Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Review Article Open Access
An Overview of Image-Guided Radiotherapy (IGRT)
| Krauss DJ* | |
| Oakland University William Beaumont School of Medicine, Department of Radiation Oncology, Royal Oak, MI 48073, USA | |
| Corresponding Author : | Krauss DJ Oakland University William Beaumont School of Medicine Department of Radiation Oncology Royal Oak, MI 48073, USA Tel: 248-551-3576 Fax: 248-551-0089 E-mail: dkrauss@beaumont.edu |
| Received May 12, 2014; Accepted June 19, 2014; Published June 26, 2014 | |
| Citation: Krauss DJ (2014) An Overview of Image-Guided Radiotherapy (IGRT). OMICS J Radiol S1:002. doi: 10.4172/2167-7964.S1-002 | |
| Copyright: © 2014 Krauss DJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
Image-guided radiotherapy (IGRT) has undergone a continuous evolution of refinements leading up to the sophisticated techniques currently implemented as standard treatment, in radiation oncology clinics. This paper aims to summarize, in a general fashion, the key advances that have resulted in the ability of radiation oncologists to deliver increased radiotherapy doses to tumors, reduce doses to normal tissues, limit treatment related toxicity/complications, and increase patient convenience.
| Keywords |
| Radiotherapy; CT imaging; Prostate cancer |
| Introduction |
| Radiotherapy (RT) in the modern era has always been image guided. Prior to the 1990’s, the standard, available imaging incorporated into RT planning and targeting consisted of 2-dimensional plain radiographs of the treated region of interest. The anatomic position of the target regions as well as critical normal tissues was inferred from bony landmarks and whatever additional procedures could be used to improve targeting confidence: e.g. administration of oral contrast for delineation of upper gastrointestinal structures, enemas or rectal markers for lower GI tissue delineation, urinary contrast/catheter placement for genitourinary delineation, etc. The inability of such techniques to precisely define soft tissue anatomy resulted in high degrees of targeting uncertainty and subsequent large margins around any area of interest requiring treatment. The large volumes of normal tissues receiving the full prescription dose frequently limited the ability to deliver tumoricidal doses and were associated with high rates of treatment failure, toxicity, and complications. |
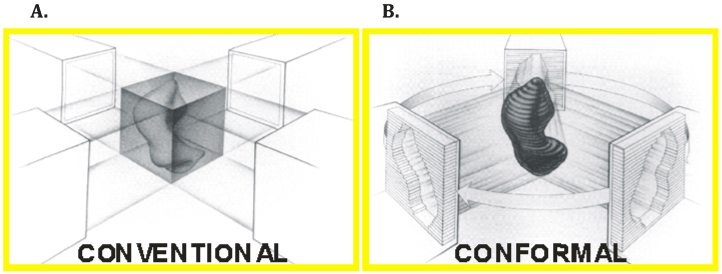
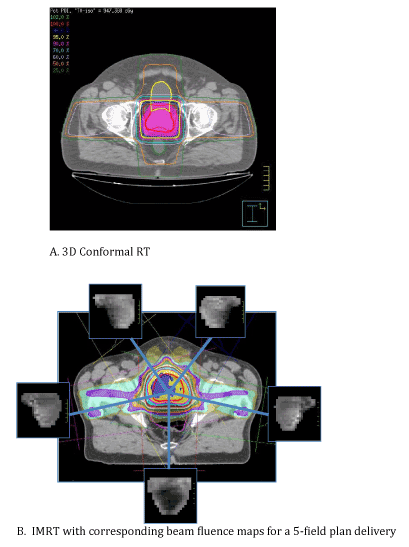
| The first major advance leading to what is regarded as modern image guided therapy was the incorporation of 3-dimensional treatment planning simulation. This generally refers to the incorporation of diagnostic-quality CT imaging as a direct part of the initial patient simulation process, allowing for accurate delineation of both target and normal critical soft tissue structures with the patient positioned and immobilized as they would be for daily RT treatment visits. Beams could now be more conformally shaped around targets and normal critical structures, and greater confidence in dose estimation with respect to both target coverage and risks to critical normal structures now existed (Figure 1). In no disease site has this concept been better illustrated than in prostate cancer where multiple prospective studies using 3D conformal RT have demonstrated improvement in clinical outcomes with the ability to safely escalate radiotherapy dose (Table 1) [1-3]. |
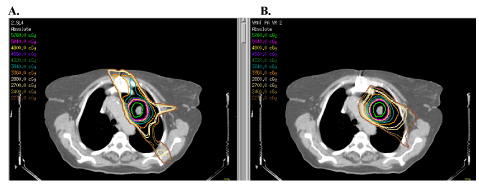
| The increased conformity afforded by 3D treatment planning was taken a step further with the routine implementation of intensity modulated radiotherapy (IMRT). IMRT took advantage of multileaf collimation of individual RT beams in that numerous beam segments could now be generated for any given beam angle. The IMRT process involves precise, 3D delineation of all relevant target volumes and critical structures based on the initial planning CT scan. A series of priority weightings are then applied based on the clinician’s specific goals of target coverage vs. normal tissue sparing. An inverse optimization algorithm is then carried out and a series of computer iterations results in an optimal solution and treatment plan. The end result (Figure 2), clinically, has been tightly conformal dose gradients and an ability to essentially “bend” isodose curves around critical structures in ways not possible with 3D conformal RT. Refinements in IMRT technique have led to techniques such as volumetric modulated arc therapy (VMAT) which has been shown in the context of lung stereotactic body radiotherapy (SBRT) to both decrease treatment time (by>50%), improve target dose coverage, and reduce total body exposure to ionizing radiation (Figure 3) [4]. It quickly became clear, however, that to fully exploit the dosimetric advantages of intensity modulation, confidence in targeting had to be maximized. |
| Off-line Image Guidance |
| Off-line image-guided radiotherapy (IGRT) refers to imaging modalities that enhance targeting accuracy without the patient physically present on the treatment table. In its most basic form, this begins with a 3D CT simulation based on which initial target volumes and critical structures may be identified. This delineation is greatly enhanced by the incorporation of other advanced imaging modalities based on the disease site being treated. Notable examples include the evolving role of MRI for prostate cancer [5] and its standard role in the delineation of primary brain tumors. Likewise, metabolic imaging in the form of positron emission tomography (PET) scans, most notably in the context of definitive RT for lung cancers, has been shown to enhance the accuracy of primary tumor delineation (differentiating from synchronous intrapulmonary pathology such as atelectasis) as well as identification of involved hilar or mediastinal lymph nodes [6,7]. |
