Research Article Open Access
An Indigenous Plant Bael (Aegle Marmelos (L.) Correa) Extract Protects Against the Doxorubicin-Induced Cardiotoxicity in Mice
| Ganesh Chandra Jagetia* and Ponemone Venkatesh | |
| Department of Zoology, Mizoram University, Aizawl-796 004, India | |
| Corresponding Author : | Ganesh Chandra Jagetia Department of Zoology Mizoram University, Tanhril, Aizawl-796 004, Mizoram, India Tel: 011-389-2330724/2330227 E-mail: gc.jagetia@gmail.com |
| Received: April 20, 2015; Accepted: May 13, 2015; Published: May 20, 2015 | |
| Citation: Jagetia GC, Venkatesh P (2015) An Indigenous Plant Bael (Aegle Marmelos (L.) Correa) Extract Protects Against the Doxorubicin-Induced Cardiotoxicity in Mice. Biochem Physiol 4:163. doi:10.4172/2168-9652.1000163 | |
| Copyright: © 2015 Jagetia GC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Doxorubicin (DOX) is an effective antineoplastic agent widely used for the treatment of various cancers; however, its clinical use is limited due the induction of dose-dependent toxicity in the hearts of patients receiving this drug. Therefore any agent that can reduce its myocardial toxicity will be very useful in clinics. The effect of hydroalcoholic extract of bael, Aegle marmelos (AME), a medicinal herb, was evaluated for its ability to reduce doxorubicin induced cardiotoxicity in mice. Based on our earlier studies 350 mg/kg AME was given to mice injected with a single dose of 15 mg/kg DOX. DOX treatment showed a significant elevation in the glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), creatine kinase (CK-MB) and lactate dehydrogenase (LDH) activities in serum of animals killed at 30 h after DOX treatment, indicating acute cardiotoxicity. Treatment of mice with AME before DOX administration significantly reduced the serum levels of CK-MB, LDH, GPT, and GOT indicating that AME protected mice against the DOX-induced acute cardiotoxicity. Pretreatment of mice with AME significantly reversed the DOX-induced decline in the antioxidant status and elevated lipid peroxidation in the myocardial tissue. In a separate experiment the effect of AME against the doxorubicin-induced cardiotoxicity was also studied by evaluating electrocardiogram and myocardial biopsies in mice administered with DDW or AME once daily for 5 consecutive days before exposure to a weekly dose of 5 mg/kg b. wt. DOX for 3 consecutive weeks. DOX treatment caused an increase in the ST interval, which was used as a measure of cardiotoxicity. AME treatment significantly reduced the ST interval indicating protection against DOX-induced chronic cardiotoxicity. The histopathological examination of heart revealed degeneration of myofibrils with mild necrosis in DOX treated group, whereas AME pretreatment drastically reduced these events in the cardiac tissue. Intraperitoneal injection of 1.25 mg/kg DOX once daily for 9 consecutive days significantly improved the survival of mice bearing Ehrlich ascites carcinoma (EAC). Treatment of EAC with 350 mg/kg AME alone did not affect the anticancer activity of DOX since AME did not alter the tumor cell growth, the median and average survival times of tumor bearing mice. The present study demonstrates that AME protects mice against the DOX-induced acute and chronic cardiotoxicity without interfering with the antineoplastic activity of DOX.
| Keywords |
| Aegle marmelos; Doxorubicin; Cardiotoxicity; Antioxidants; Electrocardiogram; Ehrlich ascites carcinoma |
| Introduction |
| Doxorubicin (Adriamycin), a water-soluble anthracycline antibiotic was first isolated from Streptomyces peucetius var caesius [1]. The DOX molecule contains an aminosugar, daunosamine, linked through a glycosidic bond to adriamycinone, a red-pigmented naphthacenequinine nucleus (Figure 1). DOX is highly lipophilic in nature and has a relatively long half-life in the body. In the chemical structure of DOX, the second ring is of special interest because of its potential for free radical generation. One electron reduction of second ring leads to the formation of a semiquinone free radical, which is relatively stable under anaerobic condition, but donates its unpaired electron to oxygen (O2) under aerobic conditions and forms a superoxide radical [2-4]. This reaction regenerates the parental Adriamycin molecule. The sequence of reactions, known as ‘redox cycling’, is potentially harmful to cells since relatively little amount of DOX would be sufficient to catalyze the formation of numerous superoxide radicals. Superoxide radicals can react with hydrogen peroxide to form highly reactive hydroxyl radicals via the iron catalyzed Haber-Weiss reaction. The secondarily derived hydroxyl radicals can cause protein and DNA damage and initiate lipid peroxidation [5]. Increased lipid peroxidation, and enhanced free radical generation in the heart have been found to be the major cause of DOX induced cardiotoxicity [6]. Iron has been reported to play an important role in the DOX-induced toxicity, because the presence of iron can facilitate the generation of oxygen free radicals by the formation of DOX-iron complexes [7]. |
