Research Article Open Access
An Overview of Low Cost Adsorbents for Copper (II) Ions Removal
Ojedokun Adedamola Titi and Olugbenga Solomon Bello*Department of Pure and Applied Chemistry, Ladoke Akintola University of Technology, P. M. B. 4000, Ogbomoso, Oyo State, Nigeria
- Corresponding Author:
- Olugbenga Solomon Bello
Department of Pure and Applied Chemistry
Ladoke Akintola University of Technology
P. M. B. 4000. Ogbomoso, Oyo State, Nigeria
E-mail: osbello06@gmail.com
Received date: July 18, 2014; Accepted date: January 30, 2015; Published date: February 06, 2015
Citation: Ojedokun At, Olugbenga SB (2015) An Overview of Low Cost Adsorbents for Copper (II) Ions Removal. J Biotechnol Biomater 5:177. doi:10.4172/2155-952X.1000177
Copyright: © 2015 Olugbenga SB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The effective removal of heavy metals from aqueous wastes is among the most important issues for many industrialized countries. Rapid industrialization and poor effluent treatment processes in many industries have led to a substantial lowering of water quality that is fed to water bodies. Presence of heavy metals is one of the many factors that lower water quality. These heavy metals cause harmful effects on health, increase environmental toxicity and affect the aesthetic quality of water. The use of agricultural products and by-products has been widely investigated as an alternative for costly methods of removing heavy metals from water and wastewater. Modification of agricultural by-products could enhance their natural capacity and add value to these by-products. This review is aimed at investigating the use of low-cost adsorbents as an alternative to expensive activated carbon adsorbents used in removing Cu(II) using batch methods from water and wastewater.
Keywords
Heavy metals; Adsorbents; Agricultural products; Low cost; Water; Wastewater
Introduction
Heavy metal pollution produced in industrial wastewater has become a major issue throughout the world. In particular, copper (II) ions, whose effects on human health and aquatic life are regarded as harmful, and of course are not biodegradable; therefore, they must be removed from effluents so as to achieve environmental quality standards. According to U.S. Environmental Protection Agency (EPA) and WHO, the permissible limit of copper in drinking water is 1.3 and 2.0 mg dm3, respectively [1]. Contamination caused by heavy metals is mostly found in waste waters that emerge from metal plating, mining, and car radiator manufacturing industries. It is also found in areas where fertilizers are intensively used. It is thus important to remove heavy metals from water and wastewater due to their persistence nature amongst others.
Copper is a widely used material. It can be found as contaminant in food. This form of contamination emanates from the use of copper material in the packaging of food. Despite the essential nature of copper to human life and health, it is potentially toxic. For instance, continuous inhalation of copper-containing spray is linked with an increase in lung cancer among exposed workers. Copper is released into the environment in a number of different ways; it finds its way into water-streams resulting in environmental contamination that poses threat to humans, animals, and plants. This can cause serious and complex problem [2,3]. Like other heavy metals, copper in trace amount is necessary for life processes. However, with higher concentrations of this element in the environment and the consequent increase in human intake, copper concentrations have reached toxic levels causing various diseases and disorders such as liver damage, Wilson disease and insomnia [4]. Various technologies for the removal of heavy metals from wastewater had been established and reported by researchers in their works. They include precipitation [5], flotation [6], biosorption [7,8], membrane separation [9], adsorption on minerals [10,11] and adsorption using activated carbon [12,13]. Among the various methods described in literature, adsorption is the most effective method that has been successfully applied in the purification and recovery of Cu (II) ions from effluents due to its high efficiency and easy handling [14]. In spite of its prolific use, activated carbon remains an expensive material since the higher the quality of activated carbon, the greater its cost. The use of complexing agents are also required to enhance the removal performance of activated carbon when used for inorganic matters [15]. This makes the use of activated carbon expensive and thus not suitable for small-scale industries.
There has been an increasing research into finding low-cost adsorbents for the removal of heavy metals from wastewaters. Agricultural by-products which have been studied for this purpose include peat, wood, pine bark, banana pith, cotton seed hulls, peanut shells, hazel nut shell, rice husk, saw dust, wool, orange peel, compost, maize cobs and guava leaves. This review is aimed at investigating the use of low-cost adsorbents as an alternative to expensive activated carbon adsorbents used in removing Cu(II) from water and wastewater.
Importance of Copper
Effect on man
Copper is a trace element which is essential to living organisms. It is essential for the proper functioning of organs and metabolic processes in humans. Ingestion of high concentrations of copper salts is associated with symptoms relating to abdominal pain, headache, nausea, dizziness, vomiting and diarrhea, tachycardia, respiratory difficulty, hemolytic anemia, massive gastrointestinal bleeding, liver and kidney failure, and death. These symptoms subside when copper in the drinking water source is reduced. Chronic copper poisoning leading to liver failure was reported in a young adult male with no known genetic susceptibility who consumed 30–60 mg/l of copper as a mineral supplement for 3 years [16].
Effect on Aquatic Life
Excess Cu(II) in water may damage marine and freshwater organisms such as fish and mollusks [17]. Fish species vary in their sensitivity to copper, with the LD50 for 96-h exposure to copper sulphate reported to be in the order of 58 mg per litre for Tilapia (Oreochromis niloticus) and 70 mg per litre for catfish (Clarias gariepinus) [18]. The chronic effect of sub-lethal concentrations of Cu(II) on fish and other creatures is damage to gills, liver, kidneys and the nervous system. It also interferes with the sense of smell in fish, thus preventing them from choosing good mates or finding their way to mating areas [19].
Agricultural wastes used as adsorbents for removing copper (II) ion from aqueous solutions
Adathoda vasica
Jafar and Shajudha [20] studied the use of Adathoda vasica stem as adsorbent to remove Cu(II) from industrial waste water. The physicochemical properties of the adsorbent are listed in Table 1 below:
| PROPERTIES | Adathodavasica Activated Carbon |
|---|---|
| Particle size (μm) | 0.75 |
| Bulk density (g/cm3) | 0.79 |
| Moisture content (%) | 0.601 |
| Ash content (%) | 11.42 |
| Fixed carbon content (%) | 73.41 |
| Matter soluble in water (%) | 2.14 |
| Matter soluble in acid (%) | 2.8 |
| pH | 6.19 |
| Surface area (m2/g) | 120.6 |
| Iron content (%) | 0.4 |
Table 1: The physico-chemical properties of Adathoda vasica stem [20].
High grade CuSO4.5H2O was used as heavy metal sample. Laboratory experimental investigation was carried out to identify the effect of pH (1.50–5.5), agitation time (30-240 min) varying temperature (30-50°C). Varying biomass quantities (2, 4, 6, 8, 10 g/L) and other co-existing ions were also examined. The adsorption process was tested with pseudo first order–Lagergren equation and first order reversible–Bhattacharya Venkobachar equation. Both Langmuir and Freundlich adsorption isotherm models fitted the experimental data best with regression coefficient r2 > 0.95 for the Cu(II). The adsorption was endothermic and the computation of the parameters ΔG°, ΔH° and ΔS° indicated that the interactions were thermodynamically favorable. The results showed that Adathoda vasica stem carbon (AVSC) was an effective and economical bio-sorbent material for the removal and recovery of heavy metal ions from waste water.
Coconut waste
Coconut husk is a low cost adsorbent which is a waste material from coconut. Coconut is abundant in Nigeria and has a high sorption capacity due to its high tannin content. Oyedeji and Osinfade [21] studied the use of coconut husk as a low-cost natural adsorbent for the removal of Cu(II) from simulated industrial waste effluent. The effects of varying adsorbent loadings, pH, contact time, metal ion concentration and temperature of adsorption on the adsorption process were determined. The adsorption of Cu (II) was maximum (92% ± 2.8) at pH range of 5 - 7, metal ion concentration of 50 ppm, temperature of 50°C and 30 minutes. 1 g of the adsorbent material was found to be optimal for the metal ion; the Freundlich isotherm was found to be suitable for the adsorption of Cu(II).
Okafor et al. [22] also explored the adsorption capacity of Coconut (Cocos nucifera L.) shell for Cu(II) from aqueous solutions. The effect of various operational parameters such as concentration, pH, temperature and sorption time on the adsorption of Cu(II) was investigated using batch process experiments. The authors found that coconut shell (CNS) can be used as a low cost adsorbent for the removal of Cu(II) in aqueous solution containing low concentrations of the metal. The percentage adsorption was found to depend on the concentration of the adsorbent present, the solution pH, temperature and the sorption. The concentration of the metal ion adsorbed increased with increase in concentrations, increase in contact time, increase in temperature and increase in pH. The rate of adsorption of the metal ions by coconut shell was rapid initially but decreased gradually due to the gradual blocking of the initial available uncovered surface area of the adsorbent. Adsorption for the metal ion increased with increase in metal ion concentration because at low concentrations, the active site on the surface of the adsorbent are not completely covered. Kinetic studies showed that the sorption of the metal ions can best be described by both pseudo-second-order and intra-particle diffusion models while the adsorption characteristics of the adsorbent followed the Freundlich adsorption isotherm. The average value of the activation energy of adsorption for CNS was found to be 3.79KJ/mol which implies that the adsorption of Cu(II) on the adsorbent is physical adsorption mechanism.
