An Innovative Surgical Management of Complicated Bile Duct Variant in Emergency Living Donor Adult Liver Transplantation: Initial Experience
Received: 04-Aug-2017 / Accepted Date: 19-Sep-2017 / Published Date: 26-Sep-2016 DOI: 10.4172/2475-7640.1000109
Abstract
Background: The incidence of biliary complications after living donor adult liver transplantation (LDALT) is still high due to the dile duct variation and necessity reconstruction of multiple small bile ducts. The current surgical management of the biliary variants is unsatisfactory. We evaluated the role of a new surgical approach in a complicated hilar bile duct variant (Nakamura type IV and Nakamura type II) under emergent right lobe LDALT for high MELD score patients. Methods: The common hepatic duct (CHD) and the LHD of the donor were transected in a right-graft including short common trunks with right posterior and anterior bile ducts, whereas the LHD of the donor was anastomosed to the CHD and the common trunks of a right-graft bile duct and the recipient CHD was end-to-end anastomosed. Results: Ten of 13 grafts (Nakamura types II, III, and IV) had two or more biliary orifices after right graft lobectomy; seven patients had biliary complications (53.8%). Later, the surgical innovation was carried out in five donors with variant bile duct (Four Nakamura type IV and one type II), and, consequently, no biliary or other complications were observed in donors and recipients during 47-53 months of follow-up, significant differences (P<0.05) were found when two stages were compared. Conclusions: Our initial experience suggests that, in the urgent condition of LDALT when an alternative live donor was unavailable, a surgical innovation of cutting part of the CHD trunks including variant RHDs in a complicated donor bile duct variant may facilitate biliary reconstruction and reduce long-term biliary complications.
Keywords: Bile duct variant; Surgical innovation; Biliary complications; LDLT
15087Introduction
Due to a shortage of cadaveric donors, living donor adult liver transplantation (LDALT) has become an effective treatment method for end-stage liver disease. However, the incidence of post-LDALT biliary complications is still high, especially in right lobe liver transplantation recipients. The bile duct variation is more common on the right lobe as a multiple-branch or split-type right hepatic duct (RHD) can be found in more than 34% of a healthy population [1]. When LDALT is carried out using right hemi-liver grafts, the difficulty of biliary reconstruction is, therefore, increased due to relatively complicated anatomic variation, such as multiple orifices of the bile duct. The variation is also a risk factor of biliary complications [2,3]. In cases of right anterior, right posterior and left hepatic ducts (LDH) converging into a “trigeminal type” duct, surgeons sometimes have to cut the bile duct closer to the LHD to get a single bile duct orifice, which makes the incidence of bile leakage and biliary strictures significantly higher in the donors postoperatively [4].
Presently, the surgical approach for multiple-branch-type bile duct orifices during LDALT includes reconstruction of two or more branches of bile duct separately, or two or more branches are joined together by ductoplasty to form a common orifice if these ductal orifices are close to each other, but in this case, the incidence of biliary leakage can be as high as 50% after the bile duct reconstruction [2]. The separate reconstruction of two or more bile duct branches, which usually have a smaller diameter, will significantly increase the incidence of biliary complications. Therefore, the current surgical management of the RHD variants is unsatisfactory and perhaps this is one of the reasons that the incidence of biliary complications is high in right lobe LDALT.
To address this problem, we devised a new surgical procedure by cutting part of the common hepatic duct (CHD) trunks, including variant RHD in complicated donor bile duct variants. Here, we report that this novel approach yields a good long-term clinical outcome as the donors and recipients showed no significant biliary complications after a follow-up of 47-53 months.
Patients And Methods
Patient selection and evaluation
From June 2006 to December 2012, 89 patients underwent LDLTs including 75 patients with right lobe LDALTs. Of the 75 right lobe LDALTs, 18 cases were split-type RHD (Nakamura II, III, and IV type). Among these 18 cases, 13 patients underwent the conventional surgical technique and bile duct reconstruction during our early study period (Jun 2006 to May 2008) whereas five patients underwent our innovative procedure of donor bile duct dissection and reconstruction between June 2008 and December 2012. The later five patients had been chosen for emergent LDALT because of the progressive deterioration of the situation and the unavailability of an alternative live donor in an emergency. The main parameters related to the severity of the five patients are shown in Table 1.
| No. | Gender | Age | Pathogenesis | Serum creatinine (µmol/L) | Total bilirubin(µmol/L) | INR | MELD# |
|---|---|---|---|---|---|---|---|
| 1 | Female | 16 | Acute liver failure (induced by drug) | 130.3 | 739 | 3.57 | 38.6 |
| 2 | Male | 30 | Idiopathic adulthood ductopenia with hepatic coma | 168 | 506 | 1.3 | 28.3 |
| 3 | Male | 38 | Decompensation of HBV-induced cirrhosis, small HCC | 162.8 | 215 | 1.96 | 29.4 |
| 4 | Male | 54 | Decompensation of HCV-induced cirrhosis, acute upper gastrointestinal bleeding | 34.8 | 588 | 9.08 | 35.6 |
| 5 | Male | 41 | Acute on chronic hepatitis B liver failure | 168.7 | 620 | 3.77 | >40 |
INR:ProthrombinInternational Normalized Ratio; MELD: Model for End-stage Liver Disease; HBV: Hepatitis B virus; HCC: Hepatocellular Carcinoma; HCV: Hepatitis C Virus.#MELD score = [0.957 ×In(serum creatinine) + 0.378 ×In(serum bilirubin) + 1.120× In(INR) + 0.643] ×10
Table 1: Baseline characteristics of five critically ill recipients.