| DOX is particularly effective against various disseminated neoplasms, such as lymphoblastic and myeloblastic leukaemias, neuroblastomas, bone marrow sarcomas, and malignant lymphomas as well as carcinomas of the breast, bladder, and thyroid [8-10]. The planar anthracycline ring of doxorubicin is capable of intercalating into DNA double helix to interfere with the reading fidelity of both DNA and RNA polymerases. It also forms sequence-specific DNA adducts that are highly susceptible to cleavage by topoisomerase II [11]. Doxorubicin (DOX) inhibits the action of topoisomerase II and stabilize a reaction, in which DNA strands are cut and covalently connected to the tyrosine residues of topoisomerase II, eventually impeding DNA resealing that results in the inhibition of DNA repair [12,13]. Regardless of the specific mechanism, DNA, RNA and protein synthesis are all inhibited, arresting cell proliferation in the G1, S-phase and G2, of the cell cycle [14,15]. |
| Doxorubicin is typically administered as an intravenous infusion of 40–75 mg/m2 repeated at 3–4 week intervals, being cautious not to exceed a cumulative dose of 450–550 mg/m2. However, the treatment of cancer patients with DOX is associated with acute toxic effects such as myelosuppression, nausea, vomiting and arrhythmias [4,16] and chronic side effects like cardiomyopathy and ultimately congestive heart failure [17,18]. The acute side effects are reversible or clinically manageable whereas chronic side effects, develop several weeks or months after repetitive doxorubicin administration, and are irreversible and have grave prognosis [19, 20]. |
| Although several hypotheses explaining the mechanisms of anthracycline-induced cardiotoxicity have been put forward, it is generally believed to be caused by the formation of oxygen free radicals and iron ions [21,22]. In biological systems, doxorubicin is known to produce highly reactive free radicals [3] and antioxidant enzymes play a critical role in inactivating these radicals [23]. It is well known that antioxidant enzymes including superoxide dismutase and catalase are present at lower levels in the cardiomyocytes than the other tissues of the body [24], which makes cardiomyocytes particularly more susceptible to free radical damage [25,26]. Therefore, a need is felt for a drug, which can protect against DOX-induced acute and chronic cardiotoxicity without compromising with its antineoplastic activity. |
| A great deal of efforts has been expended in preventing or mitigating the cardiotoxic side effects of DOX. Several agents, including dexrazoxane (ICRF-187) and amifostine, have been tried, but with a limited success [27,28]. Despite its promising protective cardiovascular effects, clinical use of dexrazoxane has been seriously questioned because it is associated with severe myelosuppression, which is actually potentiated by doxorubicin [29]. The possibility that dexrazoxane may interfere with cancer therapy has also been raised [28]. The use of antioxidants to prevent DOX-induced genotoxicity and cardiotoxicity has been advocated. Strategies have been devised to prevent DOXinduced toxicity without affecting its anti-tumor activity [20]. |
| Until more selective anticancer agents are developed, sensitive normal tissues, such as bone marrow (BM) limits the maximum tolerated doses. Thus strategies to prevent myelosuppression resulting from chemotherapy are at a high priority of research. In recent times, focus on plant research has increased all over the world and a large body of evidence has collected to show immense potential of medicinal plants used in various traditional systems. Dietary ingredients may be very useful, if they are found to protect against the deleterious effects of these antineoplastic agents on the normal tissues, as they are biocompatible, widely acceptable, and would not put an extra foreign substance into the body. Further they can be safely manipulated without toxic manifestations. Several botanicals like Crataegus oxycantha, Terminalia arjuna, Inula racemosa and Allium sativum have been found to have therapeutic benefit for the treatment of cardiovascular diseases and its cardioprotective action has been reported in vivo [30-33]. Similarly curcumin, lycopene, tomato extract, Phyllanthus urinaria, Centella asiatica and antarth a poly herbal preparation have been reported to reduce DOX-induced cardiotoxicity in vivo [34-38]. Recently a bioflavonoid naringin has been found to protect against DOX-induced cardiotoxicity in mice [39]. |
