An Introgression of Solanum habrochaites in the Rootstock Improves Stomatal Regulation and Leaf Area Development of Grafted Tomatoes under Drought and Low Root-Zone-Temperatures
Received: 02-Apr-2015 / Accepted Date: 25-Jun-2015 / Published Date: 20-Jul-2015 DOI: 10.4172/2329-8863.1000175
Abstract
Drought and low root zone temperature (RZT) hamper plant growth and leaf area development. Introgression lines (ILs) developed from a Solanum lycopersicum × Solanum habrochaites cross with the exotic parental line as donor were shown to significantly improve cold tolerance. The goal of the present study was to study agronomical and Physiological traits improving drought and, since adaptation to cold and drought is to some extent regulated by the same genetic mechanisms, low root zone tolerance in tomatoes. The parental lines of an IL library and ILs carrying quantitative trait loci (QTL) alleles for cold tolerance introgressed from S. habrochaites were used as plant material. The recurrent parent (RP) was grafted onto the IL (IL/RP) and self-grafted (RP/RP). Grafted plants were grown under two different RZTs (10°C and 20-26°C) and two soil moisture regimes (well-watered\ and drought stress). Agronomical and physiological parameters as green, non-green, and total leaf area, dry matter of plant parts, root to shoot ratio, osmotic adjustment, stomatal conductance, and stress tolerance index were measured and calculated. Plants grafted onto ILs produced higher total biomass and leaf area and probably regulated stomatal opening and closure more efficiently. The stm9 introgression from S. habrochaites LA1777 renders the scion more tolerant for low root-zone temperature and drought stress by a better stomatal regulation. Results confirm that an introgression of favourable genes from S. Habrochaites can improve cold and drought tolerance in grafted tomatoes and shoot turgor maintenance under root chilling is primarily a root-based trait.
Keywords: Drought and low root zone temperature stress; Introgression lines; Stomatal conductance; Stress recovery; Tomato; QTLs
403750Abbreviations
D: Donor; GC: Graft Combination; GLA: Green Leaf Area; IL: Introgression Lines; LCC: Leaf Chlorophyll Content; LDM: Leaf Dry Matter; NGLA: Non-green Leaf Area; OA: Osmotic Adjustment; OP: Osmotic Potential; QTL: Quantitative Trait Loci; R: S: Root Shoot Ratio; RDM: Root Dry Matter; RP: Recurrent Parent; RS: Stomatal Resistance; RZT: Root Zone Temperature; TDM: Total Dry Matter; TI: Tolerance Index; TLA: Total Leaf Area
Introduction
Extremely low and high temperatures, prolonged drought and water logging, soil salinity, etc. are very common abiotic stresses responsible mainly for low harvest [1]. Tomato (S. lycopersicum L.) is a widely distributed vegetable susceptible to suboptimal temperatures [2]. In comparison to greenhouse tomatoes, open field tomatoes are directly connected with and adversely affected by abiotic factors. Drought and temperature stress inhibit the productivity and limits the growing season and area [3]. Root hydraulic conductance of plant is substantially reduced by exposure to low temperatures. This severely decreases the conductivity of the critical membrane in roots and at the same time water viscosity increases [4]. Ultimately it reduces water absorption and may induce shoot water stress (‘chilling55 drought’). As a result, tomato leaves quickly start to wilt and photosynthesis and transpiration decreases [5]. 56 Low temperature conditions and water deficit environments are independent phenomena but cold tolerant genotypes exhibit xerophytic adaptations [6] and synthesize the plant hormone ABA with higher concentration and delivery rate in xylem sap [7] to reduce the chilling effects. Cultivated and wild tomatoes responded differently to environmental stress [8]. The shoots of chilling susceptible tomato genotypes wilted under low-root zone temperature (around 5°C) while the same treatment did not affect S. habrochaites. In S. habrochaites, stomatal resistance increased as root temperatures decreased, whereas the stomata of S. lycopersicum remained open until the RZT reached 5°C [9]. A study analyzing the post-chilling shoot re-growth capacity of S. lycopersicum, S. pimpinellifolium, S. peruvianum, and S. habrochaites revealed that its wild relatives behaved superior to cultivated tomato [10]. Osmotic adjustment (OA) has been reported as an effective component of drought resistance in plants. It assists cell turgor maintenance at a given leaf water potential and delays wilting [11]. OA demands net increase in cellular osmolality through the accumulation of osmolytes [12]. It helps to maintain cell volume and leaf turgor and supports stomatal conductance and carbon assimilation under drought stress conditions [11,13]. Genetic variation among tomato species is important to develop stress tolerant lines. Wild tomato species are useful sources of genetic materials that can be introgressed into cultivated tomato for improving tolerance to chilling temperatures, drought and salinity [14,15]. Thirteen wild tomato species have been recognized as a source material to improve productivity and fruit quality of cultivated tomatoes [16,17]. Found a minimum of three QTLs affecting growth at low temperature in an interspecific S. lycopersicum 81 x S. habrochaites backcross population. Several IL libraries with wild Solanum species as donors were developed in the past. Ninety eight ILs with S. habrochaites as donor cover more than 85% of the donor genome [18]. IL-libraries are an effective genetic pool to identify QTLs and study their effects in contrasting environmental conditions. In the recent past, a major QTL controlling chilling tolerance was identified [19]. The goal of the present study was to analyze effects of a major QTL (shoot turgor maintainer, stm9), which is believed to confer cold tolerance [19], agronomic and physiological performance of grafted tomatoes with introgression lines as rootstocks. Tomato grafting is gaining popularity among commercial growers to improve productivity and quality [20,21], and to achieve tolerances against multiple abiotic stresses [22-24]. The study hypothesized that rootstocks with introgressions on QTL regions improving abiotic stress tolerance may improve crop productivity without inferring negative effects on agronomic traits since the above ground plant part does not include introgressions and its possible negative effects on yield and product quality.
Materials and Methods
Plant materials
Introgression lines (ILs) LA3957 that represent the genome of S. habrochaites (LA1777) in the background of S. lycopersicum cv. E-6203 (LA4024), recurrent parent (RP) from the same cross (LA4024), and the donor (D) S. habrochaites were used as plant materials. During the first experiment (Expt 1), all three genotypes (IL, RP, and D) and in second experiment (Expt 2), IL and RP were used. The IL which carries the chilling tolerance QTL allele (stm9) was used as rootstock. Seeds of all genotypes were obtained from the Institute of Plant Nutrition. Two 105 experiments were conducted from 14 July - 17 August 2009 and 29 April - 2 June 2010, 34 days at the Institute of Horticultural Production System, Leibniz University Hannover in a well-ventilated greenhouse under natural light condition.
Seedling preparation
Seedlings were prepared as per practices being carried out in the Vegetable System Modelling section and guidelines described by Black et al. [25]. n Expt 1, donor was sown eight days earlier than others due to the slow growth rate of seedlings. Scions were sown three days after sowing rootstocks to get equal stem diameters of seedlings. In both experiments, seedlings for rootstocks were transplanted individually to 450 ml plastic pots. Scion seedlings were thinned to one seedling and continued growing on the same tray. Grafting was done at fourth true leaf stage that was obtained approximately 17 days after sowing. The Japanese top grafting (tube grafting) method described by Oda et al. [26] was used with slight modifications. A horizontal 90° cut across stem axis was given instead of slanting cut. Finally, four different graft combinations in the Expt 1 and two in the Expt 2 were prepared as shown in (Table 1). Graft healing was done in healing tunnels under high RH at 20°C air temperature. After day 8 grafts were hardened in greenhouse at 20°C for seven days.
| Rootstock | Scion | Graft combination | Total graft number | |
|---|---|---|---|---|
| Expt 1 | IL | RP | IL/RP | 12 |
| IL | IL | IL/IL | 12 | |
| RP | RP | RP/RP | 12 | |
| D | RP | D/RP | 12 | |
| Expt 2 | IL | RP | IL/RP | 24 |
| RP | RP | RP/RP | 24 |
Table 1: Graft combinations developed during experiments.
