An in vitro study to assess alpha amylase inhibition and antioxidant activities of the ethanol and acetone extracts of Anacardium occidentale linn (cashew) stem bark
Received: 18-Dec-2020 / Accepted Date: 10-Jan-2021 / Published Date: 10-Jan-2021 DOI: 10.4172/2168-9652.1000296
Abstract
Diabetes Mellitus (DM) is an endocrine disease due to glucose intolerance. The journey to fighting against DM is limited by the inability of some diabetic patients to have access to oral antidiabetic drugs. Alpha-amylase significantly contributes to the handling of hyperglycemia. This study investigated the DPPH free radical scavenging activity, the total phenolic content and the alpha-amylase inhibition activity of Anacardium occidentale Linn (cashew) stem bark in vitro. The total phenolic content of the extracts was found to be 3.572 ± 0.39 and 3.145 ± 0.28 mgg-1. The DPPH free radical scavenging potency (IC50) of acetone and ethanol extracts were 6.743 ± 1.16 μgmL-1 and 9.186 ± 1.06 μgmL-1 respectively while ascorbic acid was 2.796 ± 1.06 μgmL-1.
The preliminary phytochemical screening showed that terpenes, phenols, glycosides, tannins, flavonoids, alkaloids and saponins were present in all the extracts. On the contrary, sterols were tested negative in the extracts. The alpha amylase inhibitory potency (IC50) of the acetone and ethanol extracts were 46.07 μgmL-1 and 51.31 μgmL-1 respectively and acarbose (the standard drug) was 24.97 μgmL-1. The inhibition potency on α-amylase as observed together with its potential antioxidant capacity in this study proposes an efficient function of the stem bark of A. occidentale in management of DM specifically the type II and its related complications associated with oxidative stress.
Keywords: Diabetes mellitus, IC50, α-amylase inhibition, DPPH, Antioxidant, Total phenolic content, Phytochemical screening, Acarbose, Gallic acid, Ascorbic acid
Introduction
Diabetes Mellitus (DM) is an endocrine disease due to glucose intolerance. Diabetes can cause hyperglycemia, oxidative stress, polyuria, nephropathy, polyphagia, polydipsia, ketosis and disorders of the cardiac system [1].
The journey to fighting against DM is limited by the inability of some victims of DM to have access to oral antidiabetic drugs [2]. Reported that, over three hundred million people will be diabetic by 2025.
Currently, the treatment procedures for DM employ the application of oral hypoglycemic and antihyperglycemic drugs, insulin therapy, physical activity, life style, diet therapy, and xenotransplantation. Present antidiabetic drugs don’t give significant control of blood glucose [3]. For a long time, medicinal plants have performed a significant function in the treatment of human diseases which includes diabetes too. Many reported medicinal plants show anti-diabetic effect and can serve as a supplement for synthetic drugs. Examples of such drugs for management of diabetes are acarbose, miglitol, Nateglinide, Repaglinide and voglibose [4]. However, these drugs have some gastrointestinal side effects including abdominal pain, flatulence and diarrhea and other side effects such as pharyngitis, headache and nausea. Therefore, it is the need of time to identify antidiabetic substances from natural sources having fewer side effects but more efficient pharmacological response [5].
This research seeks to assess the alpha-amylase inhibitory and the antioxidant activities of ethanol and acetone extracts of Anacardium occidentale stem bark in vitro.
Materials and Method
Materials
Apparatus and equipment: Electronic balance, water bath, stirrer, mortar and pestle were obtained from University Development Studies, Navrongo campus. Whatmann No1 filter papers were purchased from Alpha chemical shop, Navrongo, spectrophotometer (Biotek Synergy H1 Hybrid Reader, USA), UV-Vis spectrophotometer (Genway 7B Series, USA), test tubes, 96 well microplate, micropipettes and the pipette tips were supplied by the faculty of pharmacy and pharmaceutical sciences of the Kwame Nkrumah University of Science and Technology, Ghana.
