific type of reactor for special purpose applications is important. Moholkar and Pandit [25] conducted a study modelling the effect of selected parameters on the cavitation intensity in liquid flow for two different flow geometries: venturi constriction and a multiperforated orifice plate.
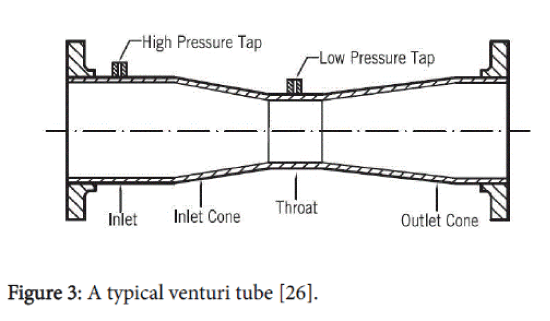
A linear pressure recovery gradient in a venturi generates a large fraction of the stabilised oscillatory radial bubble motion (Figure 3) [11,22,25].
Figure 3: A typical venturi tube [26].
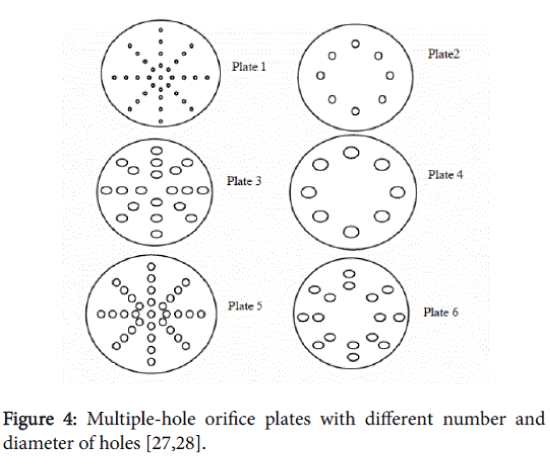
But in orifices the flow occurs both with stabilised oscillatory radial bubble motion and transient cavity behaviour, due to an additional oscillating pressure gradient caused by turbulent velocity fluctuations (Figure 4).
In addition, the magnitude of the permanent drop in pressure across the orifice is much higher compared with that across a venturi, resulting in a larger fraction of the energy being available for cavitation. Because of a higher contribution of the transient cavitation the cavitation intensity of an orifice system will be higher compared to a classical venturi [11]. Bubble dynamics simulations, using various operating/design parameters for the hydrodynamic cavitation reactor, have enabled definite trends to be established for the generated cavitation intensity.
Ozonek [4] and Gogate et al [29] investigated that the following considerable strategies have been defined for the design of hydrodynamic cavitation reactors:
• An orifice flow configuration is more suitable for applications requiring intensive cavitation conditions. For milder processes which require collapsing pressure pulses between 15-20 bar and for transformations based on physical effects a venturi configuration is more convenient and energy efficient [12].
• 2. Reducing the length of a venturi is the most economical technique for increasing cavitation intensity. But for higher flow rates there can be a limitation because of flow instability and super-cavitation possibilities. A similar data can be used when reducing the venturi constriction to pipe diameter ratio [2,12].
• 3. For an orifice flow configuration the most convenient way to control the cavitation intensity is by controlling the orifice to pipe diameter ratio (basically throttling the pump discharge through a valve), or the cross-sectional flow area by varying the number and the diameter of the perforations on the orifice plate. However, random growth of bubbles downstream from the orifice may cause splashing and vaporisation (supercavitation) [2,12,25].
• 4. An another option to have more intensive cavitation effect is to increase the pipe size downstream from the orifice. However, using pipes with a larger size requires higher volumetric flow rates to carry out the process using the same cavitation number, resulting in higher processing costs [12].