Donor selection and evaluation
The procedures involved in donor selection and evaluation conformed to the guidelines of the Regulation of Human Organ Transplantation of China and was approved by our Hospital Ethics Committee. All donors were adults aged 19-55 years with knowledge of civil rights. The evaluation was only carried out after the donor expressed a willingness to donate and learned about the advantages and risks of the operation, especially the need for donor biliary reconstruction. Written informed consent was obtained from all donors before surgery. The detailed evaluation methods, including a psychological evaluation, followed the program of Queen Mary Hospital, Hong Kong University [5] with some modified, in which MR Imaging as the sole preoperative imaging technique for LDALT donor candidates [6,7]. Our protocol includes T2- and T1-weighted imaging (in-phase and opposedphase) for detection and characterization of liver parenchymal and extraparenchymal disease; contrast-enhanced volumetric acquisitions for arterial and venous anatomy definition; and a combination of T2- weighted magnetic resonance cholangiopancreatography (MRCP) with multihance-enhanced T1-weighted MRC was used for donor biliary anatomy evaluation. In our series, traditionary intraoperative cholangiography (IOC) through cystic duct is now no longer routinely performed and is reserved only for problematic cases.
Surgical Management
The donors’ operations were performed based on the procedure described by Fan et al. [8]. Liver parenchyma was cut by Cavitron Ultrasonic Surgical Aspirator (CUSA) and the cell saver was routinely used. Briefly, the right branch of the portal vein and the right hepatic artery (RHA) were isolated, but the connective tissue between the RHD and the RHA was not isolated in order to retain the hepatic artery branches that might be present there. The peritoneum was carefully cut open between S4 segments and the convergence of RHD and LHD was identified. Then, the right triangle, right coronary, hepatorenal ligaments were dissected and the right liver was isolated. The inferior vena cava behind the liver was exposed and isolated. The right hepatic short veins were dissected and the right hepatic vein (RHV) was isolated. The RHA and the right portal vein (RPV) were occluded temporarily to produce the demarcation line between the right and left livers, with the help of intraoperative ultrasound, the location of the MHV and the liver transection plane were determined. Under the condition of central venous pressure being less than 5.0 cm H2O and the hepatic vascular inflow and outflow maintained, the liver parenchyma was dissected using CUSA. The bile ducts were divided at a predetermined line, followed by the RHA, RPV and RHV to remove the right hemiliver which was quickly transferred to a bowl containing ice and water. Ice cold (4ºC) histidine-tryptophan-ketoglutarate (HTK) solution was perfused through the portal vein and hepatic artery and the bile duct was also rinsed through. The isolated grafts were maintained in HTK solution (4ºC) until use. The liver graft was implanted using the standard method and the hepatic vein, portal vein, hepatic artery and bile duct were reconstructed, respectively.
Division and Reconstruction of Donor’s Bile Duct
Conventional method
Based on preoperative biliary images, the converging point of the RHD root, LHD and CHD near the right hepatic hilum was determined. The RHD was divided ensuring integrity of the LHD and the following surgical procedures were adopted: (i) In the trigeminal type (Nakamura II type), a part of the right lateral wall of biliary confluence was resected forming single a orifice and anastomosed to the recipient hepatic duct in an end-to-end manner (6/0 Prolene); (ii) In the case of the distance between two duct orifices being <3 mm, the two ductal orifices were joined together to form a common orifice for anastomosis; in the case of the distance between two duct orifices being >3 mm, one branch was anastomosed to the recipient CHD in an end-to-end manner (6/0 Prolene), while the other branch underwent cholangiojejunostomy, or two cholangiojejunostomies; (iii) In the case of three duct orifices, two branches were joined together to form a orifice by ductoplasty and with the third one, underwent two cholangiojejunostomies, separately.
Innovative method
The donor CHD and LHD were transversely divided at the lower edge of the convergence of the RHD to CHD and the upper edge of the LHD, respectively. The bile duct confluence including the right variant bile duct was procured. For donor bile duct reconstruction, 6/0 PDSII and Prolene (i.e., posterior wall PDS-II, anterior wall 6/0 Prolene) sutures were used to perform interrupted end-to-end sutures between the LHD and the CHD. For recipient bile duct reconstruction, based on the diameters of the recipient bile ducts, the left wall of the graft CHD trunk was opened longitudinally and anastomosed to the recipient CHD using interrupted end-to-end sutures manner (posterior wall 6/0 PDSII, anterior wall 6/0 Prolene) without support drainage. Alternatively, the upper side of the graft CHD trunk (LHD) was closed and the lower side was anastomosed to the recipient CHD in an end-to-end manner, without stenting of the anastomosis (Table 2; Figures 1-4).