Experimental set up and stress treatment
A two factorial experimental set up was designed from two RZT and two levels of soil moisture. Well-watered condition and high RZT (25 ± 2°C) was the control. The same RZT was used for the drought condition. The low RZT (10 ± 2°C) was combined with well-watered condition (hereafter referred as ‘cold treatment’). Grafted plants were completely randomized in climate chamber with 24/22°C day and night temperature and ventilation opened above 25°C. Soil temperature was maintained as described by Gosselin and Trudel [27] with modifications. In Expt 1, 0.5 litre capacity plastic pots (10 cm × 7.5 cm × 10 cm) without drainage hole were used while during Expt 2 plants 130 were grown in 1 litre pots (16.15 cm × 11.25 cm × 5.5 cm). Cold water (<8°C) from the cooling pipe circulated continuousl through double layered copper pipes in two containers in order to maintain low water bath temperature and thereby low soil temperature (10 ± 2°C) in the plastic pot. The water bath was placed on styrofoam and covered by the styrofoam to provide insulation for maintaining a constant water temperature. Four other containers were also covered by styrofoam to minimize evaporation. Soil and ambient air temperature was recorded daily using Technoterm 7300 (Testoterm, Lenzkirch, Germany). All plants were well-watered with Scotts Universol Orange water soluble fertilizer solution (500 g per 10 litre water). Once treatment started, plants in control and cold treatment were irrigated daily while plants subjected to drought were well-watered only during stress cycle break. An equal amount of water was applied to all plants of control and drought treatment. Drought stress was induced by withholding water until soil completely dried out. Length of stress cycle was affected by pot size used and weather condition. On an average, five-day long stress cycles were used in both experiments and plants were irrigated with an equal amount of water as shown in Table 2 by using pipettes fitted measuring cylinders.
| Length of stress cycles (day) | Water applied (ml) | Temperature (°C) | RH (%) | ||||
|---|---|---|---|---|---|---|---|
| Expt1 | Expt2 | Expt1 | Expt2 | Expt1 | Expt2 | Expt1 | Expt2 |
| 4 | 6 | 250 | 300 | 24.67 | 23.15 | 51.96 | 50.14 |
| 4 | 5 | 300 | 300 | 23.73 | 23.2 | 50.38 | 48.78 |
| 4 | 4 | 300 | 400 | 23.17 | 24.61 | 55.92 | 46.14 |
| 5 | 7 | 300 | 450 | 24.7 | 22.55 | 43.46 | 53.64 |
| 5 | 5 | 350 | 550 | 24.3 | 24.34 | 46.4 | 49.78 |
| 5 | 5 | 300 | 650 | 24.2 | 24.51 | 54.85 | 50.99 |
| 4 | 350 | 24.39 | 47.2 | ||||
Table 2: Length of stress cycles (day) and quantity of water applied (ml plant-1), average day temperature and relative humidity during Expt 1 and 2. Seven stress cycles were made in Expt 1 and six in 2. Associated climatic data is shown as an average calculated from daily records.
Leaf stomata resistance and chlorophyll content
The terminal leaflet of fully expanded young leaf in comparable growth stages was chosen to measure stomata resistance on abaxial surface. The leaf blade was inserted carefully into the porometer (AP4, Delta-T Devices Ltd., Cambridge) head cuvette avoiding the leaf midrib being covered. Measurements were taken in the afternoon. Additionally leaf stomatal behavior in drought treatment was recorded 3 h after each stress cycle break and expressed as sm-1. Ten measurements were averaged for lower, middle, and upper leaves for the leaf chlorophyll content as ‘SPAD index’ using chlorophyll meter (SPAD-502 Plus, Konica Minolta Sensing Inc.).
Osmotic adjustment (OA)
OA was calculated based 155 on leaf relative water content (RWC) and osmotic potential (OP). RWC and OP were estimated on the youngest fully expanded leaves. Plants of the drought, cold and control treatment were re-watered in the evening before samples were taken. For determination of RWC, leaf disks (2.5 cm) were excised using a cork borer and immediately weighed to determine fresh weight (FW). Samples were placed into covered petri dishes filled with deionized water and kept for 24 h in dim illumination at 20°C to reach full hydration. Individual leaf samples were surface dried with a paper towel and weighed immediately for the turgid weight (TW). Finally, samples were dried at 105°C to weight constant in order to obtain the dry weight (DW). Leaf RWC for cold (RWCc), well-watered (RWCw) and drought (RWCd) were calculated separately using the following formula:
RCW=FW − DW
TW – DW
For the leaf OP measurement, 0.7 cm leaf disks were detached carefully from the plants using a cork borer immediately wrapped in aluminum foil and placed in liquid nitrogen. Samples were stored at -20°C. OP was measured with pre-calibrated C-52 chambers (C- 52 Chamber; Wescor Inc., Logan, Utah). OA was determined as the difference in measured osmotic potential (OP) between non-stressed and stressed leaves. Ludlow’s full-turgor adjustment method was followed as described in Masinde et al. [28] to calculate OA. In this method bound water is neglected and leaf OP at full turgor (OP100) of both stressed and non-stressed plants is given by:
OP100w=OPw x RWCw
OP100d=OPd x RWCd
OP100c=OPc x RWCc
where subscripts ‘w’ stands for well-177 watered,
‘c’ for cold and
‘d’ for drought stress treatment.
OA for cold (OAc)
Drought (OAd) treatment was separately calculated as follows:
OAc=OP100w - OP100c
OAd=OP100w - OP100d
Wilting score
Plants in the stressed were given wilting scores from 0 to 4; 0 for fully turgid plant, 1 for slightly wilted, 2 for moderately wilted, highly wilted scored 3, and 4 for flaccid plant. Scoring was done based on visual observations before each stress cycle break.
Green, non-green and total leaf area
Leaf area meter (model 3100, Li-COR, Lincoln) was used to obtain the total leaf area of individual plant. Freshly harvested leaves were individually scanned using a flatbed color image optical scanner (EPSON Perfection 4990 2.61). Obtained images were analyzed with the image analysis software (WinRHIZO 4.0, Version 2002c, Regent Instruments Inc.). Total green and non-green leaf area were obtained from WinRHIZO and summated for each plant and subjected to further statistical analysis.
Dry mass production and partitioning, and tolerance index
Individual plant parts were divided into root, stem, and leaf consequently to get respective dry mass. Root shoot ratio (R: S) for individual plant was assessed as a ratio of root dry mass to above ground dry mass. Similarly, stress tolerance index (TI) was determined based on the method postulated by Foolad and Lin [29] as a ratio of total shoot dry matter produced in stress treatment to shoot dry mass in control and expressed as percentage. All the data obtained were presented as means and standard errors of the means. Where meaningful, data were subjected to analysis of variance (ANOVA), correlation analysis (r) using the R statistical package (Version 2.9.2, R Foundation for Statistical Computing, Vienna, Austria). For multiple comparisons of means Tukey 200 test was used. In the results and discussion sections, cold or cold treatment is frequently used for low RZT where reasonable.
Results
Results from comparison of means of measured parameters showed meaningful interrelation and interaction between factors. There was significant interaction in parameters like root dry matter (RDM), nongreen leaf area (NGLA), leaf chlorophyll index and stomatal resistance (RS). Graft x treatment interactions were not significant in parameters like leaf dry matter (LDM), total dry matter (TDM), total leaf area (TLA), and green leaf area (GLA) (Table 3) and overall graft and treatment means were compared and results interpreted accordingly. Leaf area parameters and plant parts dry matter portioning were affected by low RZT and drought.
| Factors and interactions | Dry matter partioning (g) | Leaf area (cm2) | Leaf chlorophyll content | Stomata resistance (sm-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Root | Leaf | Total | Green | Non-green | Totalleaf | Lower leaf |
Middleleaf | Upperleaf | ||
| Ex Ex | Ex Ex | Ex Ex | Ex Ex | Ex Ex | Ex Ex | Ex Ex | Ex Ex | Ex Ex | Ex Ex | |
| pt pt | pt pt | pt pt | pt pt | pt pt | pt pt | pt pt | pt pt | pt pt | pt pt | |
| 1 2 | 1 2 | 1 2 | 1 2 | 1 2 | 1 2 | 1 2 | 1 2 | 1 2 | 1 2 | |
| GC | ** ** ** |
** ** ** |
** ** ** |
** ** * |
** ** * |
** ** * |
** * * |
** ** | ** ** ** |
** ** ** |
| Trt | ** ** ** |
** ** ** |
** ** ** |
** ** ** |
** ** ** |
** ** ** |
** ** ** |
** N * S |
** ** ** |
** ** ** |
| GCx Trt |
* N S |
N N S |
N N S S S |
N N S S |
** ** * |
N N SS |
** N S |
* N | ** ** S |
** ** ** |
Table 3: Level of significance (NS, non-significant,*P<0.05,** P<0.005,** *P<0.001) of different factors and their interactions for different physiological parameters.