Chemicals and reagents
3, 5-dinitrosalicylic acid (DNS), 40% 5.31 M sodium potassium Tartrate, Folin Ciocalteau phenol reagent, 2, 2-diphenyL-1- picrylhadrazyl (DPPH), alpha amylase from porcine pancreas and potato starch were supplied by Sigma-Aldrich (St. Louis, MO, USA). Acetone and 90% ethanol were also purchased from Alpha Chemical Shop, Navrongo. Distilled water was purchased from Navrongo senior high school (Upper East Region, Ghana), absolute methanol, 6.7 mM sodium chloride, 0.02 M sodium phosphate buffer (pH 6.9), 1% sodium hydroxide, 7.5% sodium carbonate, acarbose, ascorbic acid and gallic acid were also obtained from Kwame Nkrumah University of Science and Technology (Kumasi, Ghana).
Method
Sample collection
Matured stem bark of A. occidentale was harvested from Nyoja Jembo Cashew Farm at Kpassa Jumbo No1 in the Volta region of Ghana in the month of January 2018. It was then authenticated by Dr. Imoro Wahab, a botanist in the Department of Applied Biology, Faculty of Applied Sciences, Navrongo campus of the University for Development Studies, Ghana.
Sample preparation
The matured stem bark of A. occidentale was chopped into piece, washed under running water to remove any contaminant and then shade dried. The dried pieces were pounded using mortar and pestle. It was then sieved and fine uniform powdered sample obtained.
Extraction
The extraction was carried out by maceration using a protocol previously used by Sahira & Cathrine (2015).
Phytochemical screening
The phytochemical screening was carried out using a method previously used [6].
Total phenolic content
The gallic acid solution was prepared by dissolving 10 mg (0.01g) of Gallic in 50 mL of distilled water in volumetric flask (200 μgmL- 1). The total phenolic contents present in the stem bark extracts of A. occidentale L. were determined using the Folin Ciocalteau phenol reagent colorimetric method based on redox reaction described by Waterhouse (2002) and Rodolfo et al.
1. To 100 μL of the extract in a test tube, 0.5 mL of Folin Ciocalteu phenol reagent and 1 mL of 7.5% sodium carbonate (Na2CO3) were added.
2. The content was mixed and allowed to stand for 30 min at room temperature in the dark and the absorbance measured at 700 nm using UV-vis spectrophotometer.
3. Gallic acid was used as a positive control and the total phenolic contents were expressed as milligram (mg) of gallic acid equivalents (GAE) per gram (g) of dry extracts.
α-amylase inhibition assay
This assay was carried out using a modified method [7,8]. The determination of α-amylase inhibition was carried out by quantifying the reducing sugar (maltose equivalent) liberated under the assay conditions. The enzyme inhibitory activity was expressed as a decrease in units of maltose liberated. The generation of maltose was quantified by the reduction of 3, 5-dinitrosalicylic acid to 3-amino-5-nitrosalicylic acid equivalence.
1. 1 mL of alpha amylase and 1 mL of plant extract were put together in a test tube and incubated at 37o C for 10 min.
2. After pre-incubation, 1 mL of 1% w/v starch solution was added to the content in the tube and re-incubated at 37o C for 15 min.
3. The reaction was then terminated with 2 mL of 3,5-dinitrosalicylic acid DNS reagent, closed and placed in boiling water bath for 5 min, cool to room temperature and diluted with 9 mL of distilled water.
4. The samples were then loaded into the 96 well microplates and the absorbance measured at 540 nm using a spectrophotometer (Synergy H1 Hybrid Reader, Biotek instruments, USA). Acarbose was used as positive control.
5. To eliminate the absorbance produced by plant extract, appropriate extract controls were also included. The percentage inhibition of alpha amylase activity was computed using the relation below:
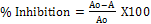
Where Ao = blank absorbance A = absorbance in the presence of the sample.
DPPH free radical scavenging assay
This assay was carried out using a modified method [9,10]. The antioxidant activity of the extracts was determined as a measurement of radical scavenging using the DPPH radical.