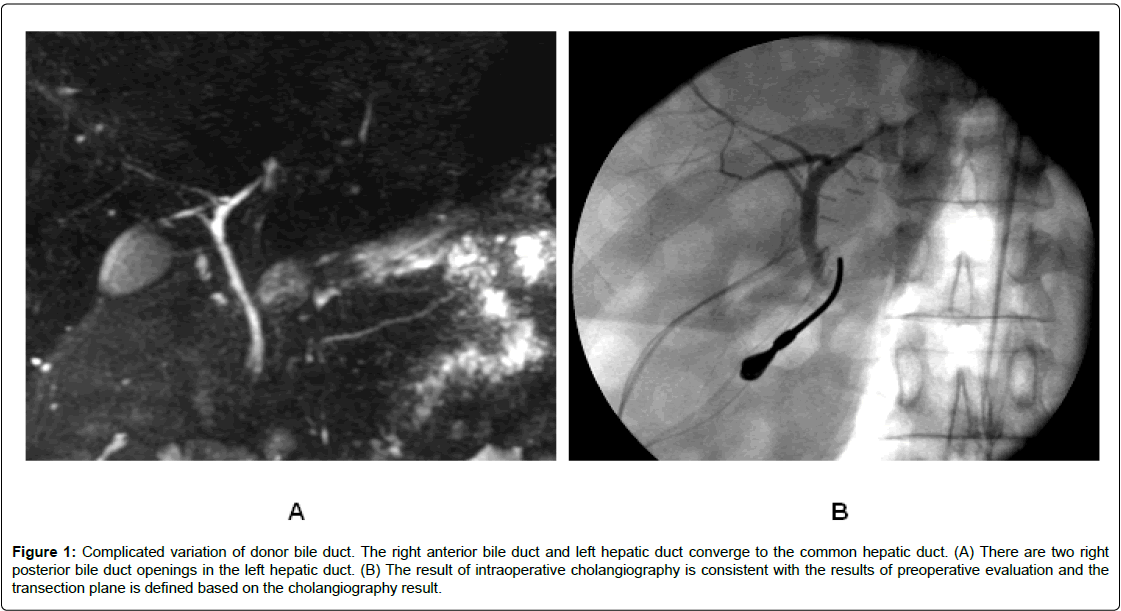
Figure 1: Complicated variation of donor bile duct. The right anterior bile duct and left hepatic duct converge to the common hepatic duct. (A) There are two right posterior bile duct openings in the left hepatic duct. (B) The result of intraoperative cholangiography is consistent with the results of preoperative evaluation and the transection plane is defined based on the cholangiography result.
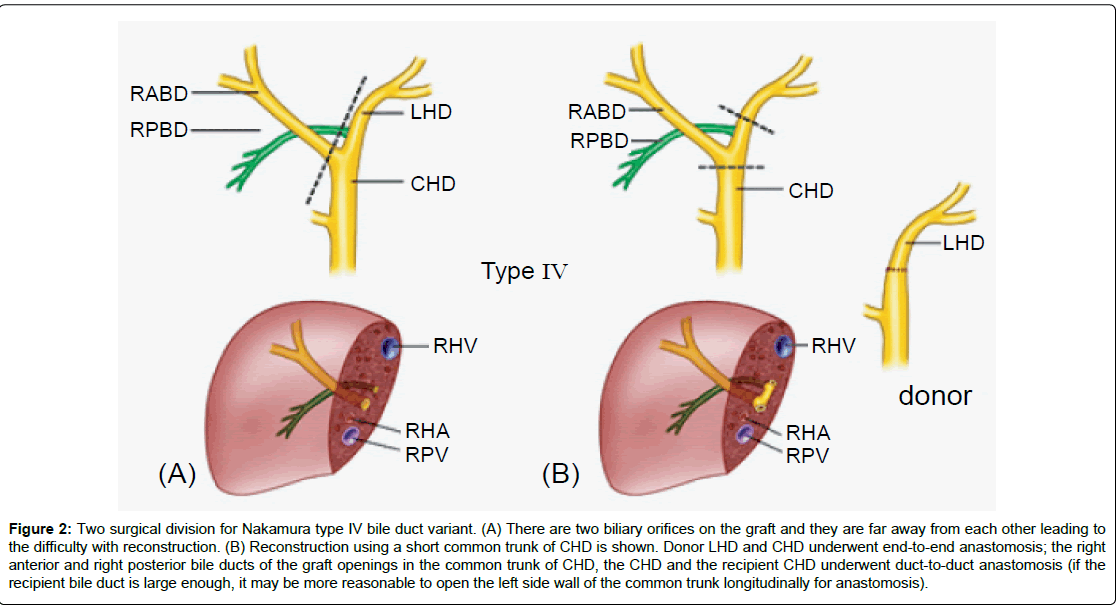
Figure 2: Two surgical division for Nakamura type IV bile duct variant. (A) There are two biliary orifices on the graft and they are far away from each other leading to the difficulty with reconstruction. (B) Reconstruction using a short common trunk of CHD is shown. Donor LHD and CHD underwent end-to-end anastomosis; the right anterior and right posterior bile ducts of the graft openings in the common trunk of CHD, the CHD and the recipient CHD underwent duct-to-duct anastomosis (if the recipient bile duct is large enough, it may be more reasonable to open the left side wall of the common trunk longitudinally for anastomosis).