Leaf area
In both experiments, the IL/RP produced significantly higher TLA (17.33%) and GLA (14.75%) than RP/RP (Table 4). Mean NGLA values between graft combinations (GCs) were not significant under the control treatment in Expt 1 and 2. In contrast to this, all four graft combinations were significantly different among each other in the cold treatment in Expt 1. RP/RP resulted to significantly higher NGLA compared to IL/RP under cold (36.8%) and drought (32.35%) treatment. The D/RP has produced the least NGLA (23.68 ± 0.573 cm-2) under cold, however fairly higher (22.3%) than IL/RP under drought in Expt 1 (Table 5). Strong positive correlation was found among GLA, TLA, LDM, RDM, and TDM (Table 6).
| Parameters | Grafting response | Treatment response | ||||
|---|---|---|---|---|---|---|
| GC | Expt1 | Expt2 | Treatment | Expt1 | Expt2 | |
| Total leaf area (cm2) | D/RP | 647.9 ±61.4c | - | Control | 1110.5 ±51.6A | 7063.2 ±172.1A |
| IL/IL | 757.7 ±74.4b | - | Cold | 532.3 ±30.9C | 1355.4 ±90.8C | |
| IL/RP | 950.7 ±82.2a | 3884.7 ±100.1a | Drought | 697.5 ±33B | 2683.8 ±86.7B | |
| RP/RP | 764.1 ±98.4b | 3517.2 ±110.6b | ||||
| Green leaf area (cm2) | D/RP | 645.8 ±78.5c | - | Control | 1180.7 ±51A | 6954.1 ±171.1A |
| IL/IL | 733.2 ±97.2bc | - | Cold | 451.9 ±26.5C | 1197.8 ±92.3C | |
| IL/RP | 940.9 ±102.9a | 3753.4 ±492.6a | Drought | 705.1 ±38.5B | 2527.2 ±90.4B | |
| RP/RP | 797 ±107.1bc | 3365.8 ±548.2b | ||||
| Leaf dry mass (g) | D/RP | 4.1 ±0.5c | - | Control | 7.7 ±0.3A | 25.8 ±0.4A |
| IL/IL | 4.6 ±0.6bc | - | Cold | 3.2 ±0.2C | 8.7 ±0.4C | |
| IL/RP | 6.2 ±0.6a | 16.6 ±1.4a | Drought | 4 ±0.2B | 12.3 ±0.4B | |
| RP/RP | 4.9 ±0.7b | 14.6 ±1.6b | ||||
| Total drymass (g) | D/RP | 7.6 ±0.9c | - | Control | 14.1 ±0.6A | 38.7 ±0.6A |
| IL/IL | 8.7 ±1b | - | Cold | 6.5 ±0.4B | 17.8 ±0.9B | |
| IL/RP | 11.7 ±1.1a | 26.6 ±2a | Drought | 7.1 ±0.3B | 18 ±0.8B | |
| RP/RP | 8.9 ±1.2b | 23.1 ±2.2b | ||||
Table 4: Means of different leaf area parameters (total and green leaf area in cm2), and dry mass portioning (leaf and total in g) produced by graft combinations under different treatment. SEM: Standard Error of the Mean. Means followed by the different letter (s) are statistically different according to Tukey (P ≤0.05). Lowercase letters denote differences among grafts and uppercase letters denote differences among treatments with in column according to Tukey (P ≤ 0.05),’-‘: not included
| Parameters | GC | Control (Mean ± SEM) | Cold (Mean ± SEM) | Drought(Mean ± SEM) | |||
|---|---|---|---|---|---|---|---|
| Non-greenleaf area (cm2) | Expt1 | Expt2 | Expt1 | Expt2 | Expt1 | Expt2 | |
| D/RP | 97.4 ± 2.7a | - | 23.6 ± 0.5a | - | 67.8 ± 4.3bc | - | |
| IL/IL | 98.9 ± 3.7a | - | 63.4 ± 2.7b | - | 84.5 ± 1.3ab | - | |
| IL/RP | 82.2 ± 6.7a | 116.3 ± 11.3a | 45.5 ± 2.3c | 131.7 ± 9b | 55.5 ± 3.6c | 138.4 ± 11.6b | |
| RP/RP | 91.4 ± 4.4a | 106.6 ± 10.7a | 101.5 ± 2.4a | 162.1 ± 11.4a | 103.1 ± 3.9a | 169.9 ± 14.1a | |
| Leaf chlorophyll content (SPAD Index) | |||||||
| Lower leaf | D/RP | 42.1 ± 0.8a | - | 29.2 ± 2.3b | - | 35.3 ± 1.1a | - |
| IL/IL | 40.8 ± 1.1a | - | 30 ± 1.1b | - | 34.1 ± 0.7a | - | |
| IL/RP | 38.4 ± 0.9a | 38.5 ± 0.6a | 34.6 ± 1.4a | 33.6 ± 1.3a | 38.3 ± 1.1a | 38.7 ± 0.6a | |
| RP/RP | 38.1 ± 0.7a | 35.2 ± 0.5b | 27.6 ± 1.1b | 31.1 ± 0.8b | 28 ± 0.7b | 36.1 ± 0.7b | |
| Middle leaf | D/RP | 54.9 ± 1.5a | - | 51.7 ± 0.4ab | - | 65.8 ± 2.6a | - |
| IL/IL | 56 ± 0.6a | - | 54 ± 0.7a | - | 61.6 ± 1.1a | - | |
| IL/RP | 53.6 ± 1a | 46.2 ± 0.5a | 55.4 ± 0.3a | 46.9 ± 1a | 61.6 ± 0.4a | 48 ± 0.1a | |
| RP/RP | 54.4 ± 0.3a | 43.6 ± 0.8a | 51.1 ± 0.7b | 44.8 ± 1.2b | 55.7 ± 1.5b | 45.8 ± 0.7b | |
| Upper leaf | D/RP | 49.6 ± 0.8a | - | 41.3 ± 0.7a | - | 43.3 ± 0.8c | - |
| IL/IL | 48.4 ± 0.5a | - | 44.6 ± 0.5a | - | 44.7 ± 1.5ab | - | |
| IL/RP | 45.7 ± 1.2a | 52.4 ± 0.7a | 43.4 ± 1.1a | 56.8 ± 0.8a | 45.2 ± 1.1a | 67.7 ± 1.3a | |
| RP/RP | 47.6 ± 0.9a | 51 ± 0.5a | 40.1 ± 1.4a | 56.2 ± 0.8a | 40.5 ± 0.6d | 61.7 ± 0.8b | |
| Stomata resistance (sm-1sup>) | D/RP | 140.6 ± 15.7a | - | 509.3 ± 24.3a | - | 5759.5 ± 22.8a | - |
| IL/IL | 147.4 ± 10.8a | - | 374.8 ± 15.3ab | - | 2979 ± 118.2c | - | |
| IL/RP | 143.1 ± 10.4a | 165.4 ± 9.8a | 518.3 ± 20.1a | 566.9 ± 37.1a | 5100.7 ± 123.2b | 5212.1 ± 149.8 a | |
| RP/RP | 142.8 ± 3.7a | 161.7 ± 9.7a | 297.2 ± 2.3b | 254.3 ± 24.4b | 2330.7 ± 34.9d | 2485.3 ± 191.1b | |
Table 5: Means of different measured parameters (NGLA in cm2), leaf chlorophyll lindex for the leaves from lower, middle and upper canopy, and stomatal resistance (sm-1) produced by different graft combinations in the cold, control and drought. SEM: Standard Error of the mean. Means followed by the different letter(s) are statistically different according to Tukey (P ≤ 0.05). Lower case letter(s)denote differences between grafts with in columns according to Tukey(P ≤ 0.05),‘-’: not included). GC: Graft Combination; IL: Introgressionline; RP: Recurrent parent; D: Donor; Exp 1: Experiment 1; Expt 2: Experiment 2.
| Leaf drymass | Root dry mass | Total drymass | Total leaf area | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Expt1 | Expt2 | Expt1 | Expt2 | Expt1 | Expt2 | Expt1 | Expt2 |
| Root dry mass | 0.79*** | 0.65*** | ||||||
| Totaldry mass | 0.98*** | 0.97*** | 0.85*** | 0.74*** | ||||
| Totalleaf area | 0.93*** | 0.97*** | 0.80*** | 0.58*** | 0.94*** | 0.93*** | ||
| Greenleaf area | 0.92*** | 0.97*** | 0.78*** | 0.58*** | 0.91*** | 0.93*** | 0.97*** | 0.99*** |
Table 6: Correlation coefficient (r value) between various agronomical parameters measured during experiment. Df=48, level of significance***P
Leaf chlorophyll content
Significant genotype x environment interaction on leaf chlorophyll content (LCC) of upper, middle, and lower canopy leaves was observed. Comparison of upper leaf LCC between IL/RP and RP/ RP in Expt 1 and Expt 2 was not significant under control and cold but IL/RP resulted significantly higher LCC (10.65%) under drought condition. Similar trend was observed 224 in middle leaf LCC in both experiments however, LCC mean difference between IL/RP and RP/RP found significant under both stress environments. IL/RP produced 6% under cold and 7.3% higher LCC under drought than RP/RP. Data on lower canopy LCC ranges from 27.6 to 42.2 and revealed the similar result pattern that was observed in case of middle and upper leaf LCC. Leaf chlorophyll content produced by IL/RP significantly higher than RP/RP under cold (16.5%) and drought (20.5%) (Table 5).