The efficacy of coconut tree sawdust (CTS) as alternative low-cost biosorbent for the removal of Cu(II) ions from aqueous solutions was investigated by Wiwid et al. [23]. Batch adsorption studies were carried out to evaluate the effects of solution pH and initial metal concentration on adsorption capacity. The optimum biosorption condition was found at pH 6.0, 0.1 g biomass dosage and at 90 min equilibrium time. The adsorption data were fitted to the Freundlich and Langmuir isotherm models. The adsorption capacity and affinity of CTS was evaluated. The Freundlich constant and separation factor values suggest that the metal ion was favourably adsorbed onto biosorbents. The maximum adsorption capacities estimated from the Langmuir isotherm model was 3.89mg/g. The characterisation studies were performed using Scanning Electron Microscope (SEM), Energy Dispersive X-ray Spectrometer (EDX) and Fourier Transform Infrared Spectrometer (FTIR). It was observed that interaction with the metal ions led to the formation of discrete aggregates on the biosorbents surface. The metal ions bound to the active sites of the biosorbents through either electrostatic attraction or complexation mechanism. The presence of functional groups in the biosorbents favoured metal ion binding. The authors concluded that the use of coconut tree sawdust (which is abundantly available at low-cost) will provide a solution to their disposal.
Gmelina arborea (Verbenaceae) Leaves
Gmelina arborea (White teak) is a fast growing deciduous tree. It is native to India and Burma, but is now planted in many tropical areas due to its good form and growth. It grows on different localities and thrives well in moist fertile valleys with an annual rainfall between 750 and 4500mm and annual mean temperature of 25- 380C. Flowering of the Gmelina arborea tree takes place during February to April while fruiting starts from May onwards up to June.
According to results obtained from various researchers, it was deduced that chemical modification improves metal ion binding capacity of the substrate thereby increasing the rate of metal ion uptake. Jimoh et al [24] compared the adsorptive capability of citric acid modified and unmodified Gmelina arborea leaves for the removal of Copper ions from aqueous solution. The experiments showed that the adsorption process using Gmelina arborea leaves was dependent on both contact time and initial metal ion concentration. It was observed that acid modified Gmelina arborea bind more metal ion than the unmodified adsorbent at every given time, this is because citric acid increases its sorption capacities. The equilibrium data for Cu (II) ion fitted well into the Freundlich adsorption isotherm, and the linearity of the plot tested the representative nature of adsorption on the adsorbent, and the correlation regression coefficient showed that Cu (II) ion adsorption was favorable using Gmelina arborea leaves.
Pomegranate Peels
Pomegranate fruits are widely consumed fresh and in processed forms as juice, jams and wine. Pomegranate peel, a by-product of the pomegranate juice industry is inexpensive. Pomegranate peel is rich in ellagitannins (ETs) such as punicalagin and its isomers, as well as lesser amounts of punicalin (4, 6-gallagylglucose), gallagic acid, ellagic acid (EA) and EA-glycosides [25]. Pomegranate peel is composed of polyphenols, ellagic tannis and gallic and ellagic acids [26]. The sorption of Cu(II) from aqueous solution by using activated carbon produced from pomegranate peel was investigated by El-Ashtoukhy et al [27]. They studied the adsorption capacity of the adsorbent using batch experiments. They also investigated the influence of pH, contact time, metal ions and adsorbent concentrations on the adsorption process. The experimental data obtained were evaluated and fitted using adsorbent equilibrium isotherms, and kinetic models.
The study showed that activated carbon prepared from chemically treated pomegranate peel was an effective adsorbent for the removal of Cu(II) from aqueous solutions. The adsorption process is a function of the adsorbent dosage and concentrations, pH and time of agitation. The effective pH for the Cu(II) removal was 5.8. Equilibrium was achieved practically in 2 h. The equilibrium sorption data are satisfactorily fitted in the order: Freundlich > Temkin > Langmuir. Adsorption kinetics follows pseudo-second order kinetic model. Kinetic results showed that both bulk and intra-particle diffusion are effective adsorption mechanisms. Thus, the results would be useful for the design of wastewater treatment plants for the removal of copper.
Rice (Oryza sativa) waste
Haluk et al. [28] assessed the ability of low-cost adsorbents such as shells of rice to adsorb Cu(II) from aqueous solution. The effect of the solution pH, temperature, contact time, initial adsorbate concentration and adsorbent doses on the removal of Cu(II) was studied. The thermodynamic parameters for the adsorption Cu(II) have also been computed and discussed. They also studied the kinetics and the factors controlling the adsorption process. Rice shell appeared to be a promising adsorbent for removal of Cu(II) from aqueous solution. Based on their results, they concluded that a process using rice shell for the removal and recovery of a heavy metal is potentially more economical than the current process technology. The adsorption of Cu(II) was dependent on its initial concentrations, the amount of adsorbent, contact time, temperature and pH of the metal solution, maximum removal of Cu(II) on rice shell are at pH about 6.0. The removal of Cu(II) increased with increase in temperature. The isothermal data of Cu(II) adsorption on the adsorbent was modeled by both Freundlich and Langmuir isotherm. These models were used to calculate the capacity of adsorbents for adsorption of Cu(II). The maximum adsorption capacities for Cu (II) on rice shell at 293, 313, and 333K temperature were found to be 9.588, 17.422, and 2.954 mg g-1, respectively.
Nasehir et.al [29] investigated the adsorption potential of rice husk based activated carbon (RHAC) to remove Cu (II) from aqueous solution using fixed-bed adsorption column. The authors determined the effects of inlet Cu (II) concentration (5-15 mg/l), feed flow rate (10-30 ml/min) and RHAC bed height (30-80 mm) on the breakthrough characteristics of the adsorption system. The highest bed capacity of 34.56 mg/g was obtained using 10 mg/l inlet Cu (II) concentration, 80 mm bed height and 10 ml/min flow rate. The fixedbed adsorption system was found to perform better with lower Cu (II) inlet concentration, lower feed flow rate and higher RHAC bed height. The adsorption data were fitted to three well-established fixed-bed adsorption models namely, Adam-Bohart, Thomas and Yoon-Nelson models. The results fitted well to the Thomas and Yoon-Nelson models with correlation coefficient, R2 ≥ 0.96. The authors discovered that RHAC prepared by ZnCl2 activation was found suitable for Cu (II) removal from aqueous solution using fixed-bed adsorption column.
Soybean waste
Soybean straw is a lignocellulosic agricultural stalk. It is abundant, inexpensive, and a renewable resource. Over 16 million metric tons of soybeans are produced annually in China [30], but most of soybean straw is arbitrarily discarded or set on fire. These disposals must result in resource loss and environmental pollution. The exploitation and utilization of soybean straw must bring obvious economic and social benefit to mankind. Bo et al. [31] modified soybean straw with citric acid after being washed with sodium hydroxide to enhance its ability to adsorb Cu(II), then they investigated the adsorption process as a function of initial pH and concentration of Cu(II). Furthermore, the mechanism of Cu(II) adsorption was studied by Fourier Transform Infrared (FTIR) analysis and the effect of washing or citric acid modification was assessed.
The amount of Cu(II) adsorbed by soybean straw was increased by modification with citric acid, regardless of whether the samples were base washed or water washed. This is due to the increase in carboxyl groups imparted onto the straw by reaction with citric acid. The Cu(II) uptake increased and percentage adsorption of the Cu(II) decreased with the increase in initial Cu(II) concentration. The Freundlich isotherm fitted the experimental data much better than the Langmuir model, but for the two kinds of citric acid modified soybean straw, there was only a slight deviation from linearity of Langmuir model. The authors suggested that further work should include performing experiments to study the effect of temperature and the thermodynamics, more detailed kinetics, adsorbent regeneration and the ultimate fate of heavy metals once removed from the adsorbent.