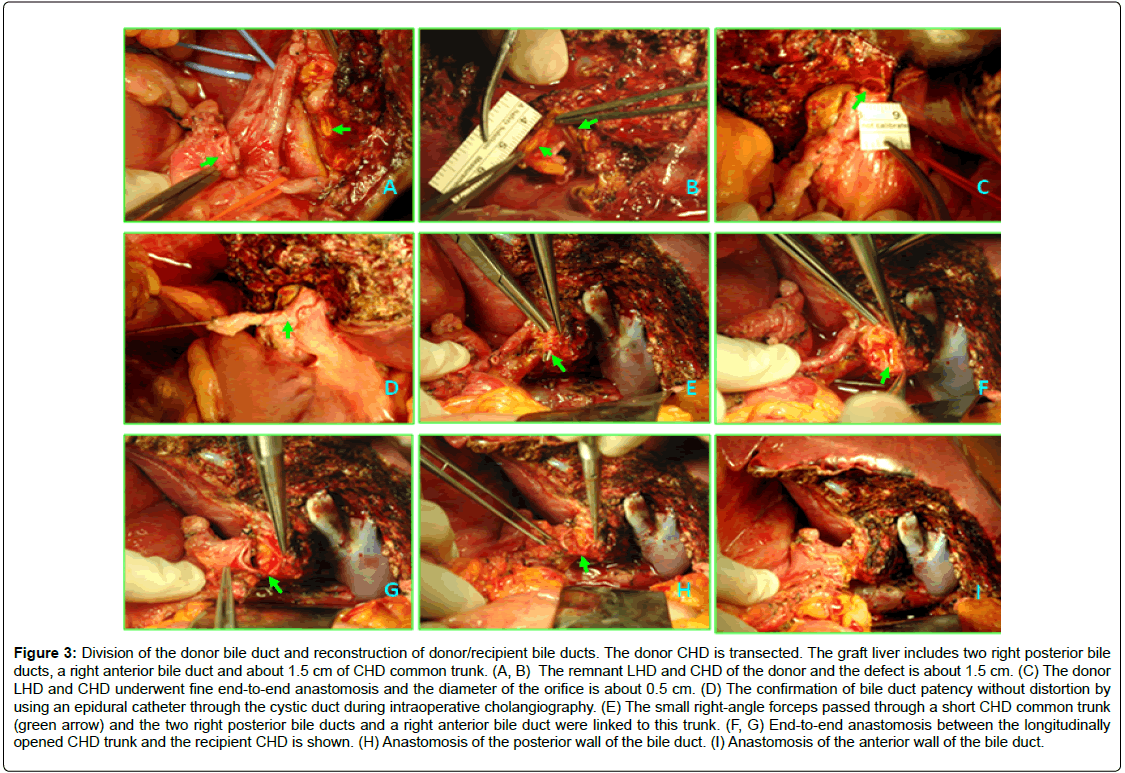
Figure 3: Division of the donor bile duct and reconstruction of donor/recipient bile ducts. The donor CHD is transected. The graft liver includes two right posterior bile ducts, a right anterior bile duct and about 1.5 cm of CHD common trunk. (A, B) The remnant LHD and CHD of the donor and the defect is about 1.5 cm. (C) The donor LHD and CHD underwent fine end-to-end anastomosis and the diameter of the orifice is about 0.5 cm. (D) The confirmation of bile duct patency without distortion by using an epidural catheter through the cystic duct during intraoperative cholangiography. (E) The small right-angle forceps passed through a short CHD common trunk (green arrow) and the two right posterior bile ducts and a right anterior bile duct were linked to this trunk. (F, G) End-to-end anastomosis between the longitudinally opened CHD trunk and the recipient CHD is shown. (H) Anastomosis of the posterior wall of the bile duct. (I) Anastomosis of the anterior wall of the bile duct.
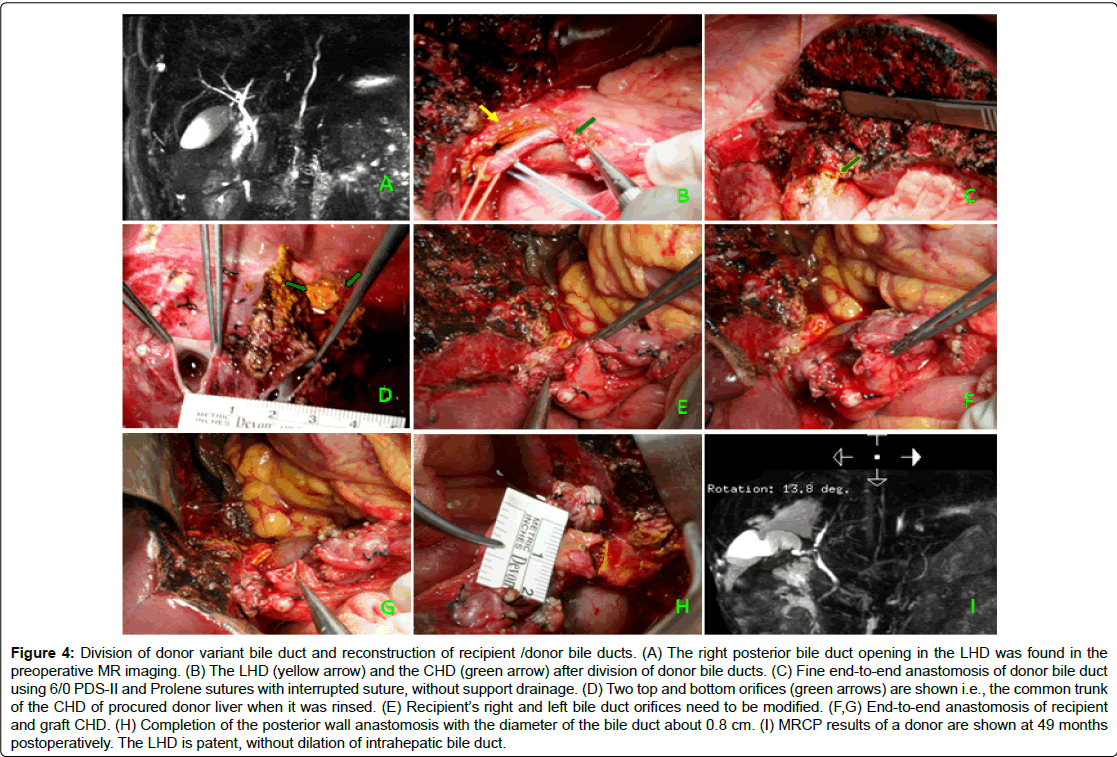
Figure 4: Division of donor variant bile duct and reconstruction of recipient /donor bile ducts. (A) The right posterior bile duct opening in the LHD was found in the preoperative MR imaging. (B) The LHD (yellow arrow) and the CHD (green arrow) after division of donor bile ducts. (C) Fine end-to-end anastomosis of donor bile duct using 6/0 PDS-II and Prolene sutures with interrupted suture, without support drainage. (D) Two top and bottom orifices (green arrows) are shown i.e., the common trunk of the CHD of procured donor liver when it was rinsed. (E) Recipient’s right and left bile duct orifices need to be modified. (F,G) End-to-end anastomosis of recipient and graft CHD. (H) Completion of the posterior wall anastomosis with the diameter of the bile duct about 0.8 cm. (I) MRCP results of a donor are shown at 49 months postoperatively. The LHD is patent, without dilation of intrahepatic bile duct.
| Cases | Graft | GRWR (%) | Variants of donor bile duct | Dimension of donor bile duct (mm)* | Dimension of recipient bile duct (mm) | Cut CHD length (mm) | Reconstruction of donor bile duct | Reconstruction of recipient bile duct |
|---|---|---|---|---|---|---|---|---|