Dry matter produced and partitioning
Accumulated dry matter in plant parts significantly varied with different growing conditions. Treatment mean comparisons for LDM, RDM, and TDM showed that grafts produced higher dry mass under control. Drought LDM mean was significantly higher than cold LDM in both experiments. However means for RDM and TDM under cold and drought stress treatment were statistically not different. Significant genotype x treatment interaction was noted in RDM in Expt 1 but not in Expt 2 (Table 3). Averaged across treatments, in both experiments IL/RP produced significantly higher LDM than RP/RP. It is 26 % higher in Expt 1, 13.75 % in Expt2 (Table 4). Significant TDM differences were observed among treatments and graft combinations (GCs) in both experiments. In Expt 1, IL/RP produced significantly higher TDM (11.703 ± 0.301) than those of D/RP (7.623 ± 0.334), IL/ IL (8.747 ± 0.334) and RP/RP (8.998 ± 0.312). A similar result was observed in Expt 2 where IL/RP produced higher TDM (26.695 ± 0.551) than RP/RP (23.083 ± 0.575). Similar result was obtained for LDM. The IL/RP produced higher RDM (1.268 ± 0.255) compared to D/RP (0.6 ± 0.094) in the cold treatment. In the control, IL/RP had significantly higher RDM (2.67 ± 0.2) than D/RP (1.138 ± 0.081). Similar result was observed in the drought where IL/RP produced significantly higher RDM (1.145 ± 0.128) than that of D/RP (0.637 ± 0.079). Other comparisons were not significant. In Expt 2, averaged 249 across treatments, IL/RP produced 44.5% higher RDM than RP/ RP (Table 4). Strong correlation was observed between TLA and TDM (r=0.935, Df=48, level of significance *** P<0.001) (Table 6). There were no marked differences in averaged root shoot ratio (R: S) data from Expt 1 although IL/RP showed slightly higher ratio than D/RP and RP/RP. In Expt 2, IL/RP produced significantly higher R: S than RP/RP which is 33.3% higher. Similarly, averaged across treatments, mean R: S value for the cold treatment was fairly higher than control in Expt 2 (Table 7).
| Rootshoot ratio (R:S) | |||||
|---|---|---|---|---|---|
| Graft combinations | Mean ±SEM | Treatments | Mean ±SEM | ||
| Expt1 | Expt2 | Expt1 | Expt2 | ||
| IL/IL | 0.15 ±0.01n.s | - | Cold | 0.15 ±0.01N.S. | 0.12 ±0.009AB |
| D/RP | 0.12 ±0.01n.s | - | Control | 0.16 ±0.01N.S. | 0.09 ±0.005C |
| IL/RP | 0.17 ±0.01n.s | 0.12 ±0.008a | Drought | 0.15 ±0.01N.S. | 0.11 ±0.01BC |
| RP/RP | 0.13 ±0.01n.s | 0.09 ±0.006b | |||
Table 7: Mean root shoot ratio produced by different graft combinations of tomato genotypes during experiments. SEM: Standard error of the mean, treatment means followed by different upper case letter(s) denote differences among treatments and graft combination mean followed by different lower case letter (s) denote differences among grafts with in columns according to Tukey (P ≤ 0.05).
Stomata conductance
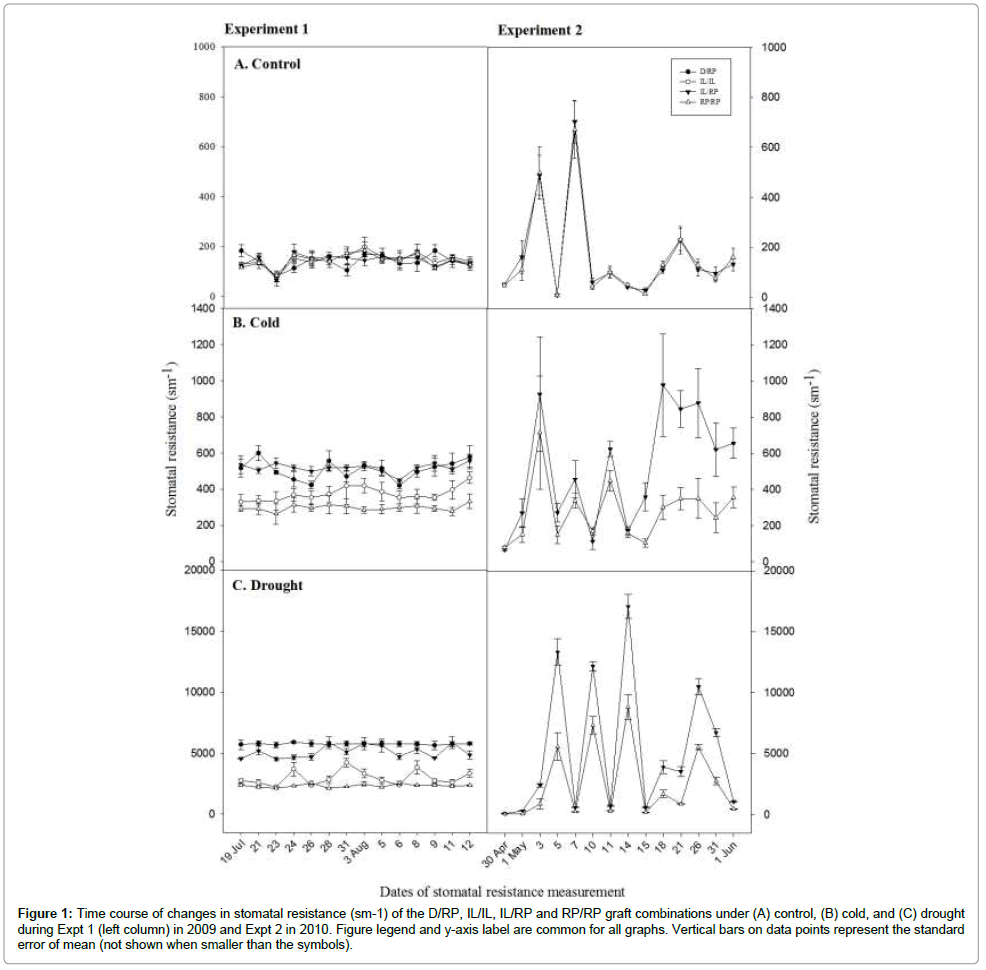
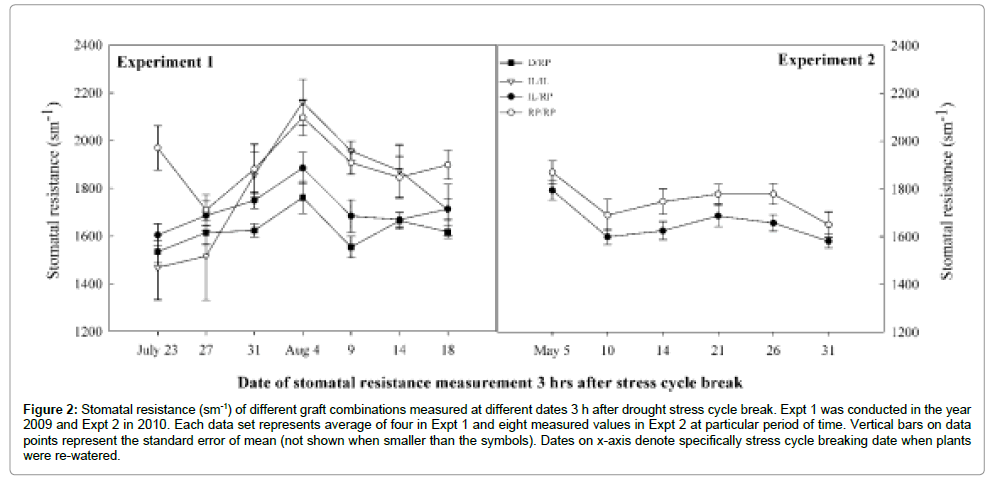
Stomatal behavior of treated plants were observed as a leaf stomatal resistance (RS) and expressed as sm-1. Test statistics on SR showed highly significant genotype x treatment interaction (P ≤ 0.05) (Table 3). Result clearly showed that IL/RP graft combination highly resisted to stomata opening compared to RP/RP under environmental stress condition. No difference between graft combinations was observed under control in both experiments. However, there was marked differences in SR under drought and cold treatments. The IL/RP showed 98% and 113.5% higher RS than RP/RP in cold and drought treatment respectively (Figure 1). Similar result observed between D/ RP and RP/RP in Expt 1, where D/RP was 109.2% more resisted than RP/RP. Data on stomatal resistance obtained 3 hours after drought stress break showed contrasting result than those incurred from regular measurement. During stress recovery process in both experiments, significantly faster recovery in stomatal conductance was noticed. In Expt 1, both D/RP (1624.5 ± 42.7) and IL/RP (1713.1 ± 50) significantly lowered down the RS than RP/RP (1901.3 ± 32.1). Similar to the result in Expt 1, in Expt 2 also IL/RP showed significantly lower RS (1657 ± 36.2) compared to RP/RP (1752.2 ± 50.9) (P ≤ 0.05) (Figure 2).