| The most detailed and sophisticated imaging, however, cannot account for variability in positions of targets and critical structures due to physiologic organ motion and technical setup variability. These factors can only be accounted for when a time factor is considered. Techniques have evolved that this may be accomplished in the off-line setting, the most notable disease site examples for which these have been employed are lung and prostate cancer [8,9]. |
| Lung Cancer–Off-Line Image Guidance |
| Tumor motion due to respiration needs to be considered every time radiotherapy is used in the management of intrathoracic tumors. 4D CT scans [8] are now routinely employed at the time of RT treatment planning simulation for primary lung cancers. This technique involves the acquisition of CT chest imaging at coordinated phases of the respiratory cycle and allows for precise quantification of the direction and amplitude of target motion. This allows for the generation of a precise integrated gross target volume to which no additional margin for tumor motion needs to be considered. Additional expansions need only consider microscopic extension of the tumor and setup inaccuracy. The end result of this process is the generation of a planning target volume to which the definitive dose prescription will be delivered. If constructed appropriately, the gross tumor plus any microscopic extension of disease should never lie outside this volume. |
| Prostate Cancer–Off-Line Image Guidance |
| It has long been recognized that the prostate is not a static target that its position in the pelvis is subject to variable anatomic shifts based most notably on the degree of rectal filling/emptying with stool and bowel gas. Historically, this has been managed using off-line adaptive techniques that have been employed and described extensively for a number of years now. This involves the integration of multiple helical CT scans acquired early in the treatment course and subsequent quantification of shifts in the prostate position due to both organ motion and setup variability. In the off-line setting, the alternative approach would be to simply apply a margin around the prostate ± seminal vesicles wide enough to account for any organ motion and setup uncertainty that may occur across the entire population. In implementing this approach, it was possible to generate patient-specific planning target volume margins that were shown, in the vast majority of cases, to reduce the volume of bladder and rectum being treated [9,10]. While not widely employed, the fact that adaptive radiotherapy for prostate cancer has been implemented since the late 1990’s has afforded the opportunity to illustrate the long-term clinical value of image guidance in terms of ability to escalate RT dose, decrease normal tissue toxicity, and improve disease control [11]. |
| On-line Image Guidance |
| Adjunct technology built into contemporary linear accelerators now routinely includes cone beam CT, a technique through which volumetric soft tissue image acquisition can be achieved with the patient precisely positioned and immobilized on the linear accelerator treatment table. This now allows for precise quantification and correction (in real-time) of interfraction variability due to organ motion and patient setup inaccuracy. While patient positioning may certainly be manually corrected by radiotherapy technical staff, accelerator technology has evolved to the point that robotic table shifts and rotations may be automatically implemented based on detected variations in 3-dimensional target location. That is, no manual correction is required and adjustments can be made remotely from the treatment console. |
| To optimize the utilization of cone beam CT, however, there is one additional variable to be considered, and that is intrafraction motion. This may be minimized using immobilization maneuvers, examples of which would include abdominal compression for thoracic tumors [12] or placement of a rectal balloon for prostate cancer [13]. Additional measures would include real-time target monitoring, which may be achieved through multiple modalities including ultrasound, infrared tracking beacons (Calypso®), [14] or continuous kilo voltage imaging of implanted fiducial markers associated with technologies such as CyberKnife®. With these measures implemented, the monitoring system is typically linked to the linear accelerator such that movement of the target beyond a pre-set tolerance will trigger a shutoff of the treatment beam. |
| In summary, modern image-guided radiotherapy has been the culmination of a series of innovations in treatment delivery that have sequentially reduced the sources of uncertainty associated with three variables: initial delineation of tumor and critical structure extent/ anatomy; target motion; and patient setup inconsistencies. Future directions to improve RT dose delivery will likely include dynamic consideration of tumor response and anatomic changes occurring on a patient-by-patient basis throughout a treatment course. While accounting for changes in general anatomy due to factors such as weight loss will only enhance the accuracy of RT delivery, adapting treatment fields/dose calculations to changes in target volumes due to tumor response will require prospective study to define the optimal timing at which to implement changes. Likewise, safe reductions in the sizes of target volumes will need to be defined specifically for different disease sites along with identifying the optimal imaging modalities required to define them. |
References |
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Post your comment
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 14472
- [From(publication date):
specialissue-2014 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 9889
- PDF downloads : 4583