| Case I | Right graft without MHV | 1.1 | RABD and LHD converge to CHD, two right posterior bile ducts opening in LHD (Nakamura IV) | RABD:1.6 RPBD?:1.2 RPBD?:1.3 CHD:3.6 | 5 | 22 | LHD and CHD interrupted sutures (6/0 PDS-II and Prolene) | Opened donor CHD trunk interrupted anastomosed with the recipient’s CHD |
| Case II | Right graft with MHV | 0.99 | RPBD opening in LHD (Nakamura IV) | RABD:1.5 RPBD:2.0 CHD:3.7 | 4 | 18 | Same as above | The upper end of the donor CHD trunk is closed, the bottom end interrupted anastomosed with recipient CHD |
| Case III | With MHV | 1.3 | RPBD opening in LHD (Nakamura IV) | RABD:1.9 RPBD:1.5 CHD:4.1 | 4 | 20 | Same as above | Same as above |
| Case IV | With MHV | 0.92 | RABD, RPBD and LHD;Trigeminal type convergence (Nakamura II) | RABD:1.4 RPBD:1.8 CHD:3.9 | 3.6 | 16 | Same as above | Same as above |
| Case V | Without MHV | 1.9 | RPBD opening in LHD (Nakamura IV) | RABD:1.3 RPBD:1.8 CHD:3.1 | 2.9 | 18 | Same as above | Same as above |
*Diameters of the bile ducts were measured by magnetic resonance imaging; GRWR: Graft Recipient Weight Ratio; RABD: Right Anterior Bile Duct; RPBD: Right Posterior Bile Duct; CHD: Common Hepatic Duct; LHD: Left Hepatic Duct
Table 2: Right liver graft harvested with complicated donor biliary variants and reconstruction of donor/recipient biliary tracts (five cases).
Follow-up
All donor’ and patient’ data have been enrolled in the China Liver Transplant Registry (CLTR) hosted in Hong Kong University and their follow-up protocol has been compiled (https://www.cltr.org/en/). Liver function tests, Doppler ultrasound and MRCP were routinely required to investigate the vascular and biliary complications in both donors and recipients. The immunosuppressive protocol of recipients included calcineurin inhibitor, mycophenolate mofetil, and steroid based triple immunosuppressive regimen.
Statistics
The data are expressed as mean ± standard deviation. Values and the variables were analyzed using the chi-square test for categorical values and student`s t-test for continuous variables. All P-values <0.05 were considered as statistically significant.
Ethics
Written informed consent was obtained from each patient and/or next of kin. The study protocol also conformed to the 1975 Declaration of Helsinki ethical guidelines and received a priori approval by the Hospital Ethics Committee.