| Aegle marmelos is a spinous tree belonging to the family Rutaceae, it has been used to treat opthalmia, ulcers, dropsy, diarrhoea, dysentery, and beriberi associated with the weakness of heart in the Ayurvedic system of Medicine in India. Aegle leaf extract has been reported to regenerate damaged pancreas (β-cells) in diabetic rats [40] and has been as effective as insulin in restoring blood glucose and body weight to normal levels [41]. Bael has been traditionally used in Ayurvedic system of medicine as a ‘cardiotonic’ and its effect is like digitalis for treatment of heart ailments [42,43]. In an earlier study AME has shown to inhibit the beating rate of heart and reduced the ratio of morphologically changed myocardial cells [44]. AME was also found to act as lipid-lowering agent in isoproterenol-induced myocardial infarction in rats [45]. Bael has been reported to reduce radiation and DOX-induced DNA damage [46,47] Aegeline, lupeol, and marmesinin active principles isolated from A. marmelos have been reported as cardioprotective in isoproterenol treated rats [48,49]. The common usage, its wide acceptability to human beings, diverse medicinal and antioxidative properties attributed to Aegle marmelos stimulated us to obtain an insight into its cardioprotective activity against the doxorubicin-induced toxicity in mice. |
| Materials and Methods |
| Chemicals |
| Doxorubicin hydrochloride (DOX) was obtained from Biochem Pharmaceutical Industries, Mumbai, India. Analytical grade cumene hydroperoxide, thiobarbituric acid (TBA), ascorbic acid, glutathione (GSH), 5,5-dithio-bis (2-nitrobenzoic acid) (DTNB), diethylenetriamine pentaacetic acid (DTPA), butylated hydroxytolune, 1-chloro-2, 4-dinitrobenzene, ethidium bromide, 2,4-dinitrophenyl hydrazine, guanidine hydrochloride, ferric chloride, ferrous sulphate and tetraethoxypropane were procured from Sigma Chemicals Co, St. Louis, MO, USA. |
| Animal Care and handling |
| The animal care and handling were done according to the guidelines issued by the World Health Organization, Geneva, Switzerland and the INSA (Indian National Science Academy, New Delhi, India). Eight to ten weeks old male Swiss albino mice weighing 30 to 36 g were selected from an inbred colony maintained under the controlled conditions of temperature (23 ± 2º C), humidity (50 ± 5%) and light (10 and 14 h of light and dark, respectively). The animals had free access to sterile food and water throughout the study period. Four animals were housed in a polypropylene cage containing sterile paddy husk (procured locally) as bedding throughout the experiment. The study was approved by Institutional Animal Ethical Committee (IAEC) of Kasturba Medical College, Manipal, where the study was carried out. |
| Extraction of AME |
| Aegle marmelos (L.) Correa was identified by Dr. Gopal Krishna Bhat, (a well known taxonomist) Department of Botany, Poorna Prajna College, Udupi, India. Mature leaves of the Aegle marmelos were collected locally during January-February of the year, cleaned, shade dried, powdered, and extracted [50]. Briefly, one hundred grams of the leaf powder was extracted with 50% ethanol at 50 to 60°C in a Soxhlet apparatus for 72 h. The cooled extract was concentrated to dryness in a lyophilizer. A 24% yield of the extract was obtained. The extract was stored at -70°C until use. Henceforth, the extract of Aegle marmelos will be called as AME. |
| Mode of administration |
| AME or doxorubicin hydrochloride (DOX) was dissolved in double distilled water (DDW), immediately before use. The animals were administered with 0.01 ml/g body weight of double distilled water (DDW) or 350 mg/kg b. wt. of AME orally (p.o.) once daily for five consecutive days using oral feeding needles (Popper & Sons Inc., New York, USA) before exposure to DOX. |
| Experimental |
| The male Swiss albino mice were divided into the following groups. A total of four animals (n = 4 animals in each group) were used in each group. |
| Acute cardiotoxicity: A separate experiment was carried out to evaluate the cardioprotective activity of bael, where the animals were divided into various groups as follows: |
| DDW group: The animals of this group were administered with 0.01 ml/g b. wt. of double distilled water, orally once daily for 5 consecutive days. |
| AME group: The animals of this group were administered with 350 mg/kg b. wt. of AME orally once daily for 5 consecutive days. |
| DOX group: The animals of this group received double distilled water, orally once daily for 5 consecutive days, and one hour after the last treatment, the animals of this group received single dose of 15 mg/ kg b. wt. DOX intraperitoneally. |
| AME + DOX group: Animals of this group were administered with 350 mg/kg b. wt. of AME orally once daily for 5 consecutive days, and one hour after the last treatment, animals received a single dose of 15 mg/kg b. wt. DOX intra-peritoneally. |