In a test tube, 3 mL of DPPH working solution was mixed with 1 mL plant extract. It was then incubated in the dark at room temperature for a period of thirty minutes and the absorbance was measured at 517 nm using UV-Vis spectrophotometer (Genway 7B Series, USA). The control (blank) contained 1mL of absolute methanol in place of the plant extract. The percent antioxidant or radical scavenging activity was calculated using the formula given below:
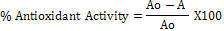
Where Ao = blank absorbance, A = absorbance in the presence of the sample.
Results and Discussion
Phytochemical screening
The results of the phytochemistry are shown in Table 1.
| Phytochemical | Solvent | |
|---|---|---|
| Acetone | Ethanol | |
| Tannins | + | + |
| Phenols | + | + |
| Flavonoids | + | + |
| Saponins | + | + |
| Sterols | - | - |
| Alkaloids | + | + |
| Terpenes | + | + |
| Glycosides | + | + |
Table 1: Phytochemical results of acetone and ethanol extracts. Key: (+) means present and (-) means absent.
The extracts were analyzed qualitatively for the presence of bioactive compounds such tannins, alkaloids, terpenes, sterols, flavonoids, phenols and glycosides. Preliminary phytochemical analysis inferred that all the extracts showed positive for glycosides, terpenes, flavonoids, phenols, tannins, saponins and alkaloids. However, sterols were negative in both extracts. This is quite different from that of [11]. In the literature, saponins, alkaloids, phenolic compounds and tannins were found in the plant extracts [12]. The difference in the qualitative chemical composition of the same plant species may occur between studies because due to the influence of factors like temperature, plant development, rainfall, seasonality, radiation, altitude and nutrients [13]. A phytochemical screening to investigate the phytoconstituents in cashew leaf, bark and fruit extracts revealed the presence of saponins, alkaloids, carotenoid (a tetraterpene), flavonoid, tannin, and polyphenols [14]. According to glycosides and phenolics are the main inhibitors of most glucosidases including alpha amylase [15]. A report emphasized that A. occidentale stem bark shows the presence of polyterpenes, phenolic compounds (lignans, phenols and flavonoids), sterols and tannins [16].
Total phenolic content
The content of phenols present in the extracts were estimated as gallic acid equivalents in milligram per gram of extract (mgg-1) (Figure 1).
They are a class of antioxidant agents which act as free radical
terminators and their activities may be attributed to their potential to
chelate metals, inhibit lipoxygenase and scavenge free radicals [17].
They are capable of inhibiting lipid peroxidation in vitro and they
have gained much attention as a result antioxidant activity [18]. The
total phenol was quantified by the use of Folin-Ciocalteu reagent (FC).
FC is composed of phosphotungstic acid and phosphomolybdic acid
which after oxidation of the phenols, is reduced to oxides of tungsten
and molybdenum. The blue coloration produced has a maximum
absorbance of 700 nm and proportional to the total quantity of
phenolic compounds originally present. Gallic acid was used as a
standard compound and the total phenols were expressed as mgg-1 gallic acid equivalent using the gallic acid calibration curve equation where y is the absorbance at 700 nm, 0.128 is the
slope and x is the total phenol in μgmL-1.
where y is the absorbance at 700 nm, 0.128 is the
slope and x is the total phenol in μgmL-1.
The total phenol ranged from 3.1453 ± 0.28 to be 3.5719 ± 0.39 mgg-1. From Table 2, the total phenols in the acetone extract was estimated to be 3.5719 ± 0.39 mgg-1 and that of the ethanol was estimated to be 3.1453 ± 0.28 mgg-1. The total phenolic content can be influenced by the process of extraction, taking into account the polarity of the solvents used [19]. Previous experiments have reported that phenolic compounds possess other biological activities such as anti-inflammatory, antiulcer, antiplasmodic and antidiarrheal (Figure 2) [20,21].
| Sample | Total Phenolic Content (mgg-1) |
|---|---|
| Acetone extract | 3.572 ± 0.39 |
| Ethanol extract | 3.145 ± 0.28 |
Table 2: The total phenolic content of the extracts.