Supaporn et. al [32] studied the efficiency of removing copper ions from copper chloride solution using soybean hulls. The soybean hulls were modified with citric acid, since it is one of several dicarboxylic acids that could form a reactive anhydride upon heating and then combine with various lignocellulosic and proteinaceous components with soybean hulls to add carboxyl for the by-product substrate. Their experiment was divided into two parts. The first part involved the determination of the time to heat soybean hulls that were soaked in citric acid. In the second part, the factors affecting copper ion adsorption by soybean hulls were determined to be initial concentration and pH of the solution, ratio of soybean hulls to copper chloride solution and size of soybean hulls. From the results of experiment, the optimum conditions were to heat for 90 minutes at an initial pH of 4.8. The ratio of soybean hulls to solution is 10 grams per 1 litre. Size of soybean hulls is 602 micrometers and the initial concentration of copper chloride solution is 50 ppm. The copper ion removal efficiency using soybean hulls is 97.68 % under these conditions and the concentration of copper ion had been reduced below the industrial standard (2.0 mg/l). The concentration of Cu(II) in the solution decreased with increasing adsorption time. The higher initial concentration of copper chloride solution resulted less adsorption capacity as shown by a higher Cu(II) concentration remaining in the solution. The reason being, that there was not enough adsorbent to adsorb more Cu(II) in the higher initial concentration of the solution. The concentration of Cu(II) in copper chloride solution increased with an increase in the size of soybean hulls for both solid/solution ratios. The smaller particle size provided more surface area for the adsorption. The ratio of soybean hulls to solution at 1:100 gm/ml showed better adsorption of Cu(II) and could remove Cu(II) below 2 ppm. No results about the kinetic and isothermal studies were provided.
Orange Peels
One of the agricultural wastes from the orange juicing industry is orange peel. It is abundant, cheap and readily available. Orange peel is largely composed of cellulose, pectin (galacturonic acid), hemicellulose, lignin, chlorophyll pigments and other low molecular weight compounds, including limonene. These components contain various functional groups, such as carboxyl and hydroxyl, which make orange peel to be a potential adsorbent material for removing metal ions from aqueous solutions. These wastes create increasing disposal and, potentially severe, environmental problems. Without significant processing, Ajmal et al. employed orange peel for metal ions removal from simulated wastewater [33-35].
Several authors reported the use of orange waste as a precursor for the preparation of adsorbent by chemical treatment [35-39]. Ningchuan et al. [40] reported the preparation of adsorbent from orange peel by means of hydrolysis of the grafted copolymer, which was synthesized by interacting methyl acrylate with cross-linking orange peel to examine its adsorption characteristics for Cu(II) from aqueous solutions. The recovery, regeneration, and comparison test and application studies were conducted to assess the practical utility of the adsorbent. The presence of poly (acrylic acid) on the biomass surface was verified by infrared spectroscopy (IR), scanning electron microscopy (SEM) and thermogravimetry (TG). Total negative charge in the biomass surface and the zeta potentials were determined.
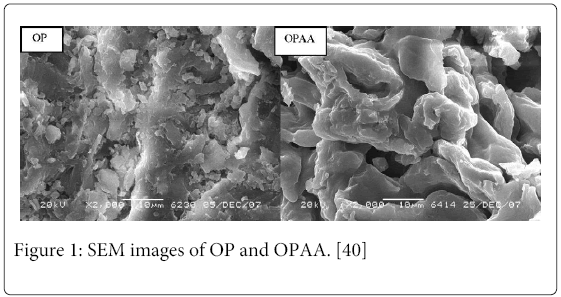
The surface features of grafted and ungrafted OP were studied by SEM. The SEM micrographs of OP and OPAA are shown in Figure 1. The surface morphology of OP is different from that of OPAA. It can be seen that the surface of the grafted OP is more uneven than that of OP, which indicates that MA is chemically bonded and/or physically adheres to the surface of OP.
The modified biomass was found to have high adsorption capacity and fast adsorption rate for Cu(II). From Langmuir isotherm, the adsorption capacity for Cu(II) was 289.0 mgg−1, which is about 6.5 times higher than that of the unmodified biomass. The kinetics for Cu(II) adsorption followed the pseudo-second-order kinetics. The adsorbent was used to remove Cu(II) from electroplating wastewater and was suitable for repeated use for more than four cycles. Sha et al. [41] also investigated the use of orange peel in removing copper (II) ion from aqueous solution. The orange peel (OP) was used as raw material to prepare two novel adsorbents: MgOP (Mg2+ type orange peel adsorbent) and KOP (K+ type orange peel adsorbent). The authors employed the use of FTIR and SEM to characterize the adsorbents.
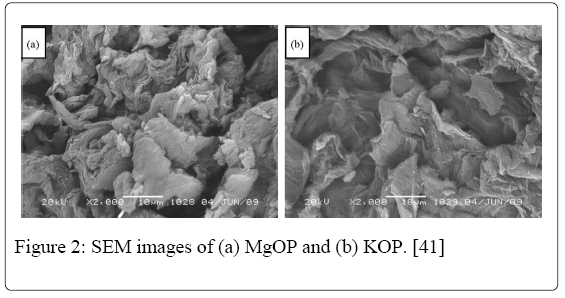
SEM micrographs of the MgOP and KOP are shown in Figure 2. Irregular and porous surfaces could be observed through these micrographs. It can thus be concluded that both adsorbents present an adequate morphology for metal adsorption. The mean diameter of MgOP and KOP was determined as 62 and 58μm, respectively.
The effects of pH, solid/liquid ratio, time and metal ion concentration on the Cu(II) adsorption by these two adsorbents were also investigated. The isotherms data were analyzed using the Langmuir, Freudlich, Temkin and Dubinin–Radushkevich models. Langmuir model provided the best correlation for the adsorption of Cu(II) by both MgOP and KOP, and the mono-layer adsorption capacity forCu(II) removal by MgOP and KOP were 40.37 and 59.77 mg/g, respectively. The adsorption efficiency of Cu(II) increased with the increase in contact time and reached equilibrium within 20 minutes.
The kinetics data were analyzed using four adsorption kinetic models: the pseudo-first and second-order equations, the Elovich equation and intraparticle diffusion equation and pseudo-secondorder equation were found to fit the experimental data very well. Thermodynamic studies showed the spontaneous and exothermic nature of the adsorption of Cu(II) by MgOP and KOP. The results of the investigation showed that orange peel adsorbent has considerable potential for the removal of Cu(II) from aqueous solutions, and K+ type orange peel (KOP) exhibits better adsorption ability than Mg2+ type orange peel (MgOP). For both adsorbents, pH has a great influence on adsorption efficiency.
Chestnut (Castanea sativa spp.) Shell
In Galicia (NW of Spain) there are large plantation areas of chestnut (Castanea sativa spp.), representing, approximately, a 5% (in m3 with bark) of the total wood stock. Chestnut fruit is processed in the food industry for the production of various food products which include marron-glace, chestnut puree, bombons glace, etc. The shell is separated as a waste product in the chestnut peeling process and used as fuel. The composition of this material and its potential for different applications which would suppose its valorization (source of natural antioxidant, adhesive component and tanning agent) were analyzed in previous works [42,43].
The use of chestnut shell treated with formaldehyde was studied by Gonzalo et al. [44]. The effects of the initial concentration, temperature, and pH were investigated using the incomplete 33 factorial design. The adsorption process fitted the Langmuir adsorption isotherm. Also, the Fourier transform Infrared and X-ray photoelectron spectroscopies revealed that carboxyl, hydroxyl, ether, alcoholic, and amino groups were involved in metal ions binding.
The biosorption of Cu(II) onto chestnut shell in a batch adsorber was studied by Yao et al. [45]. The ability of chestnut shell biosorbent to remove Cu(II) from aqueous solution was investigated in equilibrium, kinetics and thermodynamics. The equilibrium data was found to agree well with Langmuir isotherm and Redlich–Peterson isotherm models. The adsorption capacity of chestnut shell for Cu(II) was determined with the Langmuir model and was found to be 12.56mgg−1 at 293 K. The kinetic data were found to obey the pseudosecond- order model. The authors also discovered that intra-particle diffusion was not the sole rate-controlling factor. The negative values of ΔH0 and ΔG0 revealed the exothermic nature and the feasibility of adsorption. The investigation showed that chestnut shell is a promising biosorbent for Cu(II) removal from aqueous solutions.
Sugarcane bagasse
Junior et al. [46] reported the use of succinic anhydride modified sugarcane bagasse for treatment of Cu(II) from aqueous solutions. Sugarcane bagasse consists of cellulose (50%), pectin (27%) and lignin (23%). The presence of these three biological polymers causes sugarcane bagasse rich in hydroxyl and phenolic groups and these groups were modified chemically to produce adsorbent materials with new properties. According to the authors, the hydroxyl groups in sugarcane baggase can be converted to carboxylic groups by using succinic acid. During the course of the research, it was observed that sugarcane baggase treated with ethylenediamine and triethylenetetramine displayed an increase in nitrogen content compared to untreated sample. The kinetic studies of the adsorption process revealed that the equilibrium time for Cu(II) onto ethylenediamine and tetraethylenetetramine modified sugarcane bagasses was slower when compared with that modified with NaHCO3.
