Research Article Open Access
An Observational Study on Lignocaine Infusion for Intractable Chronic Pain
Amir Aslam1*, Jaipaul Singh2 and Satyan Rajbhandari2,3
1University of Calgary, Alberta, Canada
2School of Pharmacy and Biomedical Sciences, University of Central Lancashire, Preston, Lancashire, UK
3Department of Diabetology and Endocrinology, Lancashire Teaching Hospital NHS Trust, Chorley and South Ribble District General Hospital, Preston Road, Chorley, UK
- *Corresponding Author:
- Amir Aslam
Assistant Clinical Professor University of Calgary
Family Physician at Eaton Centre Medical Clinic, 751 3rd Street
Suite 417, Calgary, Alberta, Canada
Email: AmirAslam@hotmail.com
Received date: April 19, 2016; Accepted date: July 05, 2016; Published date: July 08, 2016
Citation: Aslam A, Singh J, Rajbhandari S (2016) An Observational Study on Lignocaine Infusion for Intractable Chronic Pain. J Pain Relief 5:255. doi:10.4172/2167-0846.1000255
Copyright: © 2016 Aslam A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pain & Relief
Abstract
Objective: This study assessed the efficacy of lignocaine infusion as a treatment for painful diabetic neuropathy (PDN) groups compared to chronic pain (non-PDN group) in challenging cases where conventional treatment is not helpful.
Methods: A total 11 patients participated in the study with 7 patients referred from pain clinic (non-PDN group) and 4 for patients referred from diabetic foot clinic (PDN group) for lignocaine infusion as a treatment for chronic pain. Both groups of participants were on multiple combination of pain medication with minimal help. All the subjects gave consent for participation and filled out McGill short form (SF) questionnaire before and after the lignocaine infusion.
Results: The results show 33% reduction of visual analogue pain score after lignocaine infusion in PDN group compared to 11% reduction of visual analogue pain score in non-PDN group. These data were statistically significant (p<0.05). Similarly, there was a significant (p<0.05) reduction of affective pain score with 41% after lignocaine infusion in PDN group compared to 21% in non-PDN group. In contrast, the sensory pain score reduction after lignocaine infusion was 23% in PDN group compared to 17% in non-PDN group. These data were statistically not significant (p>0.05). All 11 patients had no reported adverse effects and their observations were in normal limits throughout the lignocaine infusion.
Conclusion: Overall, the study showed that lignocaine infusion is both effective and safe in reducing chronic intractable pain when conventional treatments are intolerable or not helpful. It is more effective in painful diabetic neuropathy patients compared to other causes of chronic pain.
Keywords
Painful diabetic neuropathy; Chronic pain; Lignocaine infusion
Introduction
International association for the study of pain (IASP) defines neuropathic pain as “pain caused by a lesion or disease of the somatosensory nervous system”. Neuropathic pain is caused by direct injury or damage to, or pathological change in peripheral or central nervous system. In contrast, nociceptive pain is caused by direct injury or disease caused by tissue damage [1]. Chronic pain in general defines as pain last more than 3 to 6 months [2]. Chronic neuropathic pain is very common all over the world. It is estimated almost 6% to 8% of general population suffers from chronic neuropathic pain [3,4]. Painful diabetic neuropathic pain is the most common type of chronic neuropathic pain.
Currently, about 380 million people worldwide are living with diabetes mellitus (DM) and it is estimated that this figure will rise up to 592 million in the year 2035 [5]. The prevalence of DM-related complications is also rising. Painful diabetic neuropathy (PDN) is a common complication of DM, affecting about 1/3rd of all patients with DM [6]. It is characterized by bilateral symmetrical distal neuropathic pain in lower extremities with varied symptoms from mild pins and needles, tingling sensation, shooting pain similar to electric shock, constant burning sensation with nocturnal exacerbation and contact hyper-sensitivity-allodynia [7]. Relentless pain and allodynia affect patients both physically and mentally and they both cause disturbance in sleep, low mood, impotence and social withdrawal. In some extreme cases the patient is unable to walk [8-10].
There are a number of trials of treatment of PDN which show some reduction of pain following treatment with either with tricyclicantidepressants amitriptyline [11], selective norepinephrine uptake inhibitor including duloxetine [11,12], venlafaxine [13], anticonvulsants including pregabalin [14-17], gabapentin [18,19], carbamazepine [20], topiramate [21,22] or in severe cases opioid [23]. A met analysis on efficacy on antidepressants and anticonvulsants in combination or alone showed up to 50% reduction in pain [24]. Trial with newer medication such as tapentadol which possesses dual mode of action, opioid agonists and norepinephrine uptake inhibitor show some improvement in painful diabetic neuropathy [25]. Apart from oral treatment, there are trials with some efficacy in treating PDN with topical agents including capsaicin [26] and also isosoribide mononitrate spray [27]. Despite of treatment advancement, chronic symptoms of PDN is challenging for clinicians and distressing for patient. Boulton et al followed up 39 patients with painful diabetic neuropathy over the period of 4 years and found no significant difference in intensity of pain [28]. Another 5 year follow up study on PDN with conventional treatment reported a resolve of symptoms in only 23% patients [29].