| Serum marker enzymes: The mice were killed by decapitation 30 h after DOX treatment. The blood was collected by cardiac puncture, the sera were separated by centrifugation at 5000 rpm for 10 min and frozen at -70°C for estimation of lactate dehydrogenase (LDH) [51], creatine kinase isoenzyme (CK-MB) [52], glutamic pyruvic transaminase (GPT) and glutamic oxaloacetic transaminase (GOT) [53]. |
| Antioxidant activity: A separate experiment was conducted to evaluate the antioxidant potential of AME in vivo by dividing the animals into four groups as described above. The mice of all groups were killed at 0, 0.5, 1 and 2 h after DOX treatment by cervical dislocation. The hearts were perfused with cold PBS, removed, blot dried, weighed, homogenized in 50 mM ice cold saline (pH 7.4), and centrifuged for 5 min at 5000 g. The samples were stored at -70°C for biochemical estimations. The total proteins were measured by the modified method of Lowry [54]. The product of lipid peroxidation was measured as thiobarbituric acid reactive substances (TBARS) [55]. The activities of glutathione content [56], superoxide dismutase [57], catalase [58] and glutathione peroxidase [59] activities were determined. |
| Chronic cardiotoxicity: A separate experiment was carried out to study chronic DOX-induced cardiotoxicity where the grouping and other conditions of the experiment was essentially similar to that described in experimental section, except that mice received a single dose of 5 mg/kg DOX once a week for 3 weeks (DOX group), whereas animals of AME + DOX group received 350 mg/kg AME once daily for 5 consecutive days. One hour after the last AME treatment, the mice were administered with first dose of 5 mg/kg DOX. Thereafter a similar regimen was followed for another two weeks. Animals were monitored for 2 weeks after the last administration of DOX and subjected to ECG on the last day of observation. After recording the ECG animals were killed and their hearts removed for histopathological examination. |
| Electrocardiogram (ECG): ECG recording in mice was carried out by placing the animals under anesthesia (ether), where a mouse was kept in the supine position, and four-lead ECGs were recorded from subcutaneous 18-gauge needle electrodes implanted subcutaneously into each limb. The ECG was recorded on a Cardiart (BPL, Cardiart 108T-MK VII Instruments, AC 110 ~ 230V, 50 Hz) with a recording speed of 25 mm/s. Heart rates were calculated by counting beats from 15 sec of electrocardiogram recording and expressed as beats per minute (bpm). |
| Histopathology: Histopathological evaluation of heart of each treatment group was carried out according to the method of Billingham [60] and modified by Gabrielson et al. [61]. The hearts were fixed in phosphate-buffered 10% formalin, embedded in paraffin, sectioned (ventricular sections) at a thickness of 5 nm, and stained with hematoxylin and eosin. Qualitative analysis of heart sections was carried out under a transmitted light microscope (Photomicroscope III, Carl Zeiss, Oberkohn, Germany). |
| Effect of AME in conjunction with DOX in tumor bearing mice |
| A separate experiment was carried out to evaluate the effect of AME on the alteration in the antitumor activity of doxorubicin in the Ehrlich ascites carcinoma (EAC) bearing mice, treated with the combination of AME and DOX. |
| Tumor model: Ehrlich ascites carcinoma (EAC) procured from Cancer Research Institute (ACTREC), Mumbai, India was maintained and propagated by serial intraperitoneal transplantation into the Swiss albino mice in an aseptic environment. No spontaneous regression of EAC was observed throughout the study. The experiments were carried out by injecting 106 viable EAC cells into each animal intraperitoneally in sterile conditions and the day of tumor inoculation was considered as day 0. |
| The animals were divided into the following groups one day after tumor inoculation: |
| DDW group: The animals of this group received double distilled water, consecutively for 9 days. |
| DOX group: This group of animals was injected with 1.25 mg/kg b. wt. of DOX dissolved in sterile saline, 24 hours after tumor inoculation, consecutively for 9 days and served as positive control. |
| AME group: The animals of this group were administered p. o. with 350 mg/kg b. wt. AME 24 hours after tumor inoculation, once daily consecutively for 9 days. |
| AME + DOX group: The animals of this group were administered p.o. with 350 mg/kg b. wt. AME, 24 hours after tumor inoculation, and one hour before the administration of DOX, once daily consecutively for 9 days. |
| After the last administration of drug(s) the animals were monitored regularly for body weight changes signs of toxicity and mortality. The weight of animals was recorded every third day up to 30 days after tumor inoculation in all the groups. The tumor response of DOX was assessed on the basis of median survival time (MST) and tumor free survival. The increase in median life span (% IMLS) and increase in average life span (% IALS) were also calculated [62]. |