Plant fibres
Two types of chemical modifications on jute fibres and their effectiveness in the removal of Cu(II) were reported by Shukla and Pai [47]. The first modification involved a monochloro triazine type reactive dye, Reactive Orange 13, which was covalently loaded to the cellulosic matrix of jute fibres. The other type of modification involved the oxidation of hydroxyl groups of cellulose present in jute fibres to carboxyl group by using hydrogen peroxide. The reactive dye used for modification contains azo linkage and hydroxyl groups (a situation favourable for the formation of six membered ring chelate with metal ions). The presence of sodium sulphonate groups of the dye molecules attached covalently to the adsorbent also enhanced the metal adsorption capacity. The mechanism of ion-exchange between Na+ of the dyed material and the heavy metal ions can be represented by the following equation:
R(SO3Na)2 + M2+ R(SO3)M + 2Na+
For oxidized jute, the high uptake of heavy metal ions was due to the generation of carboxyl groups (–COOH). The authors reported that oxidation process of jute fibres was carried out under alkaline condition, therefore the carboxyl groups are in the form of carboxylate. The adsorption of heavy metal ions could also take place by ion-exchange mechanism as shown below:
2RCOONa+ + M2+ (RCOO)2M + 2Na+
Based on the Langmuir plots, maximum adsorption capacity for Cu(II) was achieved by dye loaded jute, followed by oxidized jute and unmodified jute. Chemical modification of cellulose fibre to improve its removal performance and adsorption capacity for Cu(II) using ethylenediamine was conducted by Torres et al. [48]. Cellulose consists of active hydroxyl groups present on each monomeric unit of cellulose; therefore cellulose can react with carboxyl and amine groups of organic compounds. Adsorption of heavy metal ions occurred through complexation mechanism in which the amine (–NH2) groups of ethylenediamine take part in the chelation process. It is well known that adsorption of heavy metals by cellulosic wastes depends on the contact time and temperature. Ho and Ofamaja [49] found that initial concentration of Cu(II) has great effect on the equilibrium time for copper adsorption onto HCl treated palm kernel fibre. The time to reach equilibrium was only 15 min for 50 mg l-1 copper, but increased to 60 min for 200 mg l-1.
The study revealed that Cu(II) adsorption increases with the increase of temperature which indicates that the mobility of Cu(II) increases with increase in temperature. The value of activation energy of 22kJmol-1 suggests that chemisorption was an important process in the adsorption of Cu(II) onto HCl treated palm kernel fibre. Adsorption follows pseudo-second-order kinetic model at all copper concentrations studied.
Tree barks
Three different kinds of tree barks mainly sal (Shorea robusta), mango (Mangifera indica) and jackfruit (Artocarpus integri floria) were modified using hydrochloric acid solution in order to remove Cu(II) from aqueous solutions [50]. Treated barks were able to chelate more Cu(II) than untreated ones. Extraction of soluble organic compounds which coloured the decontaminated solutions was also avoided after pretreatment process. The highest adsorption capacity of Cu(II) was shown by sal bark (51.4 mgg-1), followed by mango (42.6 mgg-1) and jackfruit (17.4 mgg-1). Binding of Cu(II) to the bark occurred through cation exchange mechanism as the pH of the effluent decreased after copper adsorption. It was reported that hydroxyl and carboxyl groups were involved in the adsorption process and the results indicate that chelation of 1 mole of copper generates about 1.6–1.8 moles of hydronium ions. Regeneration of adsorbent was more successful using higher concentration of HCl solution.
Raw rubber wood (Hevea brasiliensis)
Helen and Lima [51] worked on the adsorption of copper from aqueous solution using Hevea brasiliensis dust. In their study, they compared the adsorption capacity of the modified adsorbent with that of the unmodified adsorbent. The raw rubber wood (Hevea brasiliensis) saw dust (RSD) was modified using phosphoric acid and sodium hydroxide with different impregnation ratios (IR) to improve adsorption capacity for the removal of copper from aqueous solutions. The modified saw dusts using phosphoric acid (PMSD4, IR 1:2) and sodium hydroxide (SMSD3, IR 1:1) were evaluated through SEM and FTIR. Equilibrium and kinetic adsorption studies were carried using RSD, PMSD4 and SMSD3. The effects of various operating variables such as solution pH, adsorbent dose, initial copper concentration, temperature on the removal of copper ions were also studied.
Adsorption equilibrium data were fitted to Langmuir, Freundlich Dubinin–Radushkevich and Temkin isotherm models. The equilibrium data for RSD and SMSD3 were best represented by the Langmuir and Freundlich isotherms. The Dubinin–Radushkevich model gave the best fit for PMSD4. The SMSD3 showed higher adsorption capacity which is 53% to 68% higher than RSD. The data were also fitted to kinetic models such as pseudo first order, pseudo second order, Elovich and intraparticle diffusion models. The adsorption kinetics was found to follow the pseudo second order kinetic model. The optimum pH for the adsorption was 6. The adsorption of the metal ions increased with increasing temperature which indicated that the process is endothermic. Various thermodynamic parameters such as standard enthalpy change (ΔH°), standard entropy change (ΔS°) and standard free energy change (ΔG°) were evaluated. An appreciable increase in adsorption capacity was observed when studies were carried out using SMSD3 to PMSD4 and RSD.
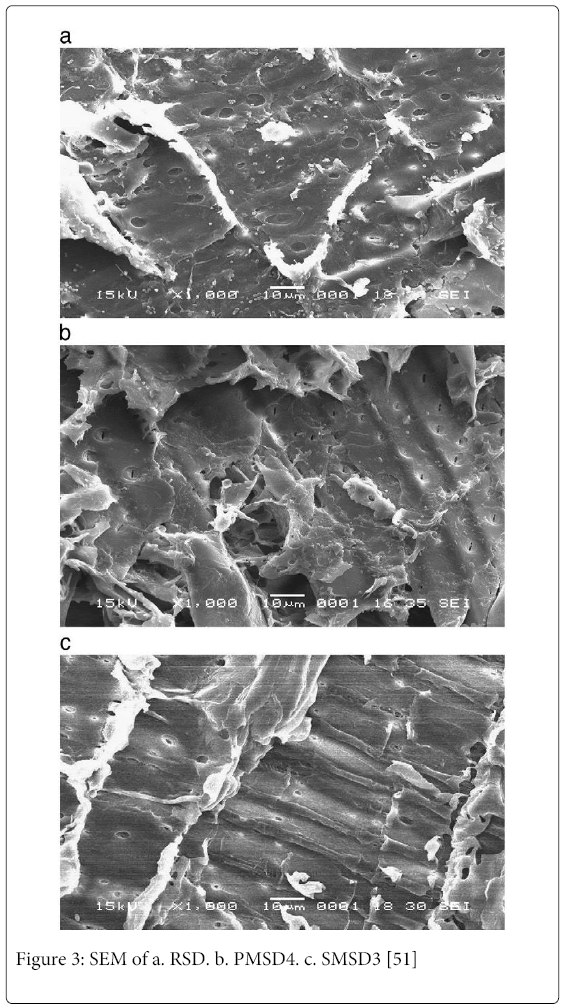
The adsorption of copper on unmodified and modified saw dust was found to be dependent on solution pH and temperature, where higher solution pH and temperature would favour the adsorption process. Scanning electron microscopy was used to observe the changes in the surface microstructures of the adsorbents due to the modifications. The scanning electron microscopic (SEM) pictures before and after modification are shown in Fig. 3a, b and c. The SEM observation of RSD (Figure 3a) indicates the common feature of porous and irregular morphology. Pores and internal surface are requisite for an efficient adsorbent. As can be observed from Fig. 3b (PMSD4) and Fig. 3c (SMSD3) there is clear difference in the surface morphology of sawdust after treatment. The difference could be due to the removal of considerable organic by-products and minerals present in the sawdust.
The authors observed that the surface modification of saw dust contributed positively to the development of micropores. Surface was more homogeneous in the case of SMSD3 in comparison to RSD and PMSD4 which shows a more heterogeneous surface. In acid treatment there are considerable small cavities and cracks on the surface forming a system of complicated pore networks. However, the alkali modification reduced the cracks and cavities on the surface of the adsorbent. Thus, the modification using NaOH could enhance the adsorption capacity of saw dust than acid modified and raw sawdust in removing copper from aqueous solutions.
Lentil shell (Lens culinaris Medik)
Haluk et al. [52] investigated the use of lentil shell (Lens culinaris Medik) as a replacement for current costly methods of removing metals from aqueous solution. The equilibrium adsorption level was determined as a function of the solution pH, temperature, contact time, initial adsorbate concentration and adsorbent doses. Adsorption isotherms of Cu (II) on adsorbents were determined and correlated with Langmuir and Freundlich models.
| Physical characteristics | Percent of the properties |
|---|---|
| Surface area (BET) (m2g-1) | 0.19 |
| Bulk density (gcm-1) | 0.49 |
| Particle size (mm) | 0.60 |
Table 2: Physical characteristics of lentil shell used in the experiment [52].