A study on chronic neuropathic pain patients with combine conventional neuropathic treatment found up to 50% response rate only [30]. Despite of treatment advances and multiple drug regime up to 50% of chronic neuropathic pain patients are resistant to conventional treatment. And some of these treatment resistant patients are in intractable pain. These challenging patients always been a challenge for physicians. Lignocaine infusion has been reported with some satisfactory response on these challenging, conventional treatment resistant patients [31-33].
Lignocaine is a sodium channel blocker. It was first synthesized by the Swedish chemist Nils Lofgren in 1943 [34]. Lignocaine is widely used as a local anaesthetic and peripheral nerve blocks. It has been used intravenously for the treatment of arrhythmias and also found effective in chronic neuropathic pain [35] and chronic pain disorders [36,37] and is not associated with significant side effects [38]. Lignocaine metabolizes in the liver and its elimination half-life following an intravenous bolus injection is typically 1.5 to 2 hours. However, conditions with chronic liver disease and congestive heart failure may prolong its half-life.
The potential beneficial use of lignocaine infusion as a treatment in PDN was first evaluated by Kastrup [31]. There are several studies which reported pain relief in PDN [31-33]. Despite of rapid half-life, the duration of pain relief reported post lignocaine transfusion was up to 28 days [31,32]. This could be due to the central de-sensitization effect of lignocaine along with peripheral action. The side effects in high doses of IV lignocaine can be sedation, hypotension and arrhythmia. However, studies found IV lignocaine infusion were very well tolerated and safe [31,32,38]. Lignocaine infusion is often preserved only for patients who have persistent excruciating pain and where other medications are not beneficial.
There are several pain assessment questionnaires available to assess the pain. This study used McGill short form (SF) questionnaire, which was developed by Melzack [39]. It is an easy, quick and an excellent tool to measure the quality of pain in three different aspects including sensory, affective and visual analogue score. It has been used as a measure of pain in variety of pain condition studies including painful diabetic neuropathy [32].
The aim of this study was to assess the efficacy of lignocaine infusion in patients with painful diabetic neuropathy (PDN group) compared to chronic pain patients (Non PDN group).
Subjects and methods
PDN subjects with chronic refractory pain for (± SD) 6.5 ± 3.42 years, not responded to standard oral and topical treatment were identified from Foot Clinic at Chorley District General Hospital (CDGH). Chronic pain subjects (non- PDN) with chronic refractory pain for (± SD) 7.75 ± 4.77 years, not responded to standard oral and topical treatment were referred for lignocaine infusion from Pain Clinic, Lancashire Hospitals NHS Trust. Both groups of patients already tried and were on multiple combination of pain medication without relief.
Both groups of subjects attended individually and admitted to coronary care unit (CCU) as a day case for 3 hours and given lignocaine infusion 0.2% (2 mg/ml), 5 mg/kg body weight over 2 hours in coronary care unit of CDGH with throughout monitoring of electrocardiogram, blood pressure, pulse and oxygen saturations. Nurse administered McGill pain short form (SF) questionnaire before and after the infusion on each subjects. All patients recalled in “neuropathy pain clinic” in 6 weeks’ time. All patients gave consent for participation.
McGill SF consisted of 15 representative words from the sensory (n=11), affective (n=4) and visual analogue score (VAS). Each word descriptor was ranked by the patient on an intensity scale of 0, none; 1, mild; 2, moderate; and 3, severe. Sensory score ranged from 0 to 33, affective score ranged from 0 to 12 and VAS range from 0 to maximum 10 [39].
Statistical analysis
Data were analysed using Graph Pad software [40]. The continuous variable were normally distributed and expressed as means, ± standard deviation (SD), median and P value. The means of McGill pain score were analysed with paired Student’s t-test compared before and after lignocaine infusion. Categorical data were expressed as frequency distribution and percentage of subjects groups and p value. The categorical data of were analysed by 2 × 2 table using Fischer’s exact test. The boxplots created with descriptive statistics using Minitab statistical software (2013). The plot showed the median (dark band) along with minimum and maximum. The box represents the lower (Q1=25%) and upper (Q3=75%) quartile rang.