| Statistical analysis: The significance between the treatments was determined using students‘t’-test for cardiotoxicity and one-way analysis of variance (ANOVA) for antioxidant activity, while the log rank test was applied for survival assay of tumor bearing mice. |
| Results |
| Acute Cardiotoxicity |
| Serum marker enzymes: Table 1 shows the effect of AME on DOX induced biochemical changes in the serum of mice. Treatment of mice with DOX significantly elevated the activities of GOT, GPT, LDH and CK-MB by more than 2 folds indicating drug-induced cardiotoxicity. Administration of 350 mg/kg b. wt. AME once daily for 5 consecutive days before DOX treatment significantly inhibited the increase in the activities of these enzymes in the serum of AME + DOX group (Table 1). |
| Antioxidant activity |
| Myocardial lipid peroxidation: Lipid peroxidation was measured as thiobarbituric acid reactive substances (TBARS). AME administration alone resulted in a significant (p<0.05) reduction in the baseline activity of TBARS in mice hearts (Table 2). The doxorubicin treatment resulted in a significant elevation in TBARS and the greatest elevation was observed at 30 min post-doxorubicin treatment. The AME administration before doxorubicin treatment caused a significant reduction in TBARS at all the post-treatment assay times (Table 2). |
| Myocardial glutathione: Treatment of mice with AME alone significantly (p<0.05) elevated the baseline GSH level at all posttreatment times when compared with the non-drug treated control group. Treatment of mice with DOX caused a significant decline in the GSH contents at all the post-treatment times. The lowest concentration of glutathione was observed at 30 min (Table 2). Treatment of mice with AME before DOX treatment significantly elevated the GSH concentration at all post-treatment times (Table 2). |
| Myocardial catalase: Treatment of mice with AME caused a significant elevation in the spontaneous level of catalase at all posttreatment times in the heart of mice (Table 2). Doxorubicin treatment caused a significant decline in the catalase activity, whereas AME treatment resulted in a significant increase (p<0.05) in the activity of myocardial catalase when compared to DOX alone (Table 2). The catalase activity also increased with increase in assay time up to 1 h (Table 2). |
| Myocardial Superoxide dismutase: AME treatment alone did not significantly alter the baseline activity of SOD at all the post-treatment assay times. The doxorubicin treatment caused a non-significant decline in the SOD activity when compared to the baseline activity (Table 2) and maximum reduction IN SOD activity was observed at 30 min post-treatment (Table 2). AME treatment before DOX administration showed a non-significant elevation (p<0.01) in SOD activity in the hearts of mice where normal levels could be observed as early as 30 min post-treatment when compared with the DOX treatment alone. |
| Myocardial glutathione peroxidase (GSHPx): AME pretreatment did not significantly alter the baseline activity of GSHPx. A significant (p<0.05) reduction in GSHPx activity was observed in the heart of mice receiving DOX treatment alone (Table 2). The myocardial tissue showed a significant (p<0.05) elevation in GSHPx activity in mice treated with AME before DOX administration. There was a time dependent marginal elevation of GSHPx in the AME + DOX group (Table 2). |
| Cardioprotective effect against chronic toxicity |
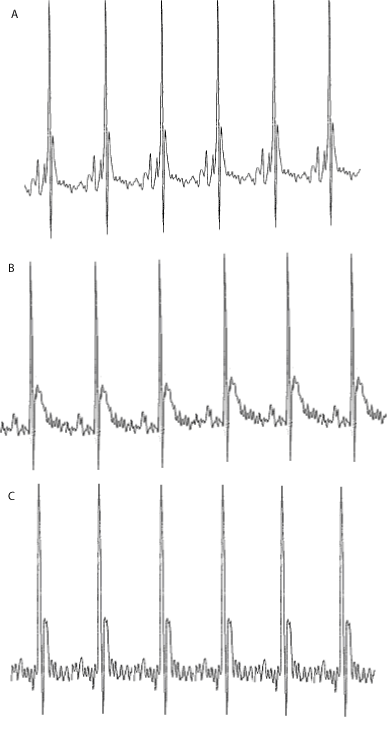
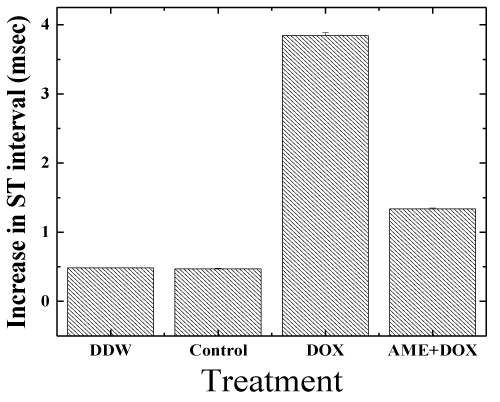
| Electrocardiogram (ECG) studies: The ECG signal in lead II deflection of a mouse is somewhat different from that of man [63]. The ECGs of the control animals did not alter during the course of the study. Doxorubicin had a profound influence on the shape of the ECG (Figure 2). The ST interval increased with time by 3.6 ± 1.2 ms. AME treatment was found to protect mice against DOX-induced ECG changes, and reduced the DOX-induced increase in the ST interval to 1.45 ± 0.8 ms (P< 0.001) relative to DOX treatment alone, which is not significantly different from control (Figure 3). In all the animals the maximum heart rate was 650-700 bpm during the entire study. |
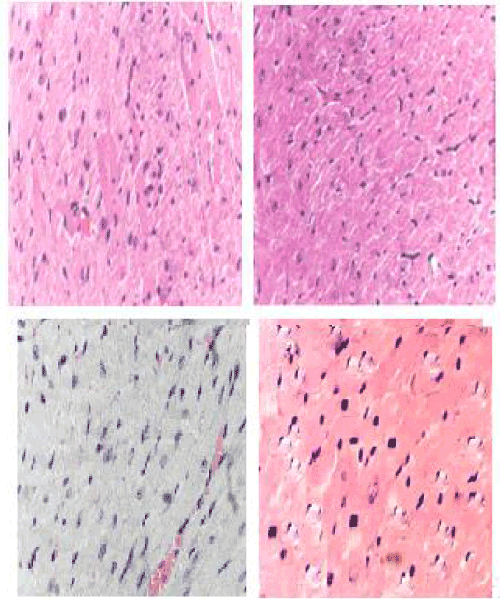
| Histopathology studies: Marked tissue injury with subendocardial loss of muscles and accumulation of acute inflammatory cells surrounded by mild edema along with degeneration of myofibrils and vacuolization of a few myocardial cells were seen in DOX group (Figure 4). DOX treatment alone showed multiple necrosis of myofibrils, whereas AME pretreatment reduced DOX-induced changes in the cardiomyocytes (Figure 4). |
| Effect of AME on antineoplastic action of DOX |
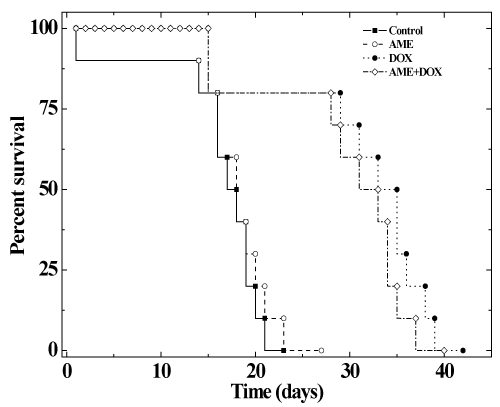
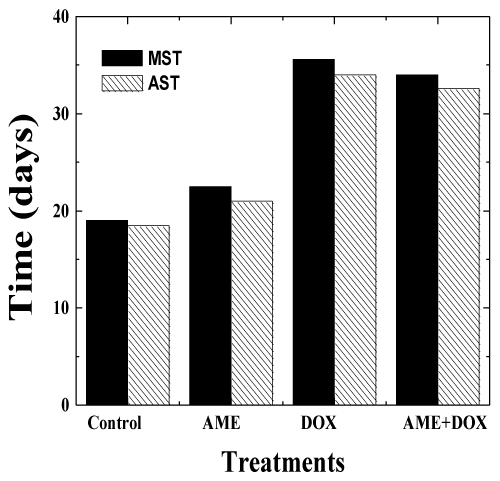