The maximum adsorption capacities for Cu (II) on the adsorbent at 293, 313 and 333K were found to be 8.977, 9.510, and 9.588 mg g-1 respectively. To predict the nature of the adsorption process, the thermodynamic parameters such as free energy (ΔG0), enthalpy (ΔH0) and entropy changes (ΔS0) for the adsorption of Cu (II) were computed.
Wheat (Triticum durum Desf) wastes
Haluk et al. [52] assessed the ability of the shell of wheat (Triticum durum Desf) to adsorb Cu (II) from aqueous solution. The effect of the solution pH, temperature, contact time, initial adsorbate concentration and adsorbent doses on the removal of Cu (II) was studied. The thermodynamic parameters, kinetics and the factors controlling the adsorption process were also studied. The isothermal data of Cu (II) adsorption on the adsorbent can be modeled by both Freundlich and Langmuir isotherm. The maximum adsorption capacities for Cu (II) on the adsorbent at 293, 313 and 333K were found to be 7.391, 16.077, and 17.422 mg g-1 respectively. To predict the nature of the adsorption process, the thermodynamic parameters such as free energy (ΔG0), enthalpy (ΔH0) and entropy changes (ΔS0) for the adsorption of Cu (II) were computed. The kinetics and the factors controlling the adsorption process were also studied. The adsorption of Cu (II) was found to be dependent on its initial concentrations, the amount of adsorbent, time of contact, temperature and pH of the metal solution, maximum removal of Cu (II) on the adsorbent are at pH about 6.0. The removal of Cu (II) increased with increase in temperature. The authors concluded that the wheat shell for the removal and recovery of a heavy metal is potentially more economical than current process technology.
| Chemical characteristics | Percent of the properties |
|---|---|
| Moisture content | 6.58 |
| Water soluble component (inorganic matter) | 18.12 |
| Insoluble components (organic matter) | 78.85 |
| Ash content | 9.18 |
| Total loss of ignition | 89.28 |
| C content | 33.53 |
| H content | 4.67 |
| pH | 5.03 |
Table 3: Chemical characteristics of lentil shell used in the experiment [52].
| Properties | Percent of the properties |
|---|---|
| Surface area (BET) (m2g-1) | 0.67 |
| Bulk density (gcm-1) | 0.36 |
| Particle size (mm) | 0.60 |
Table 4: Physical characteristics of wheat shell used in the experiment [52].
| Properties | Percent of the properties |
|---|---|
| Moisture content | 6.40 |
| Water soluble component (inorganic matter) | 22.33 |
| Insoluble components (organic matter) | 75.14 |
| Ash content | 2.58 |
| Total loss of ignition | 88.45 |
| C content | 44.59 |
| H content | 6.56 |
| pH | 6.05 |
Table 5: Chemical characteristics of wheat shell used in the experiment [52].
Wheat bran is a by-product of wheat milling industries and has been proved to be a good adsorbent for removal of many types of heavy metal ions such as Cu(II). The investigation carried out by Ozer et al. [53] showed that wheat bran is a good adsorbent for the adsorption of Cu (II) from aqueous solution.
The authors reported that the application of a strong dehydrating agent like sulfuric acid (H2SO4) can have a significant effect on the surface area of the adsorbent, thereby resulting in better efficiency of adsorption of copper ions. It was found that upon treatment with sulfuric acid, wheat bran had a much higher surface area. The authors suggested that acid treatment caused changes in surface area by increasing the conversion of macropores to micropores. Maximum adsorption capacity for Cu(II) ions was reported as 51.5 mg g-1 (at pH 5) and equilibrium time of adsorption was achieved in 30 minutes.
Sawdust
Sawdust is obtained from wood industry. It is an abundant byproduct which is easily available at negligible price. It contains various organic compounds (lignin, cellulose and hemicellulose) with polyphenolic groups that could bind heavy metal ions through different mechanisms. Sciban et al. [54] conducted an experiment on the efficiency of sawdust in the removal of Cu(II) ion. They treated two kinds of sawdust (poplar and fir wood) with NaOH (fibre-swelling agent) and Na2CO3 solutions and the adsorption capacities were compared with the untreated sawdust. For unmodified sawdust, adsorption followed Langmuir isotherm model. Equivalent amounts of adsorption capacities were recorded by both types of sawdust for Cu(II) ion, although these two adsorbents have different anatomical structure and chemical composition. A marked increase in the adsorption capacity of the adsorbents was noticed after treating with NaOH. The adsorption capacities shown by Langmuir model were 6.92 mg g-1 (poplar sawdust) and 12.70 mg g-1 (fir sawdust).
Increase in the concentration of NaOH for modification purpose however did not cause a significant increase of the adsorption capacity. No greater than 1% of concentration of NaOH solution for modification was suggested by the authors. The temperature of modification was also not a significant factor for the main increase of adsorption capacities of modified sawdust. It was observed that only a slight increase in Cu(II) adsorption occurred when the fir sawdust was treated with NaOH at higher temperature (800C). The authors also found out that the use of Na2CO3 for chemical modification is less efficient than the use of NaOH. This is due to higher number of Na+ ions in 1 g of NaOH compared to 1 g of Na2CO3. Although the authors were able to successfully describe the effect of chemical modification on the adsorption of Cu(II) from the aqueous solution, no detailed work was done on the kinetic studies.
The use of sawdust of poplar tree for Cu(II) from aqueous solution was also studied by Acar and Eren [55]. They reported the effect of sulfuric acid treatment on sawdust of poplar tree in the adsorption process. Sulfuric acid poplar sawdust possessed good removal of 92.4% Cu(II) at pH 5, while untreated sawdust could only removed 47%. The kinetic of copper binding indicated that it is a rapid process and about 70–80% of copper ions removed from the solution in 10 min. The percent of copper removed decreased with increase in metal concentration. The increase in percent of adsorption with adsorbent dose could be due to the increase in surface area and availability of more active sites. The treated poplar sawdust showed maximum adsorption capacity of 13.945 mg g-1 against 5.432 mg g-1 for untreated sawdust and the process fitted Langmuir isotherm model. The maximum adsorption capacity for sulfuric acid treated poplar sawdust is higher than to the value recorded by NaOH treated poplar sawdust reported by Sciban et al. [54].
Chubar et al. [56] studied the performance of various kinds of chemically treated cork powder obtained from cork oak tree for the removal of Cu. Treatment of cork powder with salts such as NaCl and CaCl2 caused the conversion of active binding sites from the H+ form to Na+ and Ca2+ form. The salt-modified cork powder showed greater adsorption capacity than the unmodified cork especially at higher heavy metal concentrations. It was also noted that the Na+ form cork recorded a higher adsorption capacity value than the Ca2+ form. This may be due to the different charge of cations whereby the interaction of cork powder binding sites with divalent calcium ions is stronger than the monovalent sodium ions thereby hindering the biosorption reaction of copper. Treatment of cork powder with an alkaline solution (NaOH) at high temperature increased the sorption capacity toward heavy metals by about 33%. A high concentration of NaOH however causes a decrease in the adsorption capacity.
Corncobs
Khan and Wahab [57] studied the adsorption of copper by concentrated sulfuric acid treated corncobs. It was reported that upon treatment of corncobs with sulfuric acid and heated at 1500C, the pHzpc of the adsorbent reduced from 5.2 (untreated) to 2.7 (treated), and the functional groups present in the adsorbent were mainly –OH, –COOH and –COO-. The maximum adsorption capacity obtained from Langmuir isotherm was 31.45 mg g-1. As a result of low competing effect of protons for the adsorption sites, adsorption was more favoured at higher pH value (4.5). Effect of interfering ions such as Zn(II), Pb(II) and Ca(II) was also studied. It was noticed that copper removal efficiency was reduced by 53%, 27% and 19% in the presence of Pb(II), Ca(II) and Zn(II), respectively. Regeneration study indicated that sulfuric acid treated corncobs can be regenerated by acidified hydrogen peroxide solution and as much as 90% copper could be recovered.
The study on oxidation of corncob by citric acid and nitric acid was carried out by Leyva-Ramos et al. [58]. Modification increased the surface area of the adsorbent. An increase in the amount of oxygen found in corncob was due to more oxygenated groups that were introduced on the adsorbent surface after oxidation. After oxidation, a higher proportion of acidic sites (carboxylic, phenolic and lactonic) were detected. This resulted in a reduction in the pHZPC value. It was observed that the adsorption capacities for citric acid and nitric acid oxidized corncob were much higher than unmodified corncob.