Figure 1: Time course of changes in stomatal resistance (sm-1) of the D/RP, IL/IL, IL/RP and RP/RP graft combinations under (A) control, (B) cold, and (C) drought during Expt 1 (left column) in 2009 and Expt 2 in 2010. Figure legend and y-axis label are common for all graphs. Vertical bars on data points represent the standard error of mean (not shown when smaller than the symbols).
Figure 2: Stomatal resistance (sm-1) of different graft combinations measured at different dates 3 h after drought stress cycle break. Expt 1 was conducted in the year 2009 and Expt 2 in 2010. Each data set represents average of four in Expt 1 and eight measured values in Expt 2 at particular period of time. Vertical bars on data points represent the standard error of mean (not shown when smaller than the symbols). Dates on x-axis denote specifically stress cycle breaking date when plants were re-watered.
Osmotic adjustment
Different statistics on osmotic adjustment (OA) were found among treatments and GCs. In Expt 1, mean OA (MPa) in drought treatment was significantly higher than cold. The D/RP graft combination 274 showed significantly higher OA compared to RP/RP. No significant difference was found among other GCs. In Expt 2, IL/RP tended to be higher in OA than RP/RP, and mean OA in drought stress was numerically higher than cold. However, OAs among treatments and graft combinations were not statistically different (Table 8).
| Osmotic adjustment (MPa) | |||||
|---|---|---|---|---|---|
| Graft combination | Mean ± SEM | Treatments | Mean ± SEM | ||
| Expt1 | Expt2 | Expt1 | Expt2 | ||
| D/RP | 4.4 ± 0.1a | - | Cold | 0.8 ± 0.07B | 0.08 ± 0.07N.S |
| IL/IL | 1.04 ± 0.1ab | - | Drought | 1.3 ± 0.09A | 0.12 ± 0.06N.S |
| IL/RP | 1.04 ± 0.1ab | 0.1 ± 0.07n.s. | |||
| RP/RM | 0.9 ± 0.07b | 0.09 ± 0.05n.s | |||
Table 8: Mean osmotic adjustment (MPa) for different graft combinations and treatments. SEM: Standard Error of the Mean. Graft combination (GC) mean followed by different lower case letter(s) denote differences between GC and treatment mean followed by different upper case letter(s) denote differences between treatments within columns according to Tukey (P ≤ 0.05). N.S. or n.s.: Not Significant.’-‘: not included; IL: Introgression line; RP: Recurrent parent; D: Donor; Exp 1: Experiment 1; Expt 2: Experiment 2
Wilting scores
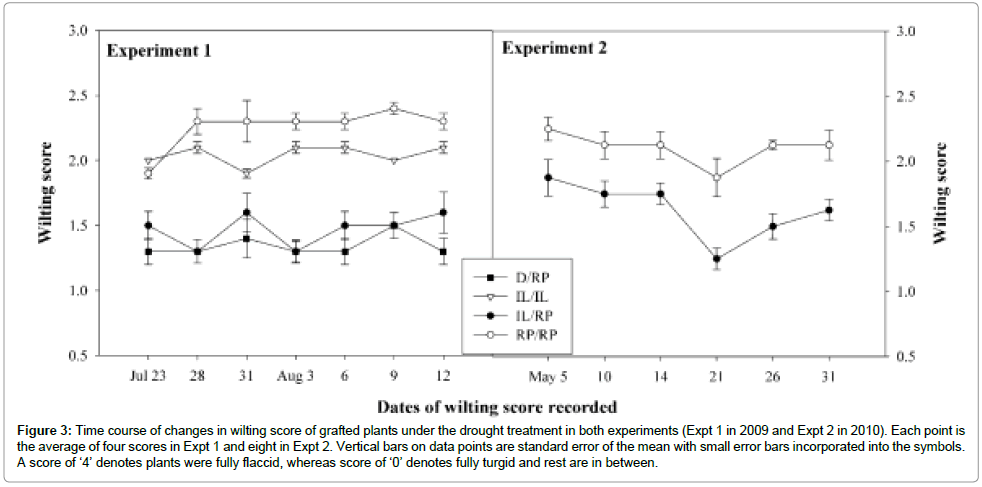
Tomato graft combinations responded differentially in wilting under the drought. The RP/RP plants responded strongly to water stress with the highest score. D/RP was less affected resulting the lowest score. Wilting scores produced by all four graft combinations were significantly different among each other. Similar result observed in Expt 2 where RP/RP affected by stress and produced significantly higher score than IL/RP (Figure 3).
Figure 3: Time course of changes in wilting score of grafted plants under the drought treatment in both experiments (Expt 1 in 2009 and Expt 2 in 2010). Each point is the average of four scores in Expt 1 and eight in Expt 2. Vertical bars on data points are standard error of the mean with small error bars incorporated into the symbols. A score of ‘4’ denotes plants were fully flaccid, whereas score of ‘0’ denotes fully turgid and rest are in between.
Tolerance index
A mean TI (%) of IL/RP was significantly higher than that of RP/ RP (P ≤ 0.05) in both experiments (Table 9). The IL/RP showed 42.33% higher TI in Expt 1 and 18.58% in Expt 2 than RP/RP. No statistical difference was found between averaged TI of cold and drought. A highly significant correlation was observed between TI and total dry matter produced under stressed environments (r=0.66 in Expt 1 and 0.96 in Expt 2, n=32, level of significance ***P<0.001).
| Tolerance index (%) ± SEM | |||||
|---|---|---|---|---|---|
| Graftcombination | Expt1 | Expt2 | Treatments | Expt1 | Expt2 |
| D/RP | 50.6 ±5.4ab | - | Cold | 47.2 ±2.8N.S. | 45.02 ±2.4N.S. |
| IL/IL | 49.5 ±2.8ab | - | Drought | 51.8 ±3.2N.S. | 45.4 ±1.8N.S. |
| IL/RP | 57.4 ±4.2a | 49.1 ±2.1a | |||
| RP/RP | 40.3 ±2.1b | 41.3 ±1.5b | |||
Table 9: Mean tolerance index for different graft combinations and treatments. SEM: Standard Error of the Mean. Graft Combination (GC) mean followed by different lower case letter(s) denote differences between GCs and treatment mean followed by different upper case letter(s) denote differences between treatments within columns according to Tukey (P ≤ 0.05). N.S. or n.s.: not significant; “-’: not included. IL: Introgression line; RP, Recurrent parent; D: Donor; Exp 1: Experiment 1; Expt 2: Experiment 2.