The degradation of persistent organic pollutants using hydrodynamic cavitation
For a long time now there has been a growing interest in a specific group of environmental pollutants, namely POPs (Persistent Organic Pollutants) [4]. These compounds, released into the environment, mainly from anthropogenic sources are characterised by their high toxicity, persistence and ability to bio-accumulate [19]. This group of organic pollutants include, amongst others: polycyclic aromatic hydrocarbons (PAHs), chlorophenols, polychlorinated biphenyls (PCBs), dioxins PCDD (Polychlorinated Dibenzodioxins) and PCDF (Polychlorinated Dibenzofurans), and some pesticides. These compounds, depending on the part of a given ecosystem in which they occur (soil, benthal sludge deposits, surface water and groundwater) may undergo slow changes due to various physical, chemical, biological or even photochemical processes [30].
Depending on the compound and medium in which they are found, as well as the environmental factors specific to the medium, decomposition processes occur at different rates, and the newly created compounds can create a burden on the environment to a greater or lesser extent. The two key mechanisms responsible for the degradation of organic pollutants using hydrodynamic cavitation are, the thermal decomposition/pyrolysis of organic pollutant entrapped in the cavities due to the generation of transient temperature pressure conditions (localized hot spots) and secondly, the reaction of free radicals with the organic pollutant occurring at the cavity–water interface [31].
Ozonek et al. [32] conducted studies into defining conditions for, and the possible removal of polycyclic aromatic hydrocarbons from sewage using hydrodynamic cavitation [33].
Raut-Jadhav et al. [10] investigated the degradation of methomyl in a hydrodynamic cavitation reactor (HC). They combined intensifying agents such as H2O2, fenton reagent and ozone (hybrid processes). In this research, firstly pH and inlet pressure were optimized to the cavitating device (circular venturi) to maximize the efficancy of hydrodynamic cavitation. At an optimum pH of 2.5 and optimum inlet pressure of 5 bar, hybrid processes have been applied to provide further degradation of methomyl. In all hybrid processes a significant synergistic effect was observed. After the combination of hydrodynamic cavitation with H2O2, fenton process and ozone, the synergetic coefficients were obtained as 5.8, 13.41 and 47.6 respectively. In terms of mineralization extent and energy efficiency, individual and hybrid processes’ efficacy has also been obtained. The most effective process was HC + Ozone process with highest synergetic coefficient, energy efficiency and mineralization extent. The study proved that for complete degradation of methomyl hydrodynamic cavitation can be applied effectively in the presence of intensifying agents.
Experimental studies
It is an acceptable method to use hydrodynamic cavitation to increase the biodegradability of organic compounds in polluted water and effluent [4]. The generation of hydroxyl radicals during this process, with the involvement of oxygen and/or air, can lead to the degradation of organic matter within the sewage and organic compounds in the polluted waters. In general, cavitation is one of the elements of an integrated treatment system, consisting of physical, chemical and biological processes. By decreasing the amount of persistent organic pollutants in wastewater treatment plant effluent, we will demonstrate the improved efficiency of treatment.
Wang et al. [34] investigated the combination of H2O2 and jetinduced hydrodynamic cavitation to decompose aqueous solution of rhodamine B. An obvious synergetic effect between hydrodynamic cavitation and hydrogen peroxide has been reported. The relative amounts of •OH radicals produced were detected by using TA as a fluorescent probe, and according to the results the production of •OH radicals in hydrodynamic cavitation can be enhanced by H2O2 addition [3]. This result suggests that the synergetic effect between hydrodynamic cavitation and H2O2 for the degradation of rhodamine B can basically be because of the contribution of additional •OH radicals production. It has been also established that increased loading of H2O2, lower medium pH, higher fluid pressures and lower initial dye concentration are more favourable for the degradation of rhodamine B. For temperature, increasing the temperature from 30°C-50°C has advantage to degradation of rhodamine B, but at 60°C the degradation rate is lower. The degradation kinetics of rhodamine B were established and reported to follow a pseudo-first-order kinetics.