Results
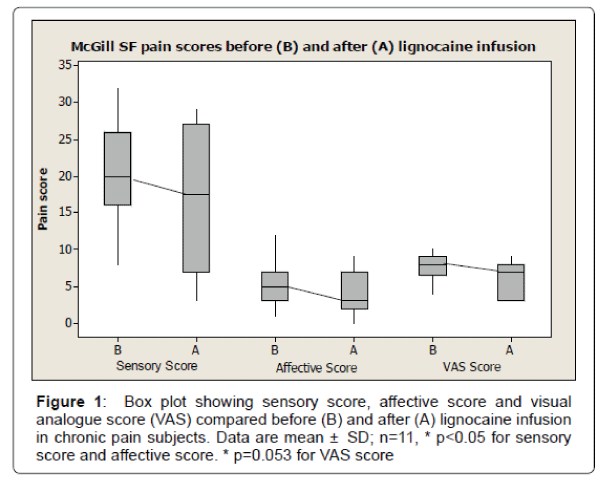
Table 1 show the demographics and baseline characteristics of patients participated in the study. A total of 11 subjects participated in the study and completed the McGill SF questionnaire before and after the lignocaine infusion. The mean age (± SD) of subject was 52 ± 13.96 years. There were total of 4 males (36%) with mean age (± SD) 58.7 ± 15 years and 7 females (64%) with mean age (± SD) of 49 ± 13 years. The mean duration of chronic pain (± SD) was 7.09 ± 4.37 years. The mean sensory score before lignocaine infusion was (± SD) 20.14 ± 7.16 compared to mean sensory score after lignocaine infusion was (± SD) 16.5 ± 9.52. There was significant reduction of sensory pain score after lignocaine infusion (p<0.05). The mean affective score before lignocaine infusion was (± SD) 5.5 ± 3.09 compared to mean affective score after lignocaine infusion was (± SD) 4.0 ± 3.13. There was significant reduction of affective pain score after lignocaine infusion (p<0.05). The mean visual analogue score (VAS) before lignocaine infusion was (± SD) 7.72 ± 1.75 compared to mean VAS score after lignocaine infusion was (± SD) 6.13 ± 2.53. There was reduction of VAS pain score after lignocaine infusion, however data were statistically not significant (p=0.053) (Figure 1). The box plot analysis in Figure 1 shows the McGill pain score in all 3 sub-categories including sensory score, affective score and visual analogue scores (VAS) which were part of McGill SF pain score either before (A) or after (B) the lignocaine infusion in all subjects with chronic pain. Before lignocaine infusion, the sensory score mean was 20.4 ± 7.16 SD (median 20) compared to sensory score mean after lignocaine infusion was 16.5 ± 9.52 SD (median 17.5) (p<0.014). Before lignocaine infusion, the affective score mean was 5.5 ± 3.09 SD (median 5) compared to affective score mean after lignocaine infusion was 4.0 ± 3.13 SD (median 3.0) (P< 0.013). Before lignocaine infusion, the VAS score mean was 7.72 ± 1.75 SD (median 8) compared to VAS score mean after lignocaine infusion was 6.13 ± 2.53 SD (median 7) (p=0.053). The plot also shows the median score (dark band) along with minimum and maximum score. The box represents the lower (Q1=25%) and upper (Q3=75%) quartile range of score. All male participants had painful diabetic neuropathy (PDN group) as a cause of pain and all female participants had non PDN cause of chronic pain. The ages in both gender- ages and groups were similarly distributed (p>0.05). The mean duration of pain (± SD) in PDN group was 6.5 ± 3.42 years compared to 7.75 ± 4.77 years in Non PDN group. The duration of pain in both groups were similarly distributed (p>0.05). Both groups had tried a combination of medications including antidepressants, antiepileptic medications, and opioid and moreover, they were on combination of medications with unsatisfactory response.