Discussion
In the present study, an attempt has been made to explore dynamics of cold and drought stress tolerance in tomato introgression lines. It studied tomato ILs together with its donor and recurrent parent and examined some major physiological traits involved in cold and drought tolerance. The main focus of this study was to compare the two graft combinations IL/RP and RP/RP under different environmental conditions. Results clearly showed the differential response of tomato genotypes to low RZT (cold) and drought. Observed variation in agronomic traits can be due to genetic 298 or morphological or environmental factors [8,30,31] or combination of them. Abiotic stress tolerance is a genetically controlled phenomena based on additive QTL-effects [29]. The present study elucidated higher green leaf area and lower non-green leaf area for IL/RP compared to self-grafted RP in stress conditions (Tables 4 and 5). A similar pattern was also observed for the leaf chlorophyll content. This, together with wilting symptoms recorded on IL/RP (Figure 3), indicates a higher tolerance of IL/RP to cold and drought compared to RP/RP. In agreement to the results, higher green area, chlorophyll content in leaf and photosynthesis rate were recorded for S. peruvianum, a chilling tolerant wild tomato, compared to cultivated tomato cultivar under chilling stress [32]. Strong variation was noticed on leaf chlorophyll content. Significantly higher chlorophyll index in lower and middle leaves were observed in IL/RP compared to RP/RP under the cold and drought treatment (Table 5) and upper leaf chlorophyll index was only different in water stress condition. This indicates that leaf chlorophyll content is also a physiological parameter affected by abiotic stress and it varies among genotypes. Compared to drought tolerant wild tomato (S. pennellii), significant yellowing of lower leaves and low chlorophyll contents were also reported by Torrecillas et al. [33] in cultivated tomato during 7 days long water stress condition. The presented data on chlorophyll content is in line with Rong-hua et al. [34] who reported that chlorophyll content was significantly higher in a drought tolerant barley genotype compared to genotypes sensitive to water stress. The data supports the hypothesis that stress tolerant genotypes slow down the wilting processes and maintain green leaf area and growth. Significant effect of suboptimal growing conditions was observed in root, leaf and stem dry matter deposition (Table 4). Averaged across the treatments, total leaf area in the cold treatment was found to be lower compared to the drought and control condition. This may be 323 due to continuous low RZT stress in the cold treatment whereas plants under drought were re-watered during stress cycle break that could have led to better leaf expansion. Though leaf expansion rate was not assessed in the current study, observed reduced leaf area found in soybean showed that severe stress reduced leaf size due to poor leaf expansion rates in cold susceptible soybean genotypes [35]. Current result on dry matter is consistent to the result by Balibrea et al. [36] working on tomato who reported that leaf area and total dry matter were significantly affected by the salinity in salt sensitive genotype. A similar result was also observed by Katerji et al. [37] in tomato. Leaf area is important because it has direct impact on photosynthesis. This can be due to the strong correlation between leaf area and carbon assimilation rate, which is directly related to the plant growth and total biomass production [38]. Strong correlation between total leaf area and total dry mass was observed in recent study (Table 6). Larger leaf area and higher dry matter produced by plant indicates that IL/RP performed better under stress condition than RP/RP. Supporting the current findings, a study has confirmed the comparable increment in net photosynthesis in cultivated tomato grafted onto S. Mammosum in contrast to selfgrafted39 tomatoes under water stress [39]. Further support comes from a study that revealed the greater capacity of S. habrochaites for CO2 uptake compared to common tomato as before and after chilling exposure [36,40,41] found significantly higher net photosynthesis rate in salt tolerant tomatoes with positive correlation between photosynthesis and plant growth. Higher total leaf area of IL/RP indicates the higher relative growth rate of IL/RP in comparison to RP/ RP under low RZT. This result is consistent with the work done by van der Ploeg et al. [42] who compared ungrafted S. pennellii, S. habrochaites, and common tomato cultivar in low temperature. Markedly reduced relative growth rate in the commercial tomato cultivar was mainly attributed to decreased leaf area. 348 The present study, however unable to compare D/RP graft performance in the second experiment due to lack of seed but the results from the Expt 1 showed significant difference in total leaf area between IL/RP and D/RP under cold and drought stress. Significant variation between graft combinations in leaf area and leaf dry mass was also pronounced in the present study. This is in agreement with the result from the study on cultivated and wild tomatoes (S. habrochaites and S. peruvianum) where interspecific comparison showed significant variations among the measured traits [43] Similar results were found in tomato grafted onto rootstocks of S. habrochaites in low RZT [24] Differential response of graft combinations to root shoot ratio (R: S) under stress was observed in this study (Table 7). The IL/RP produced significantly higher R: S compared to RP/RP in the Expt 2 while tended to be higher in the Expt 1. The finding is also resembled with findings from Torrecillas et al. [33] working on tomato. It has been reported lower R: S in cultivated tomato compared to S. pennellii. In hot pepper, significantly higher R: S was observed in the drought stressed plants compared to the well-watered [44] it can be concluded that ILs have high capacity to adjust their R: S to stress environment in comparison with the RP. Increasing R: S at 10°C RZT may adjust the root system to overcome the lower water absorption due to decreased root hydraulic conductivity and increased water viscosity [9,45]. Significant interaction between treatment and graft combinations in root dry mass indicates that the effect of the growing condition depends on the genotype used. More interestingly, IL/RP was able to produce higher root dry matter than D/RP and RP/RP under cold and drought stress. This result indicates that IL as a rootstocks can develop underground plant part better than donor rootstocks or the RP. According to the findings of Venema et al. [24] common tomato grafted onto S. habrochaites rootstock produced higher leaf and root dry matter in low RZT and root to shoot ratio (373 R: S) was also higher. Although a detailed study is required for comparative root morphological analysis observed higher root dry matter and larger R: S indicates the strong variation in root growth rate between the rootstocks used. Results from the current study elucidated higher genotypic variation in stomatal closure. Higher stomatal resistance was observed in IL/RP under both stress environment. In contrast to this, the same graft combination showed higher stomatal conductance few hours after drought stress cycle break. This indicates that drought tolerance of IL/RP is due to more efficient stomatal control. In agreement with this result, higher stomatal resistances were also reported for S. pennellii under drought stress [33], and for S. habrochaites in low RZT [9]. A similar result was found in S. cheesmaniae, S. chmielewskii, S. lycopersicum var. cerasiforme, S. neorickii and S. pimpinellifolium [46] where significantly higher stomatal resistance was observed under drought condition compared to the control. Apparently current result contradicts the comparatively larger biomass accumulation in IL/RP while persisted higher stomatal resistance. The reason is not so clear but some contextual findings in past studies may support the outcomes. Vallejos and Pearcy [47] demonstrated that S. habrochaites and its hybrid (developed by crossing with S. lycopersicum) showed faster acclimatization at low temperature by increased photosynthetic rates. Choluj et al. [48] showed a positive linear relationship between the net gas exchange and stomatal conductance in chilling tolerant tomato cultivars under short-term chilling condition. They found no significant effect of stomatal resistance in net CO2 assimilation when tolerant tomato genotype shortly exposed to chilling temperature (2°C). Similar study with a chilling tolerant maize cultivar showed that net assimilation rate was comparatively higher than that of sensitive one and it reached control values after stress release [49]. Another study on tomato 398 grafted onto S. mammosum showed the comparable increment in net photosynthesis and leaf conductance in contrast to self-grafted tomato cultivars in water stress condition [39]. More recently, Campos et al. [50] in tomato reported that carbon assimilation rate was not affected by partial drought compared to control, however, higher stomatal resistance and lower transpiration were observed. Stomatal resistance of IL/RP after stress cycle break was significantly lower than that of RP/ RP (Figure 2). Such recovery in stomatal regulation in IL/RP may lead to vegetative growth recovery which can be the reason for higher green leaf area and total leaf area, and so as higher carbon fixation. A study on wild tomatoes revealed a rapid recovery in stomatal conductance and net assimilation after breaking the water stress [46,51] Easlon, Richards, Anyia and Herzog observed the same pattern of recovery in cowpea. Water stressed plants showed higher dry matter gain than unstressed plants when re-watered after a week-long stress. Compensatory