Cassava waste
Cassava waste consists of ligands such as hydroxyl, sulfur, cyano and amino which could bind heavy metal ions. Abia et al. [59] carried out an experiment to determine the optimal concentration of thioglycollic acid (HSCH2COOH) for the removal of Cu(II) ions by cassava waste. It was noticed that the adsorption capacity of the cassava waste was improved as the concentration of thioglycollic acid was increased from 0.5 to 1.0 M due to the increase in sulfhydryl groups, –SH. Adsorption was reported to take place on the cell wall of the biomass. Optimum adsorption of the heavy metal was achieved in less than 30 minutes. However, the authors did not conduct a detail experiment on the kinetic model of adsorption.
Peanut
Activated carbons derived from peanut husk have been successfully employed for the removal of heavy metals from aqueous solutions [60]. The use of peanut hull carbon (PHC) for the adsorption of Cu (II) from wastewater was studied by Periasamy and Namasivayam [61]; their comparative study of commercial granular activated carbon (GAC) showed that the adsorption capacity of PHC was 18 times larger than that of GAC. The authors studied the adsorption behavior of peanut husk charcoal with respect to Cu(II) in order to consider its application to the purification of metal finishing wastewater. The batch method was employed; parameters such as pH, contact time, adsorbent dose and metal concentration were studied at an ambient temperature 27± 2°C. The influence of the pH of the metal ion solutions on the uptake levels of the metal ions by the adsorbents used were carried out between pH 4 and pH 11. The optimum pH for copper was 6. An equilibrium time of 2 h was required for the adsorption of Cu (II) onto the adsorbent. Adsorption parameters were determined using both Langmuir and Freundlich isotherms, but the experimental data were better fitted to the Langmuir equation than to Freundlich equation. The authors concluded that peanut husk charcoal is a low-cost adsorbent which holds potential to remove Cu(II) from industrial wastewater.
Loquat (Eriobotrya japonica) leaves
Akl and Nidà [62] studied the potential of Modified Loquat Leaves (MLL) for the removal of Cu (II) ions from aqueous solutions and electroplating wastewater. Their study was dependent on biosorption process such as pH, initial metal ions concentrations, biosorbent dose, contact time, and temperature. The equilibrium data were analyzed using Langmuir, Freundlich and Temkin isotherms. The authors also determined the characteristic parameters for each isotherm and related correlation coefficients, R2. The Langmuir biosorption isotherms provided the best correlation for the biosorption of Cu (II) onto MLL. The maximum monolayer adsorption capacity of MLL was found to be 33.33 mg of Cu (II)/g of MLL. The kinetic studies followed pseudo second-order equation. The data obtained in the determination of the optimal pH for the effective biosorption of Cu (II) onto MLL revealed the presence of many active sites on MLL surface available to bind with Cu(II) ions at low concentration. The authors concluded that since the MLL is an easily, locally available, low-cost adsorbent and has a considerable high biosorption capacity, it may be treated as an alternative adsorbent for treatment of wastewater containing copper (II) ions. Their results also indicated that MLL biosorbent can be used repeatedly in Cu (II) biosorption.
Adsorption capacities of copper (II) ions on various low-cost adsorbents are presented in Table 6 below:
| Low-cost adsorbents | Adsorption capacities (mg/g) | References |
|---|---|---|
| Diatomite | 27.55 | 63 |
| Modified diatomite | 55.56 | 63 |
| Sawdust | 1.79 | 64 |
| Peat | 12.07 | 15 |
| Low-rank Turkish coals | 1.62 | 65 |
| Tea-industry waste | 8.64 | 66 |
| Lentil Shell | 9.59 | 27 |
| Wheat shell | 17.42 | 27 |
| Rice shell | 2.95 | 27 |
| Grape stalk wastes | 0.78 | 67 |
| Citric acid modified soybean hulls | 154.90 | 68 |
| Cassava waste | 56.82 | 69 |
| Peanut hulls pellets | 9.11 | 70 |
| Banana peel | 4.75 | 34 |
| Orange peel | 3.65 | 34 |
| Cocoa shell | 2.87 | 71 |
| Teak leaves powder | 13.14 | 72 |
| Wheat shell | 10.87 | 73 |
| Herbaceous peat | 4.84 | 74 |
| Peanut hulls | 10.17 | 75 |
| Tobacco dust | 36 | 76 |
| Modified rice husk | 29 | 77 |
| Raw pomegranate peel | 1.3185 | 78 |
| Unmodified jute | 4.23 | 79 |
| Dye loaded jute | 8.40 | 79 |
| Oxidized jute | 7.73 | 79 |
| RSAC | 5.729 | 80 |
| RSD | 8.1103 | 51 |
| PMSD4 | 2.6631 | 51 |
| SMSD3 | 12.484 | 51 |
| Cotton boll | 11.40 | 81 |
| Sour orange waste | 23.47 | 82 |
| Sour orange waste (NaOH treated) | 52.08 | 82 |
| Soybean hulls | 154.9 | 83 |
| Dehydrated wheat bran | 51.51 | 84 |
| Modified orange peel | 289 | 40 |
| Rice husk | 31.85 | 85 |
| Formaldehyde modified sawdust (poplar tree) | 13.95 | 55 |
| NaOH modified sawdust (poplar tree) | 6.92 | 54 |
| Saw dust (fir tree) | 12.70 | 54 |
| HCl modified saw dust (oak tree) | 3.60 | 86 |
| Reactive orange 13 modifiedsawdust | 8.07 | 87 |
| Peanut husk | 10.15 | 88 |
| Cassava tuber bark waste | 90.90 | 89 |
| HCl modified Indian barks Sal Mango Jack fruit |
51.40 42.60 17.40 |
50 |
| Reactive orange modified jute fibre | 8.40 | 90 |
| H202 modified jute fibre | 7.73 | 90 |
| Unmodified jute fibre | 4.23 | 90 |
| Banana pith | 13.46 | 91 |
| Carrot residues | 32.74 | 92 |
| Sugarbeet pulp | 0.15 | 93 |
| Groundnut shells | 7.60 | 87 |
| Peanut hull | 8.00 | 94 |
| Corn cobs | 7.62 | 94 |
| Corn starch | 8.57 | 94 |
| Pine bark | 9.46 | 94 |
Table 6: Adsorption capacities of copper (II) ions on various low-cost adsorbents.
Conclusion and future challenges
Little efforts seem to have been made to carry out a cost comparison between activated carbon and various non-conventional adsorbents [95]. This aspect needs to be investigated further in order to promote large-scale use of non-conventional adsorbents. In spite of the scarcity of consistent cost information, the widespread use of low-cost adsorbents in industries for wastewater treatment applications nowadays are strongly recommended due to their local availability, technical feasibility, engineering applicability, and cost effectiveness. If low-cost adsorbents perform well in removing heavy metals, they can be adopted and widely used in industries not only to minimize cost inefficiency, but also improve profitability.
In addition, if the adsorbents reviewed in this article are found highly efficient for the removal of Cu(II), both the industries, living organisms and the surrounding environment will benefit from the decrease or elimination of the toxicity associated with the metal. Also, these adsorbents can be utilized in the design of adsorption columns. Thus, the use of low-cost adsorbents may contribute to the sustainability of the surrounding environment. Spectroscopic analyses involving Fourier transform infrared (FTIR), energy dispersive spectroscopy (EDS), X-ray absorption near edge structure (XANES) spectroscopy and extended X-ray absorption fine structure (EXAFS) spectroscopy are also important in order to have a better understanding on the mechanism of metal adsorption on modified plant wastes. Undoubtedly, low-cost adsorbents offer a lot of promising benefits for effective removal of Cu(II) from aqueous systems from wastewaters.
Acknowledgement
The corresponding author acknowledges the support obtained from The World Academy of Science (TWAS) in form of grant; Research Grant number:11-249 RG/CHE/AF/AC_1_UNESCO FR: 3240262674.
References
- The Council of the European Communities, Directive 82/176/EEC, Off. J. Eur. Commun., 1982, No. L 81/29.
- Papandreou A, Stournaras CJ, Panias D (2007) Copper and cadmium adsorption on pellets made from fired coal fly ash. J Hazard Mater 148: 538-547.
- Pentari D, Perdikatsis V, Katsimicha D, Kanaki A (2009) Sorption properties of low calorific value Greek lignites: removal of lead, cadmium, zinc and copper ions from aqueous solutions. J Hazard Mater 168: 1017-1021.
- Kurniawan TA, Chan GY, Lo WH, Babel S (2006) Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 366: 409-426.
- Esalah OJ, Weber ME, Vera JH, (2000) Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium di-(n-octyl) phosphinate, Can. J. Chem. Eng, 78: 948–954.
- Zouboulis AI, Matis K A, Lanara BG, Neskovic C L, (1997) Removal of cadmium from dilute solutions by hydroxy apatite. II. floatation studies, Sep. Sci. Technol., 32:1755–1767.
- Ho YS, Ng JCY, McKay G, (2001) Removal of lead (II) from effluents by sorption on peat using second-order kinetics, Sep. Sci. Technol., 36:241–261.