| Patient No | Age Years |
Gender | PDN Yes or no |
Duration of painYears |
Medication tried not helped | Current pain medications | Before Lignocaine infusion | After Lignocaine infusion | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAS | Sensory | Affective | VAS | Sensory | Affective | |||||||
| 1 | 79 | Male | Yes | 11 | Amitriptyline , Imipramine, Carbamazepine,Capsaicin cream, Tramadol, Pregabalin , Mexiletine, GTN patch, Duloxetine, MST, Acupuncture, Alphalipoic acid, Lignocaine patch | Gabapentin, Oxycontin | 7.5 | 8 | 1 | 3.5 | 3 | 3.5 |
| 2 | 60 | Male | Yes | 3 | Pregabalin, Amitriptyline Duloxetine, Clonazepam, Durogesic patch, Oramorph PRN. | Pregabalin, Amitriptyline Duloxetine, Clonazepam, Durogesic patch, Oramorph PRN. | 9 | 32 | 7 | 9 | 29 | 9 |
| 3 | 44 | Male | Yes | 5 | Gabapentin, Butrans patch, capsaicin cream, colnazepam | Pregabalin, Amitriptyline, Topiramate | 9 | 12 | 6 | 3 | 4 | 3 |
| 4 | 52 | Male | Yes | 7 | Pregabalin, Gabapentin, topical Capsaicin, Duloxetine, BuTrans patch, Tramadol, Oxycontin, | Morphine Sulphate, Amitriptyline, Sodium Valproate | 10 | 22 | 3 | 8 | 21 | 8 |
| 5 | 41 | Female | No Back Pain |
5 | Carbamazepine, Duloxetine, Amitriptyline, Pregabalin, Ropinerole. SI joint injections, Facet joint injections, Butrans patch, TENS machine |
Carbamazepine, Duloxetine, Amitriptyline, Pregabalin, Ropinerole. | 8 | 30 | 9 | 8 | 29 | 8 |
| 6 | 66 | Female | No Fibromyalgia |
3 | TENS, acupuncture, physiotherapy, Gabapentin, amitriptyline, Naproxen, Codeine, Butrans patch | Ibuprofen 400mg prn | 4 | 20 | 5 | 3 | 11 | 3 |
| 7 | 53 | Female | No Back pain |
9 | Epidural steroid injection, Gabapentin | Oxycontin, Pregabalin, Amitriptyline | 7 | 19 | 4 | 3 | 7 | 3 |
| 8 | 59 | Female | No Angiolipoma |
7 | Gabapentin, Cocodamol 30/500, SI joint injection, Facet joint injections, TENS | Carbamazepine, Duloxetine, Amitriptyline, Pregabalin, Ropinirole | 9 | 18 | 4 | 6 | 15 | 6 |
| 9 | 26 | Female | No Fibromyalgia | 18 | Amitriptyline 50 mg, Pregabalin, Gabapentin, Tramadol, psychotherapy | OxyContin, Ibuprofen, Amitriptyline, Duloxetine. | 6 | 16 | 3 | 8 | 18 | 8 |
| 10 | 45 | Female | No Demyelination |
6 | Gabapentin, Pregabalin, Nabilone, Ketamine, Butranspatch, codeine, Capsaicin cream, Lidocaine patch, Fentanyl patch, Duloxetine, Topiramate, Carbamazepine, TENS | Amitriptyline 50 mg Codeine 60 mg at night |
6.5 | 21.5 | 6.5 | 7 | 17.5 | 7 |
| 11 | 54 | Female | NoStump pain | 4 | Paracetamol , Oramorph prn, Oxycodone MR, Pregabalin, Lidocaine patches, Acupuncture, TENS, carbamzepine |
Paracetamol , Oramorph prn, Oxycodone MR, Pregabalin, Lidocaine patches | 9 | 26 | 12 | 9 | 27 | 9 |
Table 1: Demographics and baseline characteristics of patients participated in the study.
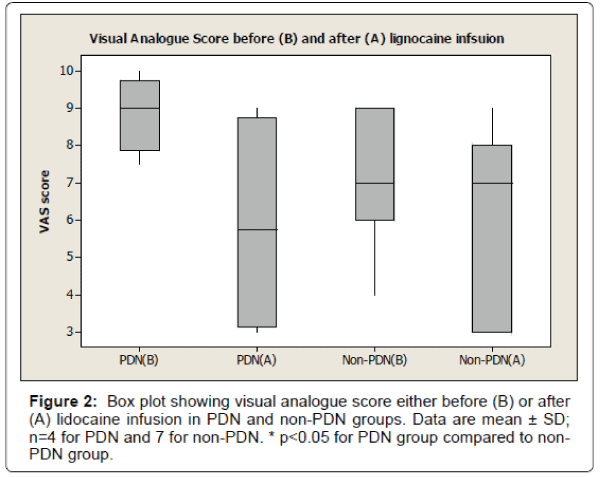
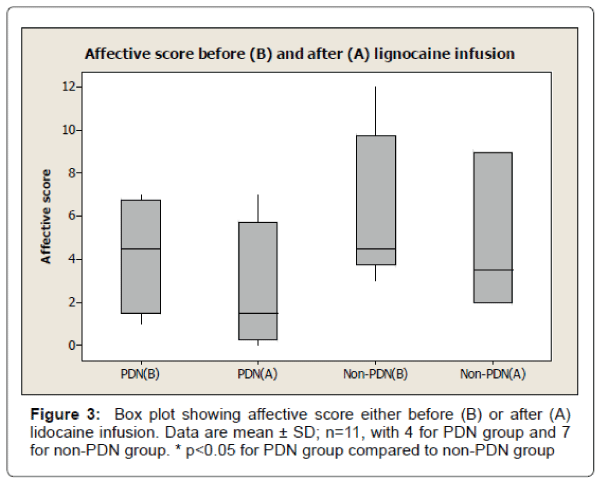
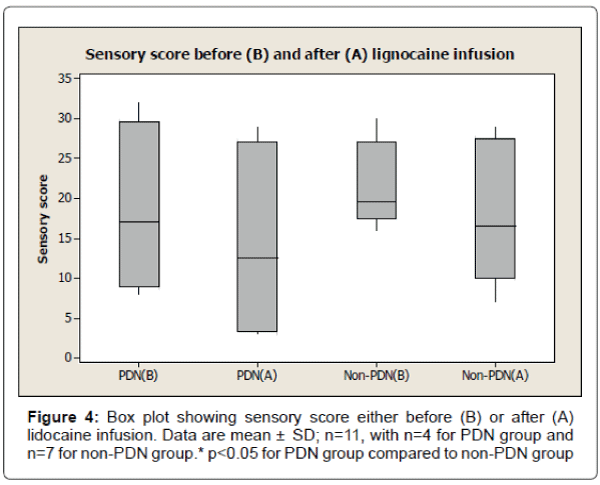
The results also show 33% reduction of visual analogue pain score after lignocaine infusion in PDN group compared to 11% reduction of visual analogue pain score in non-PDN group. The data were statistically significant (p<0.05) (Figure 2). Similarly, there was a significant (p<0.05) reduction of affective pain score, 41% after lignocaine infusion in PDN group compared to 21% in non-PDN group (Figure 3). In contrast, the sensory pain score reduction after lignocaine infusion was 23% in PDN group compared to 17% in non-PDN group. These data were statistically not significant (p>0.05) (Figure 4).