Jyoti and Pandit [35] have examined the viability of ozonation and cavitation for the disinfection of the heterotrophic plate count (HPC) bacteria and indicator microorganism in well water. It has been reported that when water is treated with 0.5 mg/l of ozone, 46% disinfection (in case of HPC bacteria) is achieved in the first 15 min of treatment, which in turn increases to 82% at the end of 60 min. When 2 mg/l ozone is used in combination with hydrodynamic cavitation, the colony forming units (CFU) count reduces by 66% (5.17 bar) in the first 15 min of treatment as against 60% with ozone alone over 60 min which clearly indicates the significant effect of the combination of hydrodynamic cavitation and ozone.
Nitrophenol is a toxic compound, which on entering into the human body, even in small quantities, causes damage to the liver, kidney or the central nervous system. It appears in the effluent from the production of herbicides, insecticides, and synthetic dyes [36,37]. Its high stability and significant solubility in water are the causes of many difficulties in its decomposition during effluent treatment. The application of cavitation reactors, for this purpose, where oxidation processes are enhanced in the presence of hydroxyl radicals, is a promising technique for the degradation of nitrophenol and other phenols.
Pradhan and Gogate [38] have investigated the removal of pnitrophenol with hydrodynamic cavitation, either individually or in combination with H2O2 and conventional Fenton process. An orifice plate and a venturi have been used and the effects of operating parameters such as initial concentration (5 g/l and 10 g/l), inlet pressure (5.7–42.6 psi) and pH (over a range 2–8) on the extent of removal has been investigated. Efficancy of removal using the combined approach was found to be strongly dependent on the operating pH and pH of 3.75 was found to be optimum. Under the optimized operating parameters, the degradation obtained using only HC was 53.4%. The obtained results indicated that the extent of removal was marginally higher for the case of venturi (53.4% removal) as compared to that obtained with orifice (51%) for 5 g/l initial pnitrophenol concentration. For the combination of HC and H2O2, extent of removal increased to 59.9% with 0.5% H2O2. In the case of combination of HC with Fenton chemistry, for 5 g/l initial p-nitrophenol concentration, the maximum removal was 63.2% whereas for 10 g/l initial concentration, the extent of degradation was 56.2%.
Bagal and Gogate [39] investigated the degradation of 2,4- dinitrophenol by using chemical and advanced oxidation processes in a combination of hydrodynamic cavitation. Under optimized operating parameters for hydrodynamic cavitation alone, it has been reported that only 12.4% degradation of 2,4-dinitrophenol takes place in 120 min(s) of reaction time. Significant intensification can be achieved when HC is combined with advanced oxidation processes such as conventional Fenton (HC/FeSO4/H2O2), advanced Fenton (HC/Fe/H2O2) and Fenton-like process (HC/CuO/ H2O2) [39]. It was reported that 100%, 54.1% and 29.80% degradation of 2,4- dinitrophenol was obtained using combination of hydrodynamic cavitation with Fenton, advanced Fenton and Fenton-like processes respectively.
Gogate and Patil [40] have observed that combination of hydrodynamic cavitation with Fenton’s reagent improves the decomposition of triazophos giving 83.12% degradation. Optimum pressure of 5 bar and pH of 3 was found to be optimum for maximum degradation of triazophos using hydrodynamic cavitation alone. Under the optimized operating conditions, hydrodynamic cavitation alone could not give complete degradation of triazophos.
Due to its capability to generate highly reactive free radicals and turbulence the hydrodynamic cavitation is used effectively in water disinfection [5,41]. In disinfection of microorganisms by cavitation the following effects are foreseen [42]. Chemical and thermal effects play supporting role to mechanical effects in microbial disinfection with hydrodynamic cavitation [5,22,41].
Litwinienko et al.[18] confirmed that hydrodynamic cavitation can also exhibit bactericidal activity during water treatment. The use of cavitation for the biological disinfection of water, polluted with 106-1010 colonies of microorganisms per 1 dm3 eliminates them with 97-99% effectiveness. Hydrodynamic cavitation also has a destructive effect on yeast, bacteria and even viruses. The neutralisation of microorganisms is caused both by the shockwaves, during cavitation bubble implosions, causing a tearing of the cell membrane, as well as by the production of hydrogen peroxide [43].