growth was evidenced during the recovery process. Sharp et al. [52] showed that tolerant tomato genotypes recovered shoot growth due to endogenous ABA accumulation. Findings of Sharp et al. support the hypothesis that higher ABA concentration in tomato leaf caused early stomata closure, and post stress ABA involvement in leaf expansion and shoot growth recovery [53,54]. Current result is in line with the results from cowpea and tomatoes and it supports the hypothesis that higher stomatal resistance during stress and low stomatal resistance during recovery result into higher biomass production in tolerant tomato genotypes. Results showed that IL/RP demonstrated higher stomatal resistance and higher biomass deposition under continuous cold treatment compared to self-grafted RP/RP plants. This could be due the genotypes used as rootstocks. ILs rootstocks carrying QTL allele, stm9, for cold tolerance may have a positive effect in stomatal behavior resulting shoot turgor and higher biomass. Although, the response of genotypes after cold stress break was not estimated, but based on the drought recovery observations 423 similar phenotypic variation among graft combinations can be presumed. Higher total leaf area, green leaf area, leaf dry matter in drought treatment compared to the cold clearly reflects the stress break affect the overall plant growth and development. Past studies uncovered the faster biosynthesis of ABA in cold tolerant maize [55] and tomato genotypes [7]. Faster ABA biosynthesis was responsible for early stomata closure. As reviewed by Blum [56] higher biomass production is associated with faster poststress recovery with reducing stomatal resistance that allows greater carbon fixation and transpiration. This recovery capacity appears to be important for the final yield. Osmotic adjustment value obtained for IL/RP was not large enough to show a difference from RP/RP but it produced higher total dry matter (Table 8). As reported by Blum [57] plants may not respond to osmotic adjustment under rapidly imposed stress environment because it is a slow process and takes more time to the adjustment depending upon the genotype. However, this result is in line with the finding of Bloom et al. [9] who reported the non-significant response of grafted tomato on shoot turgor maintenance. This result supports the hypothesis that the osmotic adjustment is part of whole to obtain stress tolerance. It may contribute to but not a major contributor for stress tolerance in plants under stress environments [58]. The nonsignificance correlation between osmotic adjustment and total dry matter from this study is consistent with the result observed by Katerji et al. [37] in tomato where non-significant correlation was found between osmotic adjustment and total dry mass under salt stress. Results showed significant wilting responses of graft combinations to drought. Significantly low wilting scores of IL/RP is in agreement with the findings from Bloom et al. [9] working in tomato. Chilling sensitive genotype resulted into higher wilting scores compared to the tolerant one when exposed to 4°C for 2 h. Present work, however, did not study the wilting response to cold but similar genotypic response 448 can be estimated based on the previous findings and current observations. Present study elucidated that IL/RP results in higher tolerance index (Table 9). The tolerance index was highly correlated with total biomass produced. This is consistent with the findings of Foolad and Lin [29] in tomatoes where such index of the filial and backcross progenies of cold tolerant parents was significantly higher than those of a cold-sensitive tomatoes. Further total dry mass and tolerance index was highly correlated. Significantly higher tolerance index of IL/RP than RP/RP indicates the superior performance of the IL/RP due to an inherent abiotic stress tolerance of ILs. Rao et al. [41] working on tomato cultivars observed significantly higher tolerance index in drought tolerant cultivar compared to susceptible one during vegetative phase. Further support to this comes from a study on Hordeum vulgare and H. chilense where Forster et al. [59] came to the similar conclusion and mentioned that salt tolerance is as a result of additive genetic control. This supports the statement that tolerance index is one of the important tools to compare the genotype based on their phenotypic performances. This study succeeded to graft recurrent parent onto the S. habrochaites rootstocks (D/RP) under normal greenhouse conditions with higher survival rate. Earlier such type of interspecific grafting was reported as incompatible [9]. Data on higher performance of IL rootstocks under suboptimal growing condition are highly consistent with previously published data and suggest that ILs can replace rootstocks in the conventional grafting practices. Under adverse growth conditions total dry matter of cultivated tomato grafted with tolerant rootstocks was considerably higher than self-grafted plants [21,24,25]. The basis for argument can be more genetic, for instance the expression of favorable alleles introgressed from the wild relative S. habrochaites. In their work on tomatoes [14,18,19,60] Goodstal, Monforte, Tanksley, Foolad and Tanksley have suggested possible crop improvement programs by using ILs.
Conclusion
This study can describe logically the agronomical parameters and physiological basis of low RZT and drought tolerance in tomato. Low RZT and drought hampered plant function and substantially reduced carbon assimilation. Grafted plants onto ILs rootstocks performed better than self-grafted RP in terms of leaf area, dry matter production, and showed better recovery ability after drought release. The parameters studied herein suggest that IL/RP efficiently controlled stomata opening and maintained growth under stress and proved comparatively higher tolerance to low RZT and drought. When tomato is grafted onto IL LA3957, the stomatal resistance increases in response to cold and drought stress so that leaf turgor (and therefore growth) can be stabilized. This in contrast to self-grafted tomato plants which show symptoms of wilting and senescence. The green and total leaf area, stomatal resistance, root shoot ratio, and tolerance index can be considered as potential physiological markers for cold and drought tolerance study in tomato breeding. ILs appeared to be a valuable gene pool for tomato breeding to improve low temperature and drought tolerant‘energy-efficient’ tomato cultivars.
Acknowledgements
Authors gratefully acknowledge the German Academic Exchange Service (DAAD) for providing scholarship to Damodar Poudyal, and recognize many colleagues who have contributed to the study to a range of problems
References
- Perez-Alfocea F, Balibrea ME, Santa-Curz A, Estan MT (1996)Â Agronomical and physiological characterization of salinity tolerance in a commercial tomato hybrid. Plant and Soil 180: 251-257.
- Hu WH, Zhoua YH, Dua YS, Xiaa JX, Yua JQ (2006)Â Differential response of photosynthesis in greenhouse- and field- ecotypes of tomato to long-term chilling under low light. Journal of Plant Physiology 163: 1238-1246.
- Venema JH, Linger P, van Heusden AW, Hasselt PR, Bruggemann W (2005) The inheritance of chilling tolerance in tomato (Lycopersicon spp.). Plant Biology 7: 118-130.
- Mahajan S, Tuteja N (2005)Â Cold, salinity and drought: an overview. Archives of Biochemistry and Biophysics 444: 139-158.
- Martin B, Donald RO, Boyer JS (1981)Â Impairment of photosynthesis by chilling temperatures in tomato. Plant Physiology 68: 329-334.
- Allen DJ, Ort DR (2001)Â Impacts of chilling temperatures on photosynthesis in warm climate plants. Trends in Plant Science 6: 36-42.
- Starck Z, Choluj D, Gawronska H (1998) The effect of drought hardening and chilling on ABA content in xylem sap and ABA delivery rate from root of tomato plant. Acta Physiologiae Plantarum 20: 41-48.
- Nakazato T, Warren DL, Moyle LC (2010)Â Ecological and geographic modes of species divergence in wild tomatoes. American Journal of Botany 97: 680-693.
- Bloom AJ, Zwieniecki MA, Passioura JB, Randall LB, Holbrook NM et al. (2004)Â Water relations under root chilling in a sensitive and tolerant tomato species. Plant, Cell and Environment 27: 971-979.
- Venema JH, Posthumus F, van Hasselt PR (1999a)Â Impact of suboptimal temperature on growth, photosynthesis, leaf pigments and carbohydrates of domestic and high-altitude wild Lycopersicon species. Journal of Plant Physiology 155: 711-718.
- Blum A(2005) Drought resistance, water-use efficiency, and yield potential- are they compatible, dissonant, or mutually exclusive? Australian Journal of Agricultural Research 56: 1159-1168.
- Blum A (1989)Â Osmotic adjustment and growth of barley genotypes under drought stress. Crop Science 29: 230-233.
- Sellin A (2001)Â Hydraulic and stomatal adjustment of Norway spruce trees to environmental stress. Tree Physiology 21: 879-888.
- Tanksley SD, Mccouch SR (1997)Â Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277: 1063-1066.
- Hanson PM, Sitathani K, Sadashiva AT, Yang RY, Graham E et al. (2007)Â Performance of Solanum habrochaites LA1777 introgression line hybrids for marketabletomato fruit yield in Asia. Euphytica 158: 167-178.
- Peralta I, Knapp S, Spooner DM (2005)Â New species of wild tomatoes (Solanum section Lycopersicon: Solanaceae) from northern Peru. Systematic Botany 30: 424-434.
- Vallejos CE, Tanksley SD(1983) Segregation of isozyme markers and cold tolerance in an interspecific backcross of tomato (Lycopersicon esculentum × Lycopersicon hirsutum). Theoretical and Applied Genetics 66: 241-247.
- Monforte AJ, Tanksley SD(2000)Â Development of a set of near isogenic and backcross recombinant inbred lines containing most of the Lycopersicon hirsutum genome in a L. esculentum genetic background: a tool for gene mapping and gene discovery. Genome 43: 803-813.