All 11 patients had no reported adverse effects and their observations including electrocardiograms, pulse, blood pressure and oxygen saturation were in normal limits throughout the lignocaine infusion. In this and subsequent figures, PDN (B) = Painful diabetic neuropathy group score before lignocaine infusion
PDN (A)=Painful diabetic neuropathy group score after lignocaine infusion; Non-PDN (B)=Non-painful diabetic neuropathy group score before lignocaine infusion; Non-PDN (A)=Nonpainful diabetic neuropathy group score after lignocaine infusion; VAS=Visual analogue score
The box plot analysis in Figure 2 shows the visual analogue score (VAS) which was part of McGill SF pain score either before (A) or after (B) the lignocaine infusion in PDN group and non-PDN group. Before lignocaine infusion, the VAS mean score was 8.87 ± 1.03 SD (median 9.0) compared to VAS mean score after lignocaine infusion which was 5.87 ± 3.06 SD (median 5.75) (33% pain reduction). In Non-PDN group (n=7), before lignocaine infusion, the mean VAS score was 7.07 ± 1.7 SD (median 7) compared to VAS mean score of 6.28 ± 2.43 SD (median 7) (11% pain reduction) after lignocaine infusion. The pain reduction of PDN group compared to Non-PDN group was statically significant (p<0.0015).
The box plot analysis in Figure 3 shows the affective score of McGill pain SF score either before (B) or after (A) the lignocaine infusion in PDN group and non-PDN group. The results show that in the in PDN group, the affective mean score was 4.25 ± 2.75 SD (median 4.5) before lignocaine infusion compared to affective mean score of 2.50 ± 3.11 SD (median 1.5) (41% pain reduction) after lignocaine infusion. In Non- PDN group (n=7), before lignocaine infusion, the mean affective score was 6.17 ± 3.54 SD (median 4.5) compared to affective mean score 4.83 ± 3.31 SD (median 3.5) (21% pain reduction) after lignocaine infusion was the pain reduction of PDN group compared to Non-PDN group was statistically significant (p<0.0036).
The box plot analysis in Figure 4 shows the sensory score of McGill pain SF score either before (B) or after (A) lignocaine infusion in PDN group and non-PDN group. In PDN group, the sensory mean score was 18.5 ± 10.75 SD (median 17.0) before lignocaine infusion compared to sensory mean score 14.25 ± 12.84 SD (median 12.5) (23% pain reduction) after lignocaine infusion. In Non-PDN group, before lignocaine infusion, the mean sensory score was 21.50 ± 5.36 SD (median 19.5) compared to sensory mean score 17.83 ± 8.73 SD (median 16.5) (17% pain reduction) after lignocaine infusion. The pain reduction of PDN group compared to Non-PDN group was not statically significant (p=0.3769).
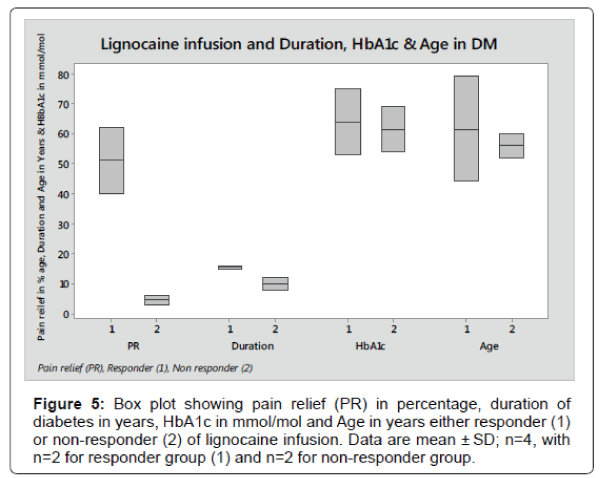
The box plot analysis in Figure 5 shows the 95% pain relief after lignocaine infusion in responder (1) and 5% in non-responder (2) of Diabetes patients. The pain reduction in responder compare to nonresponder was statistically significant (p<0.0001).