Cavitation is used for sludge pre-treatment in wastewater treatment plants. It improves and accelerates the anaerobic digestion, which means higher biogas production, mass reduction, pathogen reduction and odour removal [44,45].
Sludge digestion is the process of destroying the sludge structures (rupturing cell membranes included), accelerating cell hydrolysis by releasing the cells’ contents into the water present in the sludge, and allowing the initiation and increasing the level of biological decomposition [46]. The application of a sludge digester facilitates the hydrolysis stage producing methane (which is the essence of this process), and impacts on minimising the quantity of sludge remaining for final disposal. Sludge digestion can be used to enhance the effectiveness of the biological decomposition processes for sludge (including accelerating methane production, increasing gas production, increasing the digestion level, shortening the digestion time) by increasing the cells’ hydrolysis rate and increasing the decomposition of low biodegradable substances [47].
Another advantage derived from sludge digestion is the possibility of utilising substances released from cells, as a source of easily absorbable organic carbon in the denitrification process, when it is deficient in the incoming effluent. Favourable results for sludge digestion were obtained using cavitation nozzles [48]. A high technological effectiveness allows to intensify biogas production by using hydrodynamic cavitation. The studies used recycled activated sludge from the sewage treatment works, using advanced biological processes in the treatment of effluent, dependent on the simultaneous removal of organic compounds as well as nitrogen and phosphorus compounds.
The application of hydrodynamic cavitation in sewage treatment may be considered in two aspects:
• The breakdown of various pollutants as a result of the specific conditions inherent in the cavitating liquid.
• The combined interaction of the mechanical effects of hydrodynamic cavitation and oxidation, through the joint action of cavitation with the oxidants [4].
During the digestion (destruction) of the activated sludge cells, under cavitation conditions, into the solution of the surrounding liquid, the accumulated organic compounds and enzymes inside the cells are released, which impact the COD value (by raising it) and the products of hydrolytic decomposition. Comparing the digestion results for activated sludge it can be seen that the amount of biogas produced rose by 20% when hydrodynamic digestion was used. The study results confirmed that digestion strongly affects the amount of biogas produced, resulting in a lower dry solid content, and consequently less sludge for final disposal. The positive effect of digestion is a greater susceptibility of the sludge to drain [48].
Economic Assessment Of Hydrodynamic Cavitation Compared With Traditional Method
A successful and economical design for a cavitation reactor requires an effective conversion of mechanical, electrical, or optical energy into the energy required to break chemical bonds. The first major step is the conversion of mechanical, electrical, or optical energy into the energy required for the formation of cavities. For hydrodynamic cavitation, this is the local pressure reduction sufficient to form cavities. In a hydrodynamic cavitation reactor, the pressure loss through expansion is the major source of energy loss. The associated pumping cost is a major issue for the economics of the process. There is also energy loss associated with the formation and implosion of the cavities.
In hydrodynamic cavitation, there is a substantial energy loss in the fluid pumping process. The loss depends on the flow rate as well as the pressure level. Different pressures and different flow rates will require different types of pumps. The energy efficiency associated with cavity implosion in hydrodynamic cavitation will depend on the level of turbulence in the flow. For example, orifices with small diameters will cause larger pressure drops but also larger turbulence downstream, which will allow a larger amount of energy to be released during the cavity implosion [4].
General economic method dealing with organic compound degradation is the biochemical method. However, some components such as polyethylene terephthalate (PET), polyvinyl alcohol (PVA) and some dispersed dyes are bio-refractory or toxic. Such kind of industrial water has a low BOD/COD value and an unsuitable pH value for biochemical degradation. A simple method to evaluate the economics of hydrodynamic cavitation in wastewater treatment is to calculate its operation cost. First of all, we suppose the discharge standard is 95% of pollutant degradation. According to most previous investigations, the degradation kinetics of hydrodynamic cavitation obeys the first-order law, given as:
In C/C0=-kt (6.1)
where C (mg/L) is the pollutant concentration at time t, C0 (mg/L) is initial concentration of pollutant and k (min-1) is the rate constant [49]. The time for 95% degradation in cavitation reaction zone is:
t95(min)=2.996/k (6.2)
Electric energy (power) in kilowatt hours (kWh) required for 95% degradation is [50].