Results
Bile duct variation type, number of bile duct orifices and incidence of biliary complications
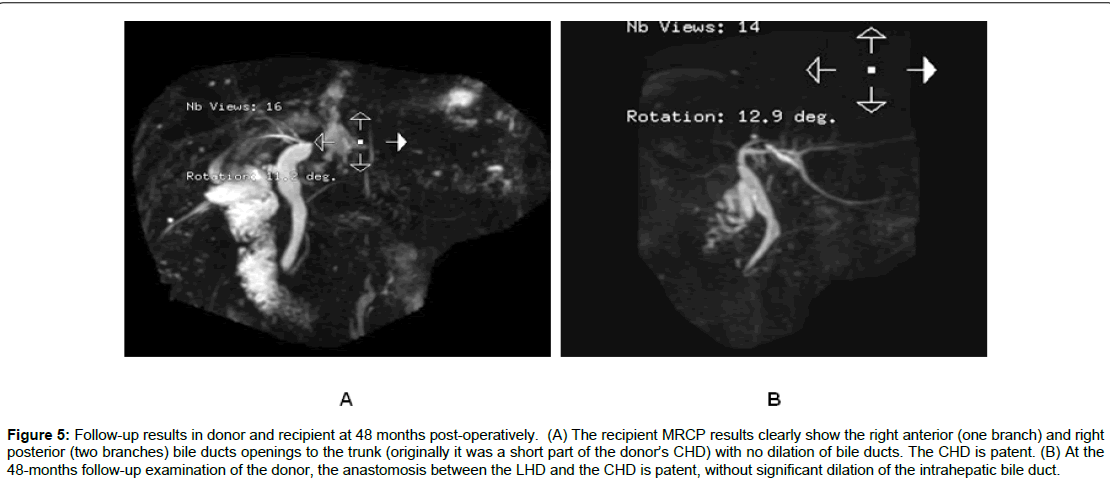
As shown in Table 3, of the 75 cases of right lobe LDALTs, 18 cases were of split-type of RHD (Nakamura II, III, IV types). Among 13 early stage cases, 10 showed two or more orifices of the right bile duct, three showed trigeminal-type of right anterior, right posterior and LHD and one orifice was formed by resecting the right side wall of the converging part of the bile duct. In these 13 recipients, the incidence of biliary complications was 53.8% (7/13). Among five later stage cases, four cases were of right posterior bile duct (one or two branches) confluence into LHD (Nakamura IV type) and one case was of “trigeminal-type”. Using our innovative method for the reconstruction of the recipient bile duct, no postoperative biliary abnormality was detected in donors or recipients by MRCP after 47-53 months of follow-up (Figures 4 and 5), and the difference between the two methods was found to be significant (P<0.05). Also, no biliary complications of donors were observed in the two groups.
Figure 5: Follow-up results in donor and recipient at 48 months post-operatively. (A) The recipient MRCP results clearly show the right anterior (one branch) and right posterior (two branches) bile ducts openings to the trunk (originally it was a short part of the donor’s CHD) with no dilation of bile ducts. The CHD is patent. (B) At the 48-months follow-up examination of the donor, the anastomosis between the LHD and the CHD is patent, without significant dilation of the intrahepatic bile duct.
| Surgical management | Type of biliary variants (n) | No. of graft bile ducts and anastomosis | n | Leakage (%) | Stricture (%) |
|---|---|---|---|---|---|
| Conventional method (n=13) | Nakamura II (3) | 1 duct/1 anastomosisa | 3 | 0 | 1 (33.3%) |
| Nakamura II (2) Nakamura III (4) Nakamura IV (4) |
2 ducts/1 anastomosisb | 4 | 1 (25%) | 1 (25%) | |
| 2 ducts or more/2 anastomosis | 6 | 2 (33.3%) | 3c(50%) | ||
| Innovational method (n=5) | Nakamura II (1) Nakamura IV (4) |
2 ducts with part of common hepatic duct/1 anastomosisd | 5 | 0* | 0* |
aResecting the partial right lateral wall of the confluence part of the biliary duct, one opening; bDuctoplasty: The two ductal orifices joined together to form a common orifice; cOne of the three patients with anastomosis stenosis followed by bile leakage; dSee (Figure 2); *P<0.05 (comparison of the conventional and innovational methods)
Table 3: Biliary variants, openings of graft bile ducts and biliary complications: comparison of conventional (n=13) and innovational (n=5) management.
Changes in donors and recipients liver function tests
In donors, transaminase recovered rapidly and returned to normal after 10 days. Bilirubin reached peak levels in three to four days and γ-glutamyltransferase returned to normal in about one month. As shown in Table S1, there was no significant difference found between early stage donors in whom the conventional method was used and later stage donors in whom the innovative method was applied.
With regard to recipients, in the novel method group, bilirubin returned to normal within one week, and transaminase and γ-glutamyltransferase returned to normal in one month after the liver transplant surgery. On the other hand, in the conventional method group, bilirubin and transaminase levels were found to be higher than those of controls due to the higher incidence of biliary complications (Table S2).
Discussion
LDALT, as compared with cadaver liver transplantation, has the main advantages of a shorter ischemia time, a higher graft quality and relatively fewer liver transplantation complications. However, the biliary complications are still very common. For example, the incidences of biliary leakage and stricture are 4.7-18.2% and 8.3-31.7%, respectively [9]. There is 55.8-73.6% variation rate in the right bile duct [10,11]. Also, the distance between the resection line of the donor RHD and the convergence is only several micrometers, and the probability of having multiple orifices on the right liver can be as high as 39.1-60.4% [12-14]. Moreover, the variant secondary grade bile ducts are tenuous and small. Undoubtedly, these variations increase the difficulty in ductoplasty and reconstruction and are also the high risk factors for biliary complications [2,3]. In addition, in the case of right anterior, right posterior and left hepatic ducts converging into a “trigeminal type” duct in donors, the transection plane deviate to the LHD to obtain a single biliary orifice can sometimes significantly increase the incidence of biliary leakage and stricture in donors [4].