- Hall C, Wales DS, Keane M A, (2001) Copper removal from aqueous systems: biosorption by pseudomonas syringae, Sep. Sci. Technol., 36:223–240.
- Canet L, Ilpide M, Seat P, (2002) Efficient facilitated transport of lead, cadmium, zinc and silver across a flat sheet-supported liquid membrane mediaed by lasalocid A, Sep. Sci. Technol., 37:1851–1860.
- Ahmed S, Khalid N, Dand M, (2002) Adsorption studies of lead minerals from aqueous media, Sep. Sci. Technol., 37:343–362.
- Buerge-Weirich D, Hari R, Xue H, Behra P, Sigg L (2002) Adsorption of Cu, Cd, and Ni on goethite in the presence of natural groundwater ligands. Environ SciTechnol 36: 328-336.
- Ravindran V, Stevens MR, Badriyha BN, Pirbazari M, (1999) Modeling the sorption of toxic metals on chelant-impregnated adsorbent, AICHE J., 45:1135–1146.
- Toles CA, Marshall WE (2002) Copper ion removal by almond shell carbons and commercial carbons: batch and column studies, Sep. Sci. Technol., 37:2369–2383.
- Kurniawan TA, Chan GY, Lo WH, Babel S (2006) Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 366: 409-426.
- Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97: 219-243.
- O'Donohue J, Reid M, Varghese A, Portmann B, Williams R (1999) A case of adult chronic copper self-intoxication resulting in cirrhosis. Eur J Med Res 4: 252.
- Van Genderen EJ, Ryan AC, Tomasso JR, Klaine SJ (2005) Evaluation of acute copper toxicity to larval fathead minnows (Pimephalespromelas) in soft surface waters. Environ ToxicolChem 24: 408-414.
- Ezeonyejiaku CD, Obiakor, MO, Ezenwelu CO (2011) Toxicity of copper sulphate and behaviourallocomotor response of tilapia (Oreochromisniloticus) and catfish (Clariasgariepinus) species. Online J. Anim. Feed Res. 1: 130–134.
- Flemming CA, Trevors J T (1989) Copper toxicity and chemistry in the environment: a review. Water, Air, Soil Pollut. 44: 143–158.
- Jafar A. Ahamed and A. Shajudha Begum (2012): Adsorption of copper from aqueous solution using low-cost adsorbent. Arch. Appl. Sci. Res., 4 (3): 1532-1539.
- Oyedeji O. Abdulrasaq and Osinfade G. Basiru, (2010): Removal of copper (II), iron (II) and lead (II) ions from mono-component simulated water effluent by adsorption on coconut husk. Afri. J. Environ. Sci. Technol. 4 (6), 382-387.
- P. C. Okafor, P. U. Okon, E. F. Daniel and E. E. Ebenso, (2012): Adsorption Capacity of Coconut (Cocosnucifera L.) Shell for Lead, Copper, Cadmium and Arsenic from Aqueous Solutions, Int. J. Electrochem. Sci., 7, 12354 – 12369.
- WiwidPranata Putra, Azlan Kamari, SitiNajiahMohdYusoff, CheFauziahIshak, Azmi Mohamed, NorhayatiHashim, IllyasMd Isa, (2014): Biosorption of Cu(II), Pb(II) and Zn(II) Ions from Aqueous Solutions Using Selected Waste Materials: Adsorption and Characterisation Studies, Journal of Encapsulation and Adsorption Sciences, 4, 25-35.
- JimohOladejoTijani, MurainaMusah and Izuelumba Blessing (2011): Sorption of lead (II) and copper (II) ions from aqueous solution byacid modified and unmodified (Eriobotyra japonica) leaves. J. Emerg. Trends Eng. Appl. Sci. (JETEAS) 2 (5): 734-740.
- Seeram N., R. Lee, M. Hardy and D. Heber (2005): Rapid large scale purification of ellagitannius from pomegranate husk, a by-product of the commercial juice. Separ. Purif. Technol., 4, 49-55.
- Ben C. Naar, N. Ayed and M. Metche (1996): Removal of lead (II) and copper (II) from aqueous solution by pomegranate peel as a new adsorbent. Desalination, 255, 165-174.
- El-Ashtoukhy E.S.Z, N.K. Amin, O. Abdelwahab (2008): Comparison of copper adsorption from aqueous solution by modified and unmodified Heveabrasiliensis saw dust. Desalination, 255, 165-174.
- Aydin H, Bulut Y, Yerlikaya C (2008) Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J Environ Manage 87: 37-45.
- Nasehir Khan E M Yahayaa, Ismail Abustana, MuhamadFaizalPakir Mohamed Latiffa, Olugbenga Solomon Bellob, MohdAzmierAhmadb, (2011): Fixed-bed Column Study for Cu (II) Removal from Aqueous Solutions using Rice Husk based Activated Carbon , Int. J. Eng. Technol., 1, 186-190.
- FAO (Food and Agriculture Organization of the United Nations), 2003.
- Zhu B, Fan T, Zhang D (2008) Adsorption of copper ions from aqueous solution by citric acid modified soybean straw. J Hazard Mater 153: 300-308.
- Supaporn Douglas, SuwassaPongamphai, SupaneeLerdtrailuck, SiriratPonin, SujitraPolchai, AcharapornKaewchana and BudsarinOsataworanun, (2006): Adsorption of Copper (II) Ion from Aqueous Solution Using Soybean Hulls, The 2nd Joint International Conference on “Sustainable Energy and Environment (SEE 2006)”
- Ajmal M, Rao RA, Ahmad R, Ahmad J (2000) Adsorption studies on Citrus reticulata (fruit peel of orange): removal and recovery of Ni(II) from electroplating wastewater. J Hazard Mater 79: 117-131.
- Annadural G, Juang RS, Lee DJ (2003) Adsorption of heavy metals from water using banana and orange peels. Water SciTechnol 47: 185-190.
- Khormaei M, Nasernejad B, Edrisi M, Eslamzadeh T (2007) Copper biosorption from aqueous solutions by sour orange residue. J Hazard Mater 149: 269-274.
- Ghimire KN, Inoue K, Yamaguchi H, Makino K, Miyajima T (2003) Adsorptive separation of arsenate and arsenite anions from aqueous medium by using orange waste. Water Res 37: 4945-4953.
- Dhakal R.P., K.N. Ghimire, K. Inoue, (2005): Adsorptive separation of heavy metals from an aquatic environment using orange waste, Hydrometallurgy, 79, 182–190.
- Li X., Y. Tang, Z. Xuan, Y. Liu, F. Luo, (2007): Study on the preparation of orange peel cellulose adsorbents and adsorption of Cd2+ from aqueous solution, Sep. Purif. Technol. 55: 69–75.
- Biswas BK, Inoue K, Ghimire KN, Ohta S, Harada H, et al. (2007) The adsorption of phosphate from an aquatic environment using metal-loaded orange waste. J Colloid Interface Sci 312: 214-223.
- Feng N, Guo X, Liang S (2009) Adsorption study of copper (II) by chemically modified orange peel. J Hazard Mater 164: 1286-1292.
- Liang S, Guo X, Feng N, Tian Q (2010) Isotherms, kinetics and thermodynamic studies of adsorption of Cu2+ from aqueous solutions by Mg2+/K+ type orange peel adsorbents. J Hazard Mater 174: 756-762.
- Vazquez G., E. Fontenla, J. Santos, M.S. Freire, J. Gonzalez-Alvarez, G. Antorrena, (2008): Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts, Ind. Crop. Prod. 28, 279–285.
- Vazquez G., J. Gonzalez-Alvarez, J. Santos, M.S. Freire, G. Antorrena, (2009): Evaluation of potential applications for chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts, Ind. Crop. Prod. 29, 364–370.
- Gonzalo Vasquez, Marcos Calvo, M. Sonia Freira, Julia Gonzalez-Alvarez, GervasioAntorrena (2009): Chestnut shell as heavy metal adsorbent: Optimization study of lead, copper and zinc cations removal. 172, 1402-1414.
- Yao ZY, Qi JH, Wang LH (2010) Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell. J Hazard Mater 174: 137-143.
- Karnitz O Jr, Gurgel LV, de Melo JC, Botaro VR, Melo TM, et al. (2007) Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse. BioresourTechnol 98: 1291-1297.
- Shukla SR, Pai RS (2005) Adsorption of Cu(II), Ni(II) and Zn(II) on modified jute fibres. BioresourTechnol 96: 1430-1438.
- Torres JD, Faria EA, Prado AG (2006) Thermodynamic studies of the interaction at the solid/liquid interface between metal ions and cellulose modified with ethylenediamine. J Hazard Mater 129: 239-243.
- Ho YS, Ofomaja AE (2006) Kinetic studies of copper ion adsorption on palm kernel fibre. J Hazard Mater 137: 1796-1802.