E(kWh)=Pm×t95/60×1000 (6.3)
If the price of electricity is P* $/kWh, the operation cost of hydrodynamic cavitation system would be:
C1=E.P* (6.4)
It should be noted that Pm and k are related with the volume of the pollutant, the geometry and operation conditions of the system [26].
Amongst the hydrodynamic cavitation equipments, high speed and high-pressure homogenizers (typically laboratory-scale equipment with capacity of 1.5 and 2.0 l respectively) have energy efficiency of 43 and 54% respectively [27]. The orifice type of hydrodynamic cavitation reactor having a capacity of 50 l (typically a pilot plant scale) has an observed energy efficiency of 60%. Conventionally speaking, hydrodynamic cavitation equipment are more energy-efficient compared to the acoustic counterparts (except for the multiplefrequency flow cells), though the exact cavitational effects may or may not follow similar trends, as the fraction of this energy utilized for the cavitational activity is different [27].
Conclusions
Hydrodynamic cavitation is a new, advanced technology for the decomposition of complex compounds and an alternative to ultrasound-induced cavitation. The use of hydrodynamic cavitation in environmental engineering technologies allows processes to be greatly effective during water and effluent treatment. A technology which utilises the cavitating liquid environment can be considered as a nonwaste technology and environmentally friendly due to the possibility of degradation of low biodegradable, hazardous and carcinogenic organic compounds, which are resistant to conventional disposal methods. Examples include pesticides, dyes, or high molecular organic compounds, which in the cavitating liquid environment become susceptible to biodegradation.
Hydrodynamic cavitation has the possible to become energy efficient technique. It can diminish recently necessary use of expensive chemical reagents for advanced treatment process. These chemicals create additional problems when deposited into environment. Finally, as nowadays a lot of attention is put upon micro pollutants such as endocrine disrupting compounds, it is expected that developed process of wastewater treatment with aid of cavitation will considerably reduce their presence in purified water.
References
- Martin MJ, Artola A, Balaguer MD, Rigola M (2003) Activated carbons developed from surplus sewage sludge for the removal of dyes from dilute aqueous solutions. ChemEng J 94: 231–239.
- Gogate PR (2010) Application of hydrodynamic cavitation for food and bioprocessing. Ultrasound technologies for food and bioprocessing, Springer, New York, pp: 141-173.
- Gogate PR (2011) Hydrodynamic cavitation for food and water processing. Food and bioprocess technology 4: 996-1011.
- Ozonek J (2012) Application of hydrodynamic cavitation in environmental engineering. Taylor & Francis Group, London.
- Gaekwad RR, Patel RL (2015) Pesticide wastewater treatment by hydrodynamic cavitation process. International journal of advance research in engineering, science & technology(IJAREST).
- Shah YT, Pandit AB, Moholkar VS (2012) Cavitation reaction engineering, Springer Science & Business Media, Germany.
- Arrojo S, Benito Y (2008) A theoretical study of hydrodynamic cavitation. Ultrasonsonochem 15: 203–211.
- Franc JP, Michel JM (2006) Fundamentals of cavitation. Springer science & business media, Germany.
- Bagieriski J (1998) Kawitacja w urzqdzeniach wodociqgowych i cieplowniczych. Wydawnictwo Politechniki Poznariskiej, Poznan.
- Raut-Jadhav S, Saini D, Sonawane S, Pandit A (2016) Effect of process intensifying parameters on the hydrodynamic cavitation based degradation of commercial pesticide (methomyl) in the aqueous solution. Ultrasonicssonochemistry 28: 283–293.
- Ozonek J, Lenik K (2011) Effect of different design features of the reactor on hydrodynamic cavitation process. Arch Mater SciEng 52: 112-117.
- Gogate PR, Pandit AB (2005) A review and assessment of hydrodynamic cavitation as a technology for the future. UltrasonicsSonochemistry 12: 21–27.