- Goodstal FJ, Kohler GR, Randall LB, Bloom AJ, St. Clair DA (2005) AÂ major QTL introgressed from wild Lycopersicon hirsutum confers chilling tolerance to cultivated tomato (Lycopersicon esculentum). Theoretical and Applied Genetics 111: 898-905.
- Khah EM, Kakava E, Mavromatis A, Chachalis D, Goulas C (2006)Â Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and openfield. Journal of Applied Horticulture 8: 3-7.
- Oda M (2007)Â Vegetable seedling grafting in Japan. Acta Horticulturae 759: 175-180.
- Santa-Cruz A, Martinez-Rodriguez MM, Perez-Alfocea F, Romero-Aranda R, Bolarin MC (2002) The rootstock effect on the tomato salinity response depends on theshoot genotype. Plant Science 162: 825-831.
- Martinez-Rodriguez MM, Estan MT, Moyano E, Gracia-Abellan JO, Flores FB et al.(2008) The effectiveness ofgrafting to improve salt tolerance in tomato when an ‘excluder’ genotype is used as scion. Environmental and Experimental Botany 63: 392-401.
- Venema JH, Dijk BE, Bax JM, Hasselt PR, Elzeng JM (2008)Â Grafting tomato (Solanum lycopersicum) onto the rootstock of a high-altitude accession of Solanum habrochaites improves suboptimal-temperature tolerance. Environmental and Experimental Botany 63: 359-367.
- Black LL, Wu DL, Wang JF, Kalb T, Abbass D et al. ( 2003)Grafting tomatoes for production in the hot-wet season. International Cooperators' Guide, AVRDC Pub 03-551.
- Oda M (1995)new grafting method for fruit-bearing vegetables in Japan. Japan Agricultural Research Quarterly 29: 187-194.
- Gosselin A, Trudel MJ(1984)Â Interactions between root-zone temperature and light levels on growth, development and photosynthesis of Lycopersicon esculentum Mill. Cultivar 'Vendor'. Scientia Horticulturae 23: 313-321.
- Masinde PW, Stützel H, Agong SG, Fricke A (2005) Plant growth, water relations and transpiration of spider plant [Gynandropsis gynandra (L.) Briq.] under water-limited conditions. Journal of the American Society for Horticultural Science 130: 469-477.
- Foolad MR, Lin GY (2001)Â Genetic analysis of cold tolerance during vegetative growth in tomato, Lycopersicon esculentum Mill. Euphytica 122: 105-111.
- Byers D (2008) Components of phenotypic variance. Nature Education 1: 161.
- Praba ML, Cairns JE, Babu RC, Lafitte HR (2009)Â Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. Journal of Agronomy and Crop Science 195: 30-46.
- Brüggemann W, Linger P (1994)Long-term chilling of young tomato plants under low light. IV. Differential responses of chlorophyll fluorescence quenching coefficients in Lycopersicon species of different chilling sensitivity. Plant and Cell Physiology 35: 585- 591.
- Torrecillas A, Guillaume C, Alarcon JJ, Ruiz-Sanchez MC (1995)Â Water relations of two tomato species under water stress and recovery. Plant Science 105: 169-176.
- Rong-Hua LI, Pei-Guo GUO, Baum M, Grando S, Ceccarelli S (2006)Â Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agricultural Sciences in China 5: 751-757.
- Bunce JA (1977)Â Leaf elongation in relation to leaf water potential in soybean. Journal of Experimental Botany 28: 156-161.
- Balibrea ME, Dell'amico J, Bolarin MC, Perez-Alfocea F (2000)Â Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiologia Plantarum 110: 503-511.
- Katerji N, van Hoorn JW, Hamdy A, Mastrorilli M (2003)Â Salinity effect on crop development and yield, analysis of salt tolerance according to several classification methods. Agricultural Water Management 62: 37-66.
- Jones HG (1992)Â Plant and microclimate: a quantitative approach to environmental plant physiology. Second edition. Cambridge University Press, Cambridge 264-290.
- Weng JH (2000) The role of active and passive water uptake in maintaining leaf water status and photosynthesis in tomato under water deficit. Plant Production Science 3: 296-298.
- Yakir D, Rudich J, Bravdo BA (1986)Â Adaptation to chilling: photosynthetic characteristics of the cultivated tomato and a high altitude wild species. Plant, Cell and Environment 9: 477-484.
- Rao NKS, Bhatt RM, Sadashiva AT (2000)Â Tolerance to water stress in tomato cultivars. Photosynthetica 38: 465-467.
- van der Ploeg A, Heuvelink E, Venema JH (2007)Â Wild relatives as a source for suboptimal temperature tolerance in tomato. Acta Horticulturae 761: 127-133.
- Venema JH, Posthumus F, De Vries M, van Hasselt PR (1999b)Â Differential response of domestic and wild Lycopersicon species to chilling under low light: growth, carbohydrate content, photosynthesis and the xanthophyll cycle. Physiologia Plantarum 105: 81-88.
- Guang-Cheng S, Na L, Zhan-Yu Z, Shuang-En Y, Chang-Ren C(2010)Â Growth, yield and water use efficiency response of greenhouse-grown hot pepper under Time-Space deficit irrigation. Scientia Horticulturae 126: 172-179.
- Equiza MA, Miravé JP, Tognetti JA(2001) Morphological, anatomical and physiological responses related to differential shoot vs. root growth inhibition at low temperature in spring and winter wheat. Annals of Botany 87: 67-76.
- Easlon HM, Richards JH (2009)Â Drought response in self-compatible species of tomato (Solanaceae). American Journal of Botany 96: 605-611.
- Vallejos CE, Pearcy RW (1987)Â Differential acclimation potential to low temperatures in two species of Lycopersicon: photosynthesis and growth. Canadian Journal of Botany 65: 1303-1307.
- Choluj D, Kalaji HM, Niemyska B(1997)Â Analysis of the gas exchange components in chilled tomato plants. Photosynthetica 34: 583-589.
- Perez DJJ, Irigoyen JJ, Sanchez-Diaz M (1997)Â Chilling of drought-hardened and non hardened plants of different chilling sensitive maize lines changes in water relations and ABA contents. Plant Science 122: 71-79.
- Campos H, Trejo C, Pena-Valdivia CB, Ramirez-Ayala C, Sanchez-Garcia P (2009)Â Effect of partial root zone drying on growth, gas exchange, and yield of tomato (Solanum lycopersicum L.). Scientia Horticulturae 120: 493-499.
- Anyia AO, Herzog H(2004)Â Water-use efficiency, leaf area and leaf gas exchange of cowpeas under mid-season drought. European Journal of Agronomy 20: 327-339.
- Sharp RE, Lenoble ME, Else MA, Thorne ET, Gherardi F(2000)Â Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. Journal of Experimental Botany 51: 1575-1584.
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ (2004)Â Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. Journal of Experimental Botany 55: 2353-2363.
- Dodd IC (2005) Root-to-shoot signaling: assessing the roles of ‘up’ in the up and down world of long-distance signaling in planta. Plant and Soil 274: 251-270.
- Capell B, Dörffling K (1993) Genotype-specific differences in chilling tolerance of maize in relation to chilling-induced changes in water status and abscisic acid accumulation. Physiologia Plantarum 88: 638-646.
- Blum A (2009)Â Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Research 112: 119-123.
- Blum A (1988)Â Plant breeding for stress environments. CRC press, Boca Raton FL 28-33.
- Taiz L, Zeiger E (2006) Stress physiology. Plant Physiology, Fourth edition. Sinauer Associates, Inc. 647-687.
- Forster BP, Phillips MS, Miller TE, Baird E, Powell W (1990)Â Chromosome location of genes controlling tolerance to salt (NaCl) and vigour in Hordeum vulgare and H. chilense. Heredity 65: 99-107.
- Foolad MR (1999)Â Genetics of salt tolerance and cold tolerance in tomato: quantitative analysis and QTL mapping. Plant Biotechnology 16: 55-64.
Citation: Poudyala D, Khatria L, Uptmoora R (2015) An Introgression of Solanum habrochaites in the Rootstock Improves Stomatal Regulation and Leaf Area Development of Grafted Tomatoes under Drought and Low Root-Zone- Temperatures. Adv Crop Sci Tech 3:175. DOI: 10.4172/2329-8863.1000175
Copyright: © 2015 Poudyala D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16327
- [From(publication date): 8-2015 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 15270
- PDF downloads: 1057