In responder group, the duration of diabetes mean score was 15.5 years ± 0.71 SD, HbA1c mean score was 64 mmol/mol ± 15.5 SD and Age means score was 61.5 years ± 24.75 SD compared to 10 years ± 2.83 SD, 66.5 mmol/mol ± 3.5 SD and 56 years ± 5.66 SD in non-responder group respectively. The data for duration of diabetes, HbA1c and Age in responder group compared to non-responder were not statically significant (p<0.116, p<0.84 and p<0.79 respectively).
Discussion
Painful diabetic neuropathy (PDN) is one of the common complications of diabetes mellitus (DM). About 1/3 of all patients with DM suffer from diabetic neuropathic pain [6] and moreover, it has a huge impact on the quality of life (QoL) of the person [8-10]. There are several trials which have reported some benefit in the improvement of symptoms of PDN with various antidepressants, anticonvulsants, opioid and topical medications [11-27]. The follow up studies reported that only 23% patients had satisfactory improvement of symptoms of PDN after conventional treatment [28,29]. Most of the patients tolerate the residual pain and live with it, however there are reports of severe PDN with constant unrelenting neuropathic pain, disturbance of sleep and even unable to walk due to severity of pain [8-10]. Lignocaine infusion has been used as a treatment when other conventional treatments were not helpful in various chronic challenging painful conditions including chronic pain syndrome [36,37], chronic neuropathic pain [35] including painful diabetic neuropathy [31-33].
The present data have shown reduction in all 3 domains of pain scores of McGill SF pain questionnaire including visual analogue score of 33%, affective scores of 41% and sensory scores of 23% in PDN group after lignocaine infusion compared to 11%, 21% and 17%, respectively in non-PDN group. The data were statistically significant for VAS and affective scores. However, the data were not statistically significant for sensory scores. This could be due to the statistically significant response of lignocaine infusion on sensory scores in both groups of patients (Figure 1).
Comparison with existing data
The data from this study have clearly shown significant reduction of McGill SF pain scores- affective score and visual analogue score after lignocaine infusion in patients with PDN compares to those patients with chronic pain (non-PDN). These results are consistent with those obtained by Viola et al. [32] and Kastrup et al. [31] who demonstrated significant reduction of both affective scores and visual analogue scores after lignocaine infusion. The present study measured effectiveness of lignocaine infusion as a treatment on PDN patients compared to chronic pain patients with other causes. In contrast, in their studies Viola et al. [32] and Kastrup et al. [31] measured the effectiveness of lignocaine infusion compared with saline infusion in patients with PDN. In our study, the reduction of McGill SF sensory pain score was 23% in PDN group compared to 17% in non-PDN group. Despite of quarter of reduction of sensory pain score in PDN group, the data were not statistically significant. In contrast Viola et al. [32] and Kastrup et al. [31] showed significant reduction of McGill sensory pain score. This discrepancy in the results could be due to the fact that the lignocaine infusion responded almost to the same level in both PDN and non-PDN groups. Therefore, the comparison difference was not significant. The present study was similar to that of Viola et al study [32] where all the patients participated with intractable pain and who failed to respond to or intolerant to conventional treatment. It is particularly noteworthy, that in this and the studies done by Viola et al study [32] and Kastrup et al. study [31], no participants had any adverse effects with lignocaine infusion of 5 mg/kg bodyweight. This observation clearly suggests that it is quite safe to use this dose of lidocaine to treat PDN. However, there is a report [41] which reported that lignocaine infusion had marked adverse effects resulting in hypotension and arrhythmia. The study is related to fibromyalgia patients and lignocaine infusion was given consecutively for 6 days. Also, the dose was increased incrementally every day to maximum of 5 mg/kg bodyweight plus 150 mg or total maximum 550 mg [41].
There are several studies reporting significant reduction of pain after lignocaine infusion in PDN and a variety of non-PDN conditions including fibromyalgia [41], headache [42], back pain [43], trigeminal neuralgia [44] and chronic pain syndrome[36,37]. Like previous investigations, the present study also showed a beneficial effect of lignocaine infusion to treat both PDN and non-PDN group. However, in patients with PDN lignocaine infusion was statistically more effective than other causes of chronic pain. PDN pathogenesis involves peripheral and central sensitization with neural plasticity [45]. The half–life of Lignocaine infusion is only 2 hours, however the effect of analgesia reported up to 28 days. This observation suggests that lignocaine infusion may affect not only peripheral, but perhaps central neural plasticity as well. This may be due to the central effect of lignocaine where it was more effective in PDN group.