- Reddy, B.R., Mirghaffari, N., Gaballah, I., (1997): Removal and recycling of copper from aqueous solutions using treated Indian barks. Resour. Conserv. Recycl. 2, 227–245.
- M. Helen Kalavathy, Lima Rose Miranda, (2010): Comparison of copper adsorption from aqueous solution using modified and unmodified Heveabrasiliensis saw dust, Desalination 255, 165–174.
- Aydin H, Bulut Y, Yerlikaya C (2008) Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J Environ Manage 87: 37-45.
- Ozer, D. Ozer, A. Ozer, (2004): The adsorption of copper (II) ions on to dehydrated wheat bran (DWB): determination of the equilibrium and thermodynamic parameters, Process Biochem., 39, 2183–2191.
- Sćiban M, Klasnja M, Skrbić B (2006) Modified softwood sawdust as adsorbent of heavy metal ions from water. J Hazard Mater 136: 266-271.
- Acar FN, Eren Z (2006) Removal of Cu(II) ions by activated poplar sawdust (Samsun clone) from aqueous solutions. J Hazard Mater 137: 909-914.
- Chubar, N., Calvalho, J.R., Correia, M.J.N., (2004): Heavy metals biosorption on cork biomass: effect of the pre-treatment. Colloids Surf. A, 238, 51–58.
- Nasiruddin Khan M, FarooqWahab M (2007) Characterization of chemically modified corncobs and its application in the removal of metal ions from aqueous solution. J Hazard Mater 141: 237-244.
- Leyva-Ramos, R., Bernal-Jacome, L.A., Acosta-Rodriguez, I., (2005): Adsorption of cadmium(II) from aqueous solution on natural and oxidized corncob. Sep. Purif. Technol., 45, 41–49.
- Abia AA, Horsfall M Jr, Didi O (2003) The use of chemically modified and unmodified cassava waste for the removal of Cd, Cu and Zn ions from aqueous solution. BioresourTechnol 90: 345-348.
- Ricordel S, Taha S, Cisse I, Dorange G. (2001): Heavy metals removal by adsorption onto peanut husks carbon: Characterization, kinetic study and modeling, Sep Purif Technol., 24(3):389–401.
- Periasamy K, Namasivayam C. (1996): Removal of copper (II) by adsorption onto peanut hull carbon from water and copper plating industry wastewater, Chemosphere, 32(4): 769–89.
- Akl M. Awwad and Nidà M. Salem, (2012): Biosorption of copper(II) and lead(II) ions from aqueous solutions by modified loquat (Eriobotrya japonica) leaves (MLL), Journal of Chemical Engineering and Materials Science, 3(1), 7-17.
- Feng D, Van Deventer JSJ, (2004): Aldrich C. Removal of pollutants from acid mine wastewater using metallurgical by-product slags. Sep. Purif. Technol. 40 (1): 61–7.
- Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption - removal of copper. J Hazard Mater 80: 33-42.
- Karabulut, S., Karabakan, A., Denizli, A., Yu’ru’m, Y., (2000): Batch removal of copper(II) and zinc(II) from aqueous solutions with low-rank Turkish coals. Sep. Purif. Technol. 18, 177–184.
- Cay, S., Uyanik, A., Ozis-ik, A., (2004): Single and binary component adsorption of copper (II) and cadmium (II) from aqueous solutions using tea-industry waste. Sep. Purif. Technol. 38 (3), 273–280.
- Villaescusa I, Fiol N, Martínez M, Miralles N, Poch J, et al. (2004) Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res 38: 992-1002.
- Marshall W.E, L.H. Wartelle, D.E. Boler, M.M. Johns, C.A. Toles, (1999): Enhanced metal adsorption by soybean hulls modified with citric acid,BioresourTechnol.6963–268.
- Abia AA, Horsfall M Jr, Didi O (2003) The use of chemically modified and unmodified cassava waste for the removal of Cd, Cu and Zn ions from aqueous solution. BioresourTechnol 90: 345-348.
- Brown P., I.A. Jefcoat, D. Parrish, S. Gill, S. Graham, (2000): Evaluation of the adsorptive capacity of peanut hull pellets for heavy metals in solution, Adv. Environ. Res. 4, 19–29.
- Meunier N, Laroulandie J, Blais JF, Tyagi RD (2003) Cocoa shells for heavy metal removal from acidic solutions. BioresourTechnol 90: 255-263.
- King P, Srinivas P, Kumar YP, Prasad VS (2006) Sorption of copper(II) ion from aqueous solution by Tectonagrandisl.f. (teak leaves powder). J Hazard Mater 136: 560-566.
- N. Basci, E. Kocadagistan, B. Kocadagistan, (2004): Desalination, 164, 135–140.
- Gündoğan R, Acemioğlu B, Alma MH (2004) Copper (II) adsorption from aqueous solution by herbaceous peat. J Colloid Interface Sci 269: 303-309.
- P. Brown, I.A. Jefcoat, D. Parrish, S. Gill, E. Graham, (2000): Adv. Environ. Res. 4, 19–29.
- Qi BC, Aldrich C (2008) Biosorption of heavy metals from aqueous solutions with tobacco dust. BioresourTechnol 99: 5595-5601.
- K.K. Wong, C.K. Lee, K.S. Low, M.J. Haron, (2003): Process Biochem. 4, 437–445.
- E.-S.Z. El-Ashtoukhy, N.K. Amin, O. Abdelwahab, (2008): Desalination, 223, 162–173.
- Shukla SR, Pai RS (2005) Adsorption of Cu(II), Ni(II) and Zn(II) on modified jute fibres. BioresourTechnol 96: 1430-1438.
- Kalavathy MH, Karthikeyan T, Rajgopal S, Miranda LR (2005) Kinetic and isotherm studies of Cu(II) adsorption onto H3PO4-activated rubber wood sawdust. J Colloid Interface Sci 292: 354-362.
- Ozsoy HD, Kumbur H (2006) Adsorption of Cu(II) ions on cotton boll. J Hazard Mater 136: 911-916.
- Khormaei M, Nasernejad B, Edrisi M, Eslamzadeh T (2007) Copper biosorption from aqueous solutions by sour orange residue. J Hazard Mater 149: 269-274.
- W.E. Marshall, L.H. Wartelle, D.E. Boler, M.M. Johns, C.A. Toles, (1999): Enhanced metal adsorption by soybean hulls modified with citric acid, Bioresour. Technol. 69, 263–268.
- Ozer, D. Ozer, A. Ozer, (2004): The adsorption of copper (II) ions on to dehydrated wheat bran (DWB): determination of the equilibrium and thermodynamic parameters, Process Biochem., 39, 2183–2191.
- Wong, K.K., Lee, C.K., Low, K.S., Haron, M.J., (2003b): Removal of Cu and Pb from electroplating wastewater using tartaric acid modified rice husk, Process Biochem. 39, 437–445.
- Argun ME, Dursun S, Ozdemir C, Karatas M (2007) Heavy metal adsorption by modified oak sawdust: thermodynamics and kinetics. J Hazard Mater 141: 77-85.
- Shukla SR, Pai RS (2005) Adsorption of Cu(II), Ni(II) and Zn(II) on modified jute fibres. BioresourTechnol 96: 1430-1438.
- Li Q, Zhai J, Zhang W, Wang M, Zhou J (2007) Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. J Hazard Mater 141: 163-167.
- Horsfall M Jr, Abia AA, Spiff AI (2006) Kinetic studies on the adsorption of Cd2+, Cu2+ and Zn2+ ions from aqueous solutions by cassava (ManihotsculentaCranz) tuber bark waste. BioresourTechnol 97: 283-291.
- Shukla SR, Pai RS (2005) Adsorption of Cu(II), Ni(II) and Zn(II) on modified jute fibres. BioresourTechnol 96: 1430-1438.
- Low, K.S., Lee, C.K., Leo, A.C., (1995): Removal of metals from electroplating wastes using banana pith, Bioresour. Technol., 51:227–231.
- Nasernejad B, Zadeh TE, Pour BB, Bygi ME, Zamani A (2005) Comparison for biosorption modeling of heavy metals (Cr(III), Cu(II), Zn(II)) adsorption from wastewater by carrot residues, Process Biochem., 40:1319–1322.
- Pehlivan E, Cetin S, Yanik BH (2006) Equilibrium studies for the sorption of zinc and copper from aqueous solutions using sugar beet pulp and fly ash. J Hazard Mater 135: 193-199.
- Reddad Z, Gerente C, Andres Y, Le Cloirec P (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ SciTechnol 36: 2067-2073.
- Nasar MM, Awwad and Nida M. Salem, (2012) Biosorption of copper (II) and lead (II) ions from aqueous solutions by modified loquat (Eriobotyra japonica) leaves. J. Eng. Mater. Sci. 3: 7-17.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 20068
- [From(publication date):
April-2015 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 14951
- PDF downloads : 5117