- Franke M, Braeutigam P, Wu Z, Ren Y, Ondruschka B (2011) Enhancement of chloroform degradation by the combination of hydrodynamic and acoustic cavitation. UltrasonSonochem 18: 888–894.
- Wojs K (2004) Kawitacja w cieczach o roznych wlasciwosciach reologicznych, Oficyna wydawnicza politechniki Wroclawskiej, Oficyna wydawnicza politechniki Wroc?awskiej.
- Cai J, Huai X, Li X (2009) Dynamic behaviors of cavitation bubble for the steady cavitating flow. Journal of Thermal Science 18: 338-344.
- Gogate PR (2008) Cavitational reactors for process intensification of chemical processing applications: a critical review. Chemical engineering and processing: process intensification 47: 515-527.
- Dular M, Griessler-Bulc, T, Gutierrez-Aguirre I, Heath E, Kosjek T, et al. (2016) Use of hydrodynamic cavitation in (waste) water treatment. UltrasonicsSonochemistry 29: 577–588.
- Litwinienko A, Nekroz A, Lukasik K (2005) Technologiczne zastosowaniekawitacji hydrodynamicznej-doswiadczenia iperspektywy. Lubelskie Towarzystwo Naukowe, Lublin.
- Kot-Wasik A, Dabrowska D, Namiesnik J (2003) Degradacja zwii^zkow organicznych w srodowisku, Nowe Horyzonty i wyzwania w analityce i monitoringu srodowiskowym. Centrum Doskonalosci analityki i Monitoringu Srodowiskowego, Gdansk, 700-722..
- Pandit AB, Mukherjee AC, Kasat GR, Mahulkar AV (2011) Method of designing hydrodynamic cavitation reactors for process intensification. US Patent Application No.12/992,038.
- Amin LP, Gogate PR, Burgess AE, Bremner DH (2010) Optimization of a hydrodinamic cavitation reactor using salicyclic acid dosimetry. Chemical engineering journal 156: 165-169.
- Chanda SK (2012) Disintegration of sludge using ozone-hydrodynamic cavitation. Electronic theses and dissertations (ETDs).
- Gogate PR (2007) Application of cavitational reactors for water disinfection: current status and path forward. Journal of environmental management 85: 801–815.
- Gogate PR (2011) Cavitation in Biotechnology. Engineering fundamentals of biotechnology 2: 957-965.
- Moholkar VS, Pandit AB (2001) Modeling of hydrodynamic cavitation reactors: a unified approach. Chemical engineering science 56: 6295-6302.
- Tao Y, Cai J, Huai X, Liu B, Guo Z (2016) Application of hydrodynamic cavitation into wastewater treatment: a review. Chemical engineering & technology.
- Gogate PR, Tayal RK, Pandit AB (2006) Cavitation: a technology on the horizon. Current science 91: 35-46.
- Bagal MV, Gogate PR (2014) Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: a review. UltrasonicsSonochemistry 21: 1–14.
- Gogate PR, Pandit AB (2000) Engineering design methods for cavitation reactors II: hydrodynamic cavitation. AIChE journal 46: 1641-1649.
- Pehkonen T, Ranta H, Tolvanen A, Laine K (2002) The frequency of the fungal pathogen Exobasidiumsplendidum in two natural stands of the host Vacciniumvitis-idaea in the subarctic timberline area. Arctic, Antarctic and Alpine Research 34: 428-433.
- Sivasankar T, Moholkar VS (2009) Mechanistic approach to intensification of sonochemical degradation of phenol. ChemEng J 149: 57–69.
- Ozonek J, Odrzywolski A, Depta M, Kujawska J (2009) Degradacja WWA wroztworzewodnym w warunkachkawitacjihydrodynamieznej, w polskainzynieriasrodowiskapi?elatpowstapieniu do uniieuropejskiej tom 1 pod redakcja: JanuszaOzonkaMalgorzatyPawtowskiejMonografieKomi let u InzynieriiSrodowiska 58: 167-175.