Strength and Limitation of the study
The study population was well defined for both groups and completed with minimal selection bias as the participations from both PDN and non-PDN groups were referred from Foot Clinic or Pain Clinic for lignocaine infusion, respectively. Moreover, both groups of participants responded 100% in filling McGill SF pain questionnaires. Both groups were similar in age, however all participants in PDN group were males and non-PDN group were females. Recall bias could exist when participants filling out questionnaire. However, most questions from McGill SF questionnaire were based on current or recent physical and mental wellbeing of person, hence recall bias were minimal. The results also show that lignocaine infusion had no significant effect on the ECG, BP, pulse rate or oxygen saturation in the both groups of patients. This was the observational study and all patients were well aware that they were having treatment with lignocaine infusion, therefore possible placebo effect cannot be ruled out. Also the sample size was very small only 4 in PDN group and 7 in non PDN group. Further randomize control trial with large sample needed to see the true results.
Conclusion
Overall, the study has shown that lignocaine infusion is both effective and safe in reducing the chronic intractable pain when conventional treatment are intolerable or not helpful in PDN and non-PDN patients. It is more effective in PDN patients compared to other causes of chronic pain. There is a need for randomize control trial to see the effect of lignocaine infusion especially in PDN. Chronic neuropathic pain including PDN causes modulation of pain at spinal level and plasticity of brain, as a result it’s more difficult to treat the refractory pain [45]. Perhaps we may need to consider, lignocaine infusion in early stage when conventional treatments are not helpful.
References
- Treede RD, Jensen TS, Campbel JN, Cruccu G, Dostrovsky JO, et al. (2008) Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 70: 1630-1635.
- Debono DJ, Hoeksema LJ, Hobbs RD (2013) Caring for patients with chronic pain: pearls and pitfalls. J Am Osteopath Assoc 113: 620-627.
- Torrance N, Smith BH, Bennett MI, Lee AJ (2006) The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain 7: 281-289.
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C (2008) Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136: 380-387.
- IDF: International Diabetes Federation (2013) IDF Diabetes Atlas. (6th edn.), International Diabetes, Federation, Belgium.
- Tesfaye S (2009) Assessment and management of painful diabetic neuropathy. Oxford University Press, New York, pp: 37-52.
- Larsen, Kronenberg (2002) Williams Textbook of Endocrinology. (10th edn.), Elsevier Science. Philadelphia, pp: 1546-1554.
- Galer B, Gianas A, Jensen M (2000) Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Research and Clinical Practice 47: 23-128
- Gardner D, Shoback D (2007) In Greenspan’s Basic and Clinical Endocrinology, (8th edn) McGraw-Hill Medical,pp: 727-733.
- Quattrini C, Tesfaye S (1996) Understanding the impact of painful diabetic neuropathy. Diabetes Metab Res Rev 19: S2-S8.
- Kaur H, Hota D, Bhansali A, Dutta P, Bansal D, et al. (2011) A comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: a randomized, double-blind, cross-over clinical trial. Diabetes Care 34: 818-822.
- Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S (2005) Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 116: 109-118.
- Kadiroglu AK, Sit D, Kayabasi H, Tuzcu AK, Tasdemir N, et al. (2008) The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. J Diabetes Complications 22: 241-245.
- Rosenstock J, Tuchman M, LaMoreaux L, Sharma U (2004) Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 110: 628-638.
- Lesser H, Sharma U, LaMoreaux L, Poole RM (2004) Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology 63: 2104-2110.
- Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, et al. (2005) Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 6: 253-260.
- Freyhagen R, Strojek K, Griesing T, halen E, Balkenohl M (2005) Efficacy of pregabalinin neuropathic pain evaluated in a 12 week, randomised controlled, double blind , multicentre, placebo-controlled trial of flexible and fixed dose regimens. Pain 115: 254-263
- Backonja MM (1999) Gabapentin monotherapy for the symptomatic treatment of painful neuropathy: a multi-centre, double-blind, placebo-controlled trial in patients with diabetes mellitus. Epilepsia 40: 557-559.
- Vinik A, Fonseca V, LaMoreaux L (1998) Neurontin (Gabapentin, GBP) improves quality of life (qol) in patients with painful diabetic peripheral neuropathy. Diabetes 47: A374.
- Badran AM, Aly MA, Sous ES (1975) A clinical trial of carbamazepine in the symptomatic treatment of diabetic peripheral neuropathy. J Egypt Med Assoc 58: 627-631.