Current surgical approaches for multiple bile duct orifices are as follows: (i) When two or multiple branches are in close proximity, they can be formed into one single orifice and then reconstructed [4,8,10], but after ductoplasty, the incidence of postoperative biliary leakage can be as high as 50% [2]. (ii) When two or multiple branches are far away from each orifice, the graft orifices can be anastomosed to the recipient’s LHD and RHD, or anastomosed to the recipient’s CHD and cystic duct [4,15,16]. As the diameters of multiple bile ducts are far smaller than those of a single orifice, and also because of the artery communicating arcade of the hilar bile duct is damaged in varying degrees during surgical procedures, the blood supply of the bile duct will probably be affected [17,18]. Therefore, the incidences of bile duct necrosis and biliary leakage are high when following the anastomotic procedure of two biliary openings to the recipient’s LHD and RHD separately. The cystic duct has a spiral valve and a small diameter and is usually not suitable for reconstruction. (iii) If it is difficult to perform multiple bile ducts duct-to-duct anastomosis, then one bile duct end-to-end anastomosis and a cholangiojejunostomy are performed; alternatively, two or more cholangiojejunostomies are performed in multiple bile ducts patients [12,13]. However, cholangiojejunostomy involves certain disadvantages. First, the loss of Oddi’s sphincter leads to the loss of prevention function of intestinal fluid reflux which may lead to ascending cholangitis. Second, cholangiojejunostomy involves intestine operations and, therefore, it increases the incidence of infection and the duration of surgery. If bile leakage occurs, the outcome is more devastating because unlike the situation of duct-to-duct anastomosis, intestinal content also leaks into the peritoneal cavity. Third, cholangiojejunostomy breaches the bilebowel physiological continuity leading to difficulties in postoperative endoscopic intervention and thus limits the treatment options for biliary complications. Given that, the current clinical outcome of right LDALT with the variation of right bile duct is not satisfied.
In the present study, in the five patients with a complicated variation of the bile duct (four cases with Nakamura IV type, and one case of “trigeminal type” duct), use of the conventional approach would have produced two or three bile duct orifices. Because the diameters of bile ducts are smaller (1.5 mm-2 mm), either end-to-end biliary anastomosis or cholangiojejunostomy is carried out which often leads to a high incidence of postoperative biliary complication. We had gained experience from the management of hepatic artery variations during LDALT. For example, the middle hepatic artery (MHA) which supplies the left inner lobe is from the RHA. The proper hepatic artery (PHA) and RHA above the MHA are transversely dissected in the donor during left lobe LDALT. The RHA and PHA of the donor is end-to-end anastomosed and graft PHA is used for recipient hepatic artery reconstruction. Similarly, based on a full understanding of the biliary anatomy, we used a new surgical approach by first transecting donor CHD and LHD, the bile duct of the right liver graft including a short common trunk of CHD (1.6-2.2 cm), and then donor LHD and CHD was end-to-end anastomosed using 6/0 PDS-II and Prolene sutures without support drainage. The graft CHD and recipient CHD was duct-to-duct anastomosed for recipient biliary reconstruction. Of note, this approach ensures a single, larger caliber of the duct-to-duct anastomosis and less likelihood of stenosis. Importantly, liver function recovered stably and the bile duct patency was confirmed by MRCP or ultrasound during 47-53 months of follow-up.
One of the major concerns about the use of this new approach is the reconstruction of donor bile duct and some critical queries need to be addressed. First, can the defect between LHD and CHD (1.6- 2.2 cm; Table 2) be anastomosed and reconstructed? Second, will the incidence of postoperative bile leakage and duct stricture increase? In our practice, we find that transecting a certain length of converging portion of bile duct poses no tension in the bile duct anastomosis. This is due to increased mobility of the hepatoduodenal ligament after resection of the right hemiliver. Moreover, without the traction from the RHD, the angle between the LHD and CHD is larger leading to the direct anastomosis and reconstruction without the need to free and loosen bile duct. Importantly, however, it is prohibited to isolate liver in the left hepatic hilum and CHD to protect the blood supply of the LHD during the right lobectomy. The reconstruction of bile duct requires an accurate anastomosis technique in which 6/0 PDS-II is used in the posterior wall, 6/0 Prolene suture is used in the anterior wall duct-to-duct interrupted sutures and keeping prompt margins and sufficient distance between needles avoids bile leakage and biliary ischemia due to too tight sutures. No biliary drainage tube is required in this procedure.
Another major concern about the use of this new approach is that if the increased donor risk will be worthwhile to have this change. We summarized as follows: (i) All our patients belong to critical illness, the median MELD scores was 34.38 (Table 1), three of five patients were in a delirious situation, the mortality rate will be very high if an effective treatment was not performed. (ii) The multiple biliary orifices on the right graft increase the difficulty in ductoplasty and reconstruction and are also the high risk factors for biliary complications. The higher biliary complication rates are a marker for a lower posttransplant life quality, health-care spending, graft failure, and an increased risk of death. (iii) All donors were the closest relatives of recipients, such as husband-wife, parents-daughter or son, etc. If there is a flash of hope, they all used full efforts to strive for the last choice of the patients’ survival. Moreover, all the donors positively expressed a willingness to donate and learned about the advantages and risks of the operation, especially the need for donor biliary reconstruction. (iv) The approach provides an effective alternative option for treatment of this critical illness during LDLT when an alternative donor is unavailable. However, it is noteworthy that the reconstruction of donor bile duct needs highly specialized surgical skills and an extensive experience with bile duct anastomosis, otherwise, the occurrence of postoperative biliary complications may still be a problem to deal with. Therefore, this method can only be carried out at a hepatobiliary surgery center where surgeons have extensive biliary surgery experience and the access to advanced surgical facilities, which guarantee the risk of donor, is controllable.