| The effects of AME on the antitumor efficacy of DOX, was assessed in mice bearing Ehrlich ascites carcinoma. No spontaneous regression was observed in the mouse injected with EAC cells during the study period and the animals exhibited a constant increase in the weight due to tumor cell multiplication. The first death was observed on day 14 and all the control animals died by day 23 post-tumor inoculation (Figure 5-6). The median survival time (MST) and the average survival time (AST) were found to be 19 and 18.5 days, respectively (Table 3). Treatment of 1 day old tumors with 1.25 mg/kg DOX inhibited weight gain in animals indicating arrest of tumor cell proliferation and growth (Table 3). DOX treatment increased the MST and AST up to 35.6 and 34 days, respectively accompanied by an increase in the mean life span (IMLS) and the average life span (IALS) up to 87.3% and 83.7%, respectively. Treatment of 1 day old tumors with 350 mg/kg AME alone did not inhibit weight gain in animals indicating that drug as such did not affect tumor cell multiplication and growth. The MST of 22.5 and AST of 21 days remained unaltered in the AME group when compared with the control group (Table 3). Similarly, treatment of EAC mice with 350 mg/kg of AME before administration of 1.25 mg/kg DOX did not significantly alter the MST of 34 and AST of 33 days when compared with the DOX group treatment alone indicating that AME did not interfere with the antineoplastic activity of DOX (Table 3). A similar effect was noticed for the increase in mean life span (IMLS) and the average life span (IALS) that remained unaltered to 78.9 % and 76.2 %, respectively (Table 3). |
| Discussion |
| The use of cytoprotective agents represents an alternative strategy to reduce radio- and chemotherapeutic toxicity in normal tissues [64,65], which will prevent the risk of potentially genotoxic effects leading to secondary tumor formation [66]. For those agents whose maximum tolerated dose is limited primarily by myelosuppression, several strategies are under evaluation to permit dose escalation in the hope of obtaining better clinical results with currently available chemotherapeutic drugs. The major acute toxicity induced by DOX is bone marrow suppression, and its long-term clinical usefulness is limited by a cumulative dose-dependent irreversible chronic cardiotoxicity, which manifests itself as congestive heart failure or cardiomyopathy [65,67]. Therefore, it is essential to screen chemical agents that can protect the normal cells against DOX-induced cumulative toxicity without compromising its neoplastic activity. Plants by virtue of their wide usage in the traditional medicine and less toxic implications have attracted the attention of researchers around the world and WHO has recognized complimentary and alternative medicine as part of treatment modalities. The herbal drugs have been used since time immemorial to treat various disorders and offer an alternative to the synthetic drugs, as they have been considered either nontoxic or less toxic than their synthetic counterparts. Ayurveda [Ayu = life, veda = knowledge] the classical Indian system of medicine is based on the principle of balance and counterbalance and it extensively uses plantderived compound formulations for the treatment of various ailments. AME has been traditionally used in Ayurveda as a cardiotonic drug for treating various heart ailments. Therefore the present study was designed to evaluate the cardioprotective effect of bael (Agele marmelos) in mice treated with doxorubicin. |
| Elevation of serum CK has been reported to be a reliable indicator of DOX-induced cardiotoxicity [68]. The DOX treatment significantly increased the acute cardiotoxicity manifested by elevated levels of serum CK-MB, LDH, GOT and GPT. A similar rise in these enzymes has been previously reported [34,38,39,63]. AME has been able to reduce the DOX-induced cardiotoxicity in mice, as indicated by a significant reduction in the serum GOT, GPT, CK-MB and LDH. These findings are in agreement with earlier studies where AME was reported to reduce the CK-MB and LDH levels significantly and protect rat myocardium against lipid peroxidation and membrane damage [45,49]. The isolated periplogenin from Agele marmelos has been found to reduce these enzymes in rat serum [69]. In earlier studies AME has been reported to stabilize cardiac cell membranes and preserve their integrity by regulating cardiac enzyme release [42,44]. Similarly, other pharmacological agents such as antarth (a composite herbal preparation), curcumin, lycopene, naringin, tomato extract, Phyllanthus urinaria and Centella asiatica have been reported to protect against DOX-induced cardiotoxicity [34-39]. ICRF-187, 7-monohydroxyethylrutoside and Frederine have also been reported to reduce DOX-induced cardiotoxicity [67,70-72]. The polyphenol, resveratrol, has been reported to protect against the DOX-induced elevation in LDH, and GOT in Wistar rats [73]. The bioflavonoid narigin has been reported to protect DOX-induced cardiotoxicity by reducing the activity of GOT, GPT, CK-MB and LDH in mice [39]. |