- Edwards K, Glantz MJ, Button J (2000). Efficacy and safety of topiramate in the treatment of painful diabetic neuropathy: a double-blind placebo-controlled study. Neurology S4: A8.
- Donofrio PD, Raskin P, Rosenthal NR, Hewitt DJ, Jordan DM, et al. (2005) Safety and effectiveness of topiramate for the management of painful diabetic peripheral neuropathy in an open-label extension study. Clin Ther 27: 1420-1431.
- Harati Y, Gooch C, Swenson M, Edelman S, Greene D, et al. (1998) Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 50: 1842-1846.
- Rudroju N, Bansal D, Talakokkula ST, Gudala K, Hota D, et al. (2013) Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician 16: E705-714.
- Vinik AI, Shapiro DY, Rauschkolb C, Lange B, Karcher K, et al. (2014) A Randomized-Withdrawal, Placebo-Controlled Study Evaluating the Efficacy and Tolerability of Tapentadol Extended Release in Patients With Chronic, Painful Diabetic Peripheral Neuropathy. Diabetes Care37: 2302-2309.
- Low PA, Opfer-Gehrking TL, Dyck PJ, Litchy WJ, O'Brien PC (1995) Double-blind, placebo-controlled study of the application of capsaicin cream in chronic distal painful polyneuropathy. Pain 62: 163-168.
- Yuen KC, Baker NR, Rayman G (2002) Treatment of chronic painful diabetic neuropathy with isosorbide dinitrate spray: a double-blind placebo-controlled crossover study. Diabetes Care 25: 1699-1703.
- Boulton AJ, Armstrong WD, Scarpello JH, Ward JD (1983) The natural history of painful diabetic neuropathy--a 4-year study. Postgrad Med J 59: 556-559.
- Daousi C, Benbow SJ, Woodward A, MacFarlane IA (2006) The natural history of chronic painful peripheral neuropathy in a community diabetes population. Diabet Med 23: 1021-1024.
- Tesfaye S1, Wilhelm S, Lledo A, Schacht A, Tölle T, et al. (2013) Duloxetine and pregabalin: high-dose monotherapy or their combination? The "COMBO-DN study"--a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain.Pain 154: 2616-2625.
- Kastrup J, Petersen P, Dejgård A, Angelo HR, Hilsted J (1987) Intravenous lidocaine infusion--a new treatment of chronic painful diabetic neuropathy? Pain 28: 69-75.
- Viola V, Newnham HH, Simpson RW (2006) Treatment of intractable painful diabetic neuropathy with intravenous lignocaine. J Diabetes Complications 20: 34-39.
- Bach FW, Jensen TS, Kastrup J, Stigsby B, Dejgård A (1990) The effect of intravenous lidocaine on nociceptive processing in diabetic neuropathy. Pain 40: 29-34.
- Löfgren N, Lundqvist B (1946) Studies on local anaesthetics II. Svensk Kemisk Tidskrift 58: 206-217
- Tremont-Lukats IW, Hutson PR, Backonja MM (2006) A randomized, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain 22: 266-271.
- Cahana A, Shvelzon V, Dolberg O, Magora F, Shir Y (1998) Intravenous lignocaine for chronic pain: an 18-month experience. Harefuah 134: 692-694, 751, 750.
- Wallace MS, Ridgeway BM, Leung AY, Gerayli A, Yaksh TL (2000) Concentration-effect relationship of intravenous lidocaine on the allodynia of complex regional pain syndrome types I and II. Anesthesiology 92: 75-83.
- Challapalli V, Tremont-Lukats IW, McNicol ED, Lau J, Carr DB (2005) Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev : CD003345.
- Melzack R (1987) The short-form McGill Pain Questionnaire. Pain 30: 191-197.
- Graphpad Inc USA.
- Raphael JH, Southall JL, Kitas GD (2003) Adverse effects of intravenous lignocaine therapy in fibromyalgia syndrome. Rheumatology (Oxford) 42: 185-186.
- Rosen N, Marmura M, Abbas M, Silberstein S (2009) Intravenous lidocaine in the treatment of refractory headache: a retrospective case series. Headache 49: 286-291.
- Park CH, Jung SH, Han CG (2012) Effect of intravenous lidocaine on the neuropathic pain of failed back surgery syndrome. Korean J Pain 25: 94-98.
- Arai YC, Hatakeyama N, Nishihara M, Ikeuchi M, Kurisuno M, et al. (2013) Intravenous lidocaine and magnesium for management of intractable trigeminal neuralgia: a case series of nine patients. J Anesth 27: 960-962.
- Aslam A, Singh J, Rajbhandari S (2014) Pathogenesis of painful diabetic neuropathy. Pain Res Treat 2014: 412041.
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 11894
- [From(publication date):
July-2016 - Jul 09, 2025] - Breakdown by view type
- HTML page views : 10964
- PDF downloads : 930