Conclusions
Our initial experience suggest that, in the urgent condition of LDALT when an alternative live donor is unavailable, a surgical innovation by cutting part of CHD trunks including variant RHD in complicated donor bile duct variant may facilitate in biliary reconstruction and reduce long-term biliary complications. However, although the advantages of recipient biliary reconstruction observed in this small series are noteworthy, more long-term evidence-based outcomes of donor biliary complications will be necessary to prove its safety, and the need for a larger and prospective study is warranted.
Financial Support
This study was supported by the Natural Sciences Foundation of China (Grants # 30772136; 30972950; 81070383).
Conflict of Interest
The authors have no conflict of interest with regard to this study.
Acknowledgments
The authors would like to thank Ma Fen Xi for her excellent assistance with schematic drawing.
References
- Couinand C (1999) Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg 16: 459-467.
- Nakamura T, Tanaka K, Kiuchi T, Kasahara M, Oike F, et al. (2002) Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation 73: 1896-1903.
- Testa G, Malagó M, ValentÃn-Gamazo C, Lindell G, Broelsch CE (2000) Biliary anastomosis in living related liver transplantation using the right liver lobe: techniques and complications. Liver Transpl 6: 710-714.
- Ishiko T, Egawa H, Kasahara M, Nakamura T, Oike F, et al. (2002) Duct-to-duct biliary reconstruction in living donor liver transplantation utilizing right lobe graft. Ann Surg 236: 235-240.
- Fan ST (2008) Estimation of Donor. Living Donor Liver Transplantation(1stedn.) HongKong: Takubfpao Publish Co. Ltd.pp: 6-18.
- Limanond P, Raman SS, Ghobrial RM, Busuttil RW, Lu DS (2004) The utility of MRCP in preoperative mapping of biliary anatomy in adult-to-adult living related liver transplant donors. J Magn Reson Imagin 19: 209-215.
- Schroeder T, Malagó M, Debatin JF, Goyen M, Nadalin S,et al. (2005) All-in-one†imaging protocols for the evaluation of potential living liver donors:comparison of magnetic resonance imaging and multideterctor computed tomography.Liver Transpl 11: 776-787.
- Fan ST, Lo CM, Liu CL (2000) Technical refinement in adult-toadult living donor liver transplantation using right lobe graft. Ann Surg 231: 126-131.
- Yazumi S, Chiba T (2005) Biliary complications after a right-lobe living donor liver transplantation. J Gastroenterol 40: 861-865.
- Ohkubo M, Nagino M, Kamiya J, Yuasa N, Oda K, et al. (2004) Surgical anatomy of the bile ducts of the hepatic hilum as applied to living donor liver transplantation. Ann Surg 239: 82-86.
- Varotti G, Gondolesi GE, Goldman J, Wayne M, Florman SS, et al. (2004) Anatomic variations in right liver living donors. J Amer Coll Surg 198: 577-582.
- Gondolesi GE, Varotti G, Florman SS, Muñoz L, Fishbein TM, et al. (2004) Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation 77: 1842-1848.
- Kasahara M, Egawa H, Takada Y, Oike F, Sakamoto S, et al. (2006) Biliary reconstruction in right lobe living-donor liver transplantation: comparison of different techniques in 321 recipients. Ann Surg 243: 559-566.
- Dulundu E, Sugawara Y, Sano K, Kishi Y, Akamatsu N, et al. (2004) Duct-to-duct biliary reconstruction in adult living-donor liver transplantation. Transplantation 78: 574-579.
- Suh KS, Choi SH, Yi NJ, Kwon CH, Lee KU, et al. (2004) Biliary reconstruction using the cystic duct in right lobe living donor transplantation. J Amer Coll Surg 199: 661-664.
- Asonuma K, Okajima H, Ueno M, Takeichi T, Zeledon Ramirez ME, et al. (2005) Feasibility of using the cystic duct for biliary reconstruction in right-lobe living donor liver transplantation. Liver Transpl 11: 1431-1434.
- Gunji H, Cho A, Tohma T, Okazumi S, Makino H, et al. (2006) The blood supply of the hilar bile duct and its relationship to the communicating arcade located between the right and left hepatic arteries. Am J Surg 192: 276-280.
- Tohma T, Cho A, Okazumi S, Makino H, Shuto K, et al. (2005) Communicating arcade between the right and left hepatic arteries:evaluation with CT and angiography during temporary balloon occulusion of the right or left hepatic arteries. Radiology 237: 361-365.
Citation: Ye S, Dong JH, Duan WD, Ji WB, Liang YR (2016) An Innovative Surgical Management of Complicated Bile Duct Variant in Emergency Living Donor Adult Liver Transplantation: Initial Experience. J Clin Exp Transplant 1: 109. DOI: 10.4172/2475-7640.1000109
Copyright: © 2016 Ye S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15072
- [From(publication date): 12-2016 - Apr 17, 2025]
- Breakdown by view type
- HTML page views: 14144
- PDF downloads: 928