DPPH free radical scavenging activity
The DPPH assay stands on the basis of an antioxidant to donate a hydrogen radical or an electron to DPPH radical which is stable free radical with deep violet color. When an odd electron becomes paired in the presence of free radical scavenger of antioxidant agent, DPPH radical gets reduced to corresponding hydrazine, DPPH-H form and the solution gets decolorized from its initial deep violet to light yellow color [22]. The results of the DPPH radical scavenging activities of the acetone extract, ethanolic extract of cashew stem bark and ascorbic acid are presented in Table 3. The acetone extract (IC50 of 6.743 ± 1.16 μgmL-1) and ethanol extract (IC50 of 9.186 ± 1.06 μgmL-1) exhibited a significant percentage DPPH radical scavenging activity not comparable to that of ascorbic acid (IC50 of 2.796 ± 1.06 μgmL-1). In the ethanol extract, the DPPH scavenging activity increased with increasing extract concentration. The acetone extract and ethanol extract exhibited antioxidant activity but not as higher as that of ascorbic acid as demonstrated by their ability to scavenge free radical. It has been previously suggested that the antioxidant effect probably may be due to the phenolic content in the extract [23]. Also other phytochemicals such flavonoids, alkaloids, phenolic acids, tannins, anacardic acid and carotenoids have equally been reported to enhance antioxidant potentials against free radicals and oxidative damage in the body [24].
| Sample | IC50 (µgmL ) -1 |
|---|---|
| Ascorbic acid | 2.796 ± 1.06 |
| Acetone extract | 6.743 ± 1.16 |
| Ethanol extract | 9.186 ± 1.06 |
Table 3: The IC50 of the free radical scavenging ability of acetone extract, ethanolic extract and ascorbic acid.
The lower the IC50 value the higher will be its free radical scavenging power. Observed that oxidative capacity of 92 plant extracts did not completely depend on their total phenolic content [25]. Many times, the relationship between DPPH, total phenolic content and total flavonoid content depend on their chemical structure, polarities and solubility in the testing medium [26]. Also Folin Ciocalteu reagent measures other components like amino acids, ascorbic acids and sugar in the plant extract which may not give an accurate amount of phenolic or flavonoid compounds. Therefore, the observed free radical scavenging activities by the extracts suggest a possible synergistic interaction of phenolic compounds with other antioxidants that may not be phenolic in nature [27].
Alpha amylase inhibition assay
In all the extracts, the blank absorbance of 0.64 was used to compute the percentage inhibition (Table 4).
| Sample | IC50 (µgmL ) -1 |
|---|---|
| Acarbose | 24.97 |
| Acetone extract | 46.07 |
| Ethanol extract | 51.31 |
Table 4: The IC50 of the alpha amylase inhibition ability of extract and acarbose.
The α-amylase enzyme catalyzes the hydrolysis of α-1, 4 glycosidic linkages of polysaccharides to yield maltose units which are in turn acted upon by other glucosidases down the GIT to produce glucose residues [28]. In the α-amylase inhibition assay, acetone extract (IC50 of 46.07 μgmL-1) was more potent compared to that of ethanolic extract (IC50 of 51.31 μgmL-1). Percentage alpha amylase inhibition of the two plant extracts was plotted as function of concentration in comparison with acarbose as shown in Figure 3. For the two extracts of A. occidentale stem bark, it was found that acetone extract shows a better α-amylase inhibition compared to the ethanolic extract. A previous study by Dineshkumar et al., proposed that, the inhibition of α-amylase activity by plant extracts may be due to the presence of potential α-amylase inhibitors such as flavonoids, alkaloids, terpenes and glycosides.
Conclusion
The study shows that extracts obtained from the stem bark of A. occidentale are rich in bioactive secondary metabolites, exerting a potential α-amylase inhibition. The investigation on α-amylase inhibition of A. occidentale suggested that, all investigated extracts could inactivate α-amylase with different degrees of inhibition. The inhibition was found to be dose-dependent. In addition, the extracts exhibited antioxidant activity but not as significantly potent as that of ascorbic acid.
