An Overview of Energy Storage Development
Received: 11-Oct-2017 / Accepted Date: 11-Oct-2017 / Published Date: 18-Oct-2017
Introduction
Over the generations human society is depending on fossil fuel energy sources leading to the emission of CO2 into atmosphere. It is at most important and urgent need for the replacement of fossil fuel energy sources with renewable and sustainable energy sources which can leave low or no carbon foot prints. In this regard energy storage place major role after producing energy using various technologies like solar, wind, tidal, etc., before distributing to the customer. On other hand energy storage devices like rechargeable batteries became part of life in the modern society to power portable devices like phones, laptops, etc. Starting from Frog leg experiment by Luigi Galvani in 1780’s various electrochemical techniques and materials were developed to realise the capability of these techniques and materials in developing electrochemical devices. After observation of possible Ag+ ion transport in Ag2S solids by Faraday lead scientific interest in energy storage devices.
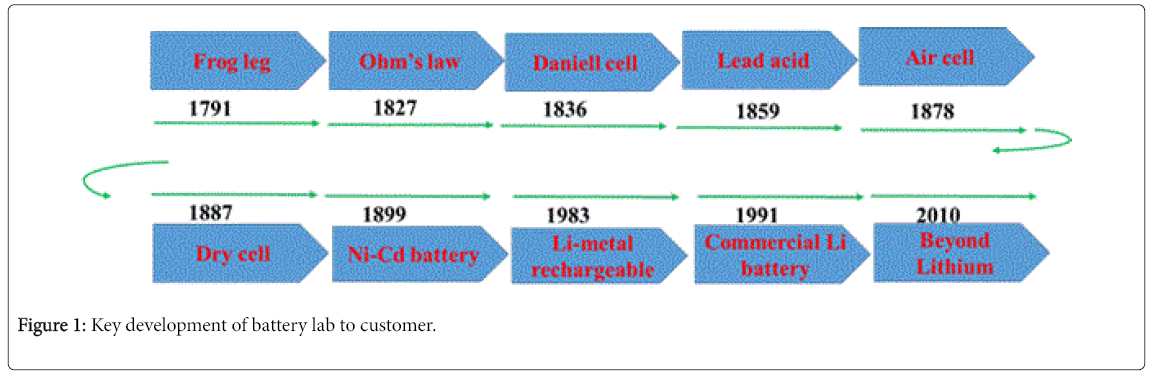
Lead acid battery attracted major scientific and industrial community from 1860’s till now. Nickel cadmium battery also played significant role from 1899 along with Lead acid batteries for military and consumable applications. In spite of their high theoretical energy density (Lead acid: 171 Wh/kg and NiCd: 210 Wh/Kg) the practical limited capacity about 55 Wh/kg and 70 Wh/Kg for Lead acid and Nickel Cadmium respectively, lead to the continuous search to develop new materials which can store more energy. Another breakthrough in energy storage is the commercialisation of Lithium battery by Sony in 1991. Lithium battery technology left its major impact by powering portable devices like mobile phone and laptops due to their high practical energy density (up to 210 Wh/Kg, LiCoO2/ cathode, C as anode), which is about 3 to 4 times higher than the technologies available by then. Below figure shows the mile stones in the development of battery (Figure 1).
To further enhance the storage capacity and reduction in the cost of lithium battery LiFePO4, LiVPO4, LiNi1/3Co1/3Mn1/3O2, LiFePO4F, with layer, spinel, Perovskite, etc., crystal type structures [1]. These cathodes can help in gain give a potential ranges from 2.5 to 4.5 V in theory. Realization of full specific capacity is one of the challenge for industry. Nano technology helped to some extent in enhancing the possible storage and rates of charge and discharge. Scaling up of nanomaterials with uniform particle size, morphology, and surface coatings as prepared in laboratory is a major challenge in bringing the lab inventions to customer use.
Li-air is one of the emerging technologies for stationary energy storage applications. There batteries can help in storing energy density up to 5217 Wh/kg theoretically. Initially researchers used liquid electrolytes carbon for electronic conductivity and platinum as catalyst to enhance reaction kinetics at the cathode side. Many used O2 at different constant pressures by keeping the cells in closed boxes. Replacement of solid electrolytes like Li1.5Al0.5L1.5Ge0.5(PO4)3 pave way for used of low pH solutions, which can help in realising higher voltage Li-air batteries.