- Szulzyk-Cieplak J, Fijalkowski S, Ozonek J (2005) Wykorzystaniezjawiskakawitacjihydrodynamieznej w technologiioczyszczaniawodyisciekow, Materialy II KongresuInzynieriiSrodowiska tom 1 MonografieKomitetuInzynieriiSrodowiska PAN 33: 279-282.
- Wang X, Wang J, Guo P, Guo W, Wang C (2009) Degradation of rhodamine B in aqueous solution by using swirling jet-induced cavitation combined with H2O2. J hazard mater 169: 486–491.
- Jyoti KK, Pandit AB (2004) Ozone and cavitation for water disinfection. Biochemeng J 18: 9-19.
- Vasilieva NB, Ryazantsev AA, Batoeva AA (2007) Nitrophenol oxidation in water with the use hydrodynamic cavitation. Chemistry for sustainable development 15: 705-710.
- Batoeva AA, Khandarkhaeva MS, Sizykh MR, Ryazantsev AA (2010) Cavitional activation of the galvanochemical oxidation of phenol. Russian journal of applied chemistry 83: 72-75.
- Pradhan AA, Gogate PR (2010) Removal of p-nitrophenol using hydrodynamic cavitation and Fenton chemistry at pilot scale operation. ChemEng J 156: 77–82.
- Bagal MV, Gogate PR (2013) Degradation of 2, 4-dinitrophenol using a combination of hydrodynamic cavitation, chemical and advanced oxidation processes. Ultrason. Sonochem 20: 1226–1235.
- Gogate PR, Patil PN (2015) Combined treatment technology based on synergism between hydrodynamic cavitation and advanced oxidation processes. Ultrasonicssonochemistry 25: 60-69.
- Brahmbhatt JI, Patel RL (2015) Treatability study of pharmaceutical wastewater by hydrodynamic cavitation process. International journal of engineering research and general science 3: 74-78.
- Gogate PR, Kabadi AM (2009) A review of applications of cavitation in biochemical engineering/biotechnology. Biochemical engineering journal 44: 60-72.
- Margulis MA (1995) Sonochemistry and cavitation. Gordon and Breach Publishers, Amsterdam.
- Pilli S, Bhunia P, Yan S, LeBlanc RJ, Tyagi RD, et al. (2011) Ultrasonic pretreatment of sludge: a review. Ultrasonicssonochemistry 18: 1-18.
- Petkovšek M, Mlakar M, Levstek M, Strazar M, Širok B, et al. (2015) A novel rotation generator of hydrodynamic cavitation for waste-activated sludge disintegration. Ultrasonicssonochemistry 26: 408-414.
- Zhang G, Zhang P, Yang J, Chen Y (2007) Ultrasonic reduction of excee sludge from the activated sludge system. J hazard mater 145: 515-519.
- Gronroos A, Kyllonen H, Korpijarvi K, Pirkonen P, Paavola T, et al. (2005) Ultrasound assisted method to increase soluble chemical oxygen demand (SCOD) of sewage sludge for digestion. Ultrasonicssonochemistry 12: 115-120.
- Suschka J, Grubel K, Machnicka A (2007) Mozliwosci intensyfikacji procesu fermentacji beztlenowej osadow sciekowych poprze dezintegracje osadu czynnego w procesie kawitacji mechanicznej Gaz, Woda i Technika Sanitarna 26-31.
- Mehrjouei M, Müller S, Möller D (2011) Degradation of oxalic acid in a photocatalyticozonation system by means of Pilkington ActiveTM glass. Journal of photochemistry and photobiology A: Chemistry 217: 417–424.
- Adewuyi YG, Peters RW (2013) Fundamental developments and economic feasibility of AOPS involving ultrasound for environmental remediation.
Citation: Dindar E (2016) An Overview of the Application of Hydrodinamic Cavitation for the Intensification of Wastewater Treatment Applications: A Review. Innov Ener Res 5: 137.
Copyright: ©2016 Dindar E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 23778
- [From(publication date): 6-2016 - Jul 08, 2025]
- Breakdown by view type
- HTML page views: 21509
- PDF downloads: 2269