| Generation of free radicals with subsequent lipid peroxidation in cardiac tissues is an important mechanism by which DOX induces cardiotoxicity [3,74]. The increase in MDA is likely to follow DOXinduced free radicals generation with decrease in antioxidant enzymes. GSH is involved in both enzymatic and non-enzymatic detoxification of a variety of free radicals including reactive oxygen species; several investigations have explored the role of GSH in cardiac protection against DOX-induced toxicity [75,76]. Administration of exogenous GSH to mice significantly inhibited the acute myocardial toxicity of DOX [75]. Several studies have shown that natural ingredients like garlic, Terminalia arjuna and powerful antioxidants like curcumin, probocol, natural flavanoids and captopril have been reported to protect against DOX-induced toxicity [31,33,34,39,77]. The damage to mouse myocardium may be due to DOX-induced oxidative stress in the present study. AME has been reported to scavenge various free radicals earlier [46] and the reduced cardiotoxicity may be due the ability of AME to scavenge DOX-induced free radicals in vivo. |
| In the present study a single dose of DOX (15 mg/kg) caused a significant increase in the myocardial TBARS and subsequent decrease in myocardial GSH, catalase, SOD and GSHPx at all the post-DOX treatment times indicating an increase in oxidative stress. Our results are in agreement with earlier studies where DOX has been reported to decrease the concentration of cardiac GSH and increase in TBARS [34,69,78]. AME administration before DOX treatment significantly raised the GSH, catalase, SOD and GSHPx levels and a consequent decline in the TBARS induced by DOX at all post DOX treatment times. Increase in catalase activity is accompanied by increase in SOD and GSHPx activities in the myocardial tissue of mice administered with AME before DOX treatment. Any increase in SOD activity of the tissue is beneficial in the event of free radical generation. However it has been reported that a rise in SOD activity, without a concomitant rise in catalase or GSHPx activity might be detrimental [79]. It is due to the fact that the SOD generates hydrogen peroxide, which is a cytotoxic metabolite and has to be scavenged by catalase or GSHPx. The cardioprotective effect of AME might be attributed to increase in the GSH, catalase, SOD and GSHPx activities with subsequent decline in the TBARS in the present study. An identical effect has been observed earlier, where AME and antarth treatment elevated the activity of antioxidant enzymes accompanied by a decline in the TBARS in isoproterenol treated rats or DOX treated mice [38,45,49]. AME has also been reported to elevate antioxidant status in irradiated mice [80,81]. A similar rise in antioxidants has been reported in the heart of mice receiving narinign before DOX treatment [39]. |
| DOX is known to cause a decrease in heart function in both humans and experimental laboratory animals [82, 83]. Electrocardiographic changes associated with DOX-induced cardiomyopathy initially include various reversible arrhythmias, most commonly sinus tachycardia [84,85]. ECG profiles after two weeks of chronic DOX treatment in mice showed a largest peak, which represents depolarization of ventricular myocardium resulting in heart contraction in the present study. Immediately following the main peaks is the T wave which represents the repolarization of the heart. Broadening and decreasing T wave, correlates with the development of DOX-induced cardiotoxicity in mice [83]. DOX treatment widened the ST intervals in mice. An identical effect has been observed in rats treated with isoproterenol [49]. AME pretreatment significantly reduced the ST interval and restored it to almost normal (Figure 2). A similar effect has been observed in isoproterenol-treated rats with marmesinin, an active component present in AME [49]. Likewise, simvastatin has been reported to significantly reduce the ST interval and restore heart beat in DOX treated rats [86]. Further AME has been reported to significantly reduce the ratio of morphologically changed myocardial cells originated from calcium load [44]. The widening of ST interval in DOX treatment may be explained by prolongation of action potential. The action potential is prolonged in Purkinje fibers after incubation with DOX [87]. |
| The diagnostic test with greatest specificity and sensitivity for DOX-induced cardiomyopathy is endomyocardial biopsy [88]. The endomyocardial biopsy from the ventricle shows typical histopathological changes like loss of myofibrils, distention of sarcoplasmic reticulum, and vacuolization of the myocardial cells. Marked tissue injury with subendocardial loss of muscles and accumulation of acute inflammatory cells surrounded by mild edema after DOX treatment alone, whereas only focal necrosis of muscle fiber with moderate edema was noted after AME pretreatment indicating protection against DOX-induced cardiac injury. An identical effect has been reported by garlic and tomato against the DOX-induced histological alterations in the cardiac muscles [33,35]. |