Li-S batteries, where carbon is replace with highly abundant sulphur, with up to 5 times more theoretical energy density than conventional li-ion batteries also one of the important technologies under consideration [2]. Elemental sulphur cannot be used as cathode due to its electronic resistive nature. Many research groups demonstrated the use of carbon in the form of nano particles, nano rods, and sandwiched sulphur with carbon sheets. These studies could show a capacity of 1100 mAh/g at 168 mA/g current density. Encapsulation of sulphur with TiO2 yolk shell architecture showed the possibility of about 800 mAh/g with about 75% retention of initial capacity at 0.5 C rate after 1000 cycles [3]. All these batteries suffer with dissolution of polysulfide in electrolytes. Some researchers replaced liquid electrolyte with solid electrolytes, which can help in minimising the dissolution of polysulphides, and demonstrated the possibility of preparing all-solid-state lithium sulphur battery [4-6]. Another approach considered in the literature is the replacement of sulphur with lithium polysulfide dissolved in non-polar solvents using solid electrolyte membrane. Such batteries could show a possible specific capacity of about 600 mAh/g after 20 cycles [7]. Still problems exist related to the electrolyte and electrode interface.
To address the problems of volume expansion of cathode, replace organic flammable liquid electrolyte, to minimise the battery shot circuit due dendrite formation, solid electrolytes are considered as potential alternatives. Any researchers are showing interest in developing high ionic conducting oxide, sulphide and oxysluphide electrolyte, which can not only replace liquid electrolyte, but also can work as anode protecting membrane, remove the necessity of polymer porous separator. Among the solid electrolyte sulphide based compounds like Li10GeP2S12, Li6PS5Cl, Li6PS5Br, Li9(Si5/3P4/3)S11Cl1/3Δ2/3 (Where Δ is defect) etc., can offer high ionic conductivity upto 1 mS/cm at room temperature [8-10].
Still enormous problems like safety, cost, energy density, power density and integration of battery with devises, need to be addressed by both academic and industrial community.
References
- Reddy MV, Subba Rao GV, Chowdari BVR (2013) Metal oxides and oxysalts as anode materials for Li ion batteries. Chem Rev 113: 5364-5457.
- Bruce PG, Freunberger SA, Hardwick LJ, Tarascon JM (2012) Li-O2 and Li-S batteries with high energy storage. Nat Mater 11: 19-29.
- Seh ZW, Li W, Cha JJ, Zheng G, Yang Y, et al. (2013) Sulphur-TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat Commun 4: 1331
- Hayashi A, Ohtomo T, Mizuno F, Tadanaga K, Tatsumisago M (2003) All-solid-state Li/S batteries with highly conductive glass–ceramic electrolytes. Electrochem commun 5: 701-705.
- Chen M, Rao RP, Adams S (2014) The unusual role of Li6PS5Br in all solid state CuS/Li6PS5Br/In-Li batteries. Solid State Ion 268: 300-304.
- Chen M, Rao RP, Adams S (2014) High capacity all solid state Cu-Li2S/Li6PS5Br/In batteries. Solid State Ion 262: 183-187.
- Prasada Rao R, Adams S (2016) Membranes for rechargeable lithium sulphur semi-flow batteries. ‎J Mater Sci 51: 5556-5564.
- Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, et al. (2011) A lithium superionic conductor. Nat Mater 10: 682-686.
- Kato Y, Hori S, Saito T, Suzuki K, Hirayama M, et al. (2016) High power all solid state batteries using sulfide superionic conductors. ‎Nat Energy 1: 16030.
- Deiseroth HJ, Kong ST, Eckert H, Vannahme J, Reiner C, et al. (2008) Li6PS5X: A class of crystalline Li-rich solids with an unusually high Li+ mobility. Angew Chem Int Edit 47: 755-758.
Citation: Rao RP (2017) An Overview of Energy Storage Development. J Mater Sci Nanomater 1: e108.
Copyright: © 2017 Rao RP. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3123
- [From(publication date): 0-2017 - Nov 24, 2024]
- Breakdown by view type
- HTML page views: 2461
- PDF downloads: 662