| We were also interested to know whether AME treatment alters the antinneoplastic action of DOX and our studies on EAC mice indicate that AME treatment did not interfere with normal tumor development, as the MST and AST remained unchanged in this group when compared with the non-AME treated controls. A Similar effect has been observed earlier in EAC mice receiving antarth or naringin [38,39]. Our results suggest that use of AME and DOX may be a good paradigm to reduce DOX-induced cardiotoxicity and utilize the full antineoplastic action of DOX in clinical situation. |
| The exact mechanism of protection by AME against the doxorubicininduced cardiotoxicity is not known. The AME is a pleotropic molecule, which may have utilized multiple putative mechanisms to protect against the DOX-induced myocardial toxicity. The DOX induces free radicals and scavenging/inhibition of free radical generation by DOX seems to one of the important mechanisms of reduction in the myocardial injury. AME has been reported to scavenge free radicals in a concentration dependent manner [46]. The increased antioxidant status indicated by the elevation in GSH, catalase, superoxide dismutase, and GSHPx may have also played a crucial role in protecting heart against DOX-induced toxicity. The DOX act by inhibiting the action of topoisomerase –II enzyme and fixing the damage into the DNA and AME would have restored the action of topoisomerase –II enzyme thereby reducing the DNA damage. Our earlier studies have shown that AME redeuced the DOX-induced DNA damage [47]. The DOX has been reported to form covalent adducts with DNA particlualry 8-OHdG and mtDNA adducts in rats [89, 90]. In our earlier study we have also reported that DOXinduced 8-OHdG DNA adducts in the mice heart [39] and inhibition of DNA adduct formation by AME could also be responsible for reduction in the DOX-induced cardiotoxicity. Naringin has been reported to protect mice heart by reducing DOX-induced 8-OHdG DNA adducts earlier [39]. Poly (ADP-ribose) polymerase (PARP) overactivation has been reported as a major cause of myocardial injury as well as damage to other tissues and DOX treatment has been found to increase PARP activation in mice heart [39,91]. The AME pretreatment may have protected mice heart by suppressing the DOX-induced activation of PARP in the present study. DOX increases the activation of NF-κB in cultured cardiomyocytes and inhibition of NF-κB by AME may have inhibited the inflammation and thereby reduced the DOX-induced cardiotoxicity [92,93]. |
| Bael has been reported to contain aegeline, aegelenine, marmelosine, marmelin/ marmesinin, periplogenin, o-methyl hayordinol, alloimperratorin methyl ester, o-isopentanyl hayordinol, linoleic acid, cineole, p-cymene, citronella, citral, cuminaldehyde, D-limonene, eugenol, tannins like ellagic acid and gallic acid, phlobatannins, flavon-3-ols, rutin, leucoanthocyanins, anthocyanins and flavonoid glycoside [43]. Most of these compounds have been reported to possess antioxidative and free radical scavenging activities [69,94,95]. The observed increase in antioxidant status may be due to the presence of the above components in AME, which would have restored the “endogenous antioxidant reserves” and thus protected against DOXinduced cardiotoxicity. The reaction of endogenous antioxidants has been reported to improve myocardial structure and function. Although mechanisms for AME-induced increase in antioxidants are not clear, the study clearly demonstrates that AME may have protected mice heart by acting as an antioxidant as well as by promoting endogenous antioxidants. |
| Our study demonstrates that AME protected mice against the DOX-induced cardiotoxicity by scavenging of free radicals and raising the antioxidant status of mice heart. The other plausible mechanism of caridoprotection by bael may be due to restoration of topoisomerase –II enzyme activity, reduction in the DOX-induced DNA damage, suppression of DNA adduct formation, PARP and NF-κB activation. The AME protected mouse heart without interfering with the DOX antineoplastic activity, suggesting that AME may be a suitable candidate to reduce DOX-induced toxicity in clinical situations. |
| Acknowledgements |
| The financial support from the Indian Council of Medical Research, Government of India, New Delhi to carry out this study is thankfully acknowledged. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 15146
- [From(publication date):
September-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10663
- PDF downloads : 4483