References
- Gandjbakhch I, Leprince P, D’Alessandro C, Ouattara A, Bonnet N, et al. (2005) Coronary bypass graft surgery in patients with diabetes. Bulletin de I’Academie Nationale de Med J 189: 257-266.
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for 2000 and projections for 2030. Diabetes Care 47: 50- 56.
- Serup P, Madsen OD, Mandrup-Poulsen TT (2001) Islet and stem cell transplantation for treating diabetes. British Med J 322: 29-32.
- Exarchou V, Nenadis N, Tsimidou M, Gerothanassis IP, Boskou D (2002) Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage and summer savory. J Agric Food Chem 50: 5294-299.
- Najmussahar K, Khan Z, Mular S, Nazia DK, Syed S (2017) In vitro assay of α-amylase inhibitory activity of different vegetables. Inter J Advan Res Develop 2: 22-23.
- Rothit KB (2015) Preliminary tests of phytochemical screening of crude ethanolic and aqeous extracts of moringa pterygosperma Gaertin. J Pharmacog Phytochem 4: 7-9.
- Bhutkar MA, Bhise S (2012) In vitro assay of alpha amylase inhibitory activity of some indigenous plants. Inter J Chem Sci 10: 457-462.
- Varum K, Aakanksha J, Ravinder S (2013) In vitro study on alpha amylase inhibitory activity and phytochemical screen of few Indian medicinal plants having anti-diabetic properties. Inter J Sci Res Publications 3: p 1.
- Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant. LWT-Food Sci Tech 28: 25-30.
- Dildar A, Muhammad MK, Ramsha S (2015) Comparative Analysis of Phenolics, Flavonoids, and Antioxidant and Antibacterial Potential of Methanolic, Hexanic and Aqueous Extracts from Adiantum caudatumLeaves. Open access: Antioxidant 4: 394-409.
- Rajaram SS, Ashvin G, Shabha JD (2013) Comparative screening of acetonic extract of fruits of Terminalia catappa Linn and Anacardium occidentale Linn. Assian J Plant Sci Res 3: 150-153.
- Akinjogunla OJ, Adenugba IT, Jumbo OM (2012) In-vitro antibacterial evauation of ethanolic stem crude extracts if Anacardium occidentale Linn (Anacardiaceae) on streptococcus mutant associated with Dental caries. Scientific J Microbiology 1: 71-81.
- Smania A, Delle MF, Smania EF, Cuneo RS (1999) Antibacterial qctivity of steroidal compounds isolated from Ganoderma applanatum (pers) pat. (Aphyllophoromycetideae) fruit body. Inter J Mol Med 1: 325-330.
- John OO, Gabriel I, Peter YF (2017) Comparative studies of the phytochemical, antioxidant and antibacterial properties of cashew leaf, bark and fruit extracts. Am J Food and Nutrition 5: 115-120.
- Moradi-Afrapoli F, Asghari B, Saeidnia S, Ajani Y, Mirjani M, et al. (2012) In vitro α-glucosidase inhibitory activity of phenolic constituents from aerial parts of Polygonum hyrcanicum. DARU J Pharmal Sci 20: 37.
- Tchikaya FO, Guy BB, Kouakou-Siransy G, Jaques YD, Paul AY, et al. (2011).
- A. occidental Linn (Anacardiaceae) stem bark extracts induces hypotensive and Cardio-inhibitory effect in experimental animal models. Afr J Trad Complement and Alternative Med 8: 452-461.
- Roya K, Fatemeh G (2013) Screening of total phenol and flavonoids content, antioxidant and antibacterial activities of the methanolic extracts of three Silene species from Iran. Inter J Agricultural crop Sci 5: 305-312.
- Haslam E (1996) Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J products 59: 205-215.
- López A, Rico M, Rivero A, DeTangil M (2011) The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Biochemistry 125: 1104-1109.
- Neweze EL, Okafor JL, Njoku O (2004) Anitmicrobial activity of methanolic extracts of Trune guineesis (Schumm thorn) and Morida icida Benth used in Nigerian Herbal Medicine Practice. J Bio Res Bio 2: 39-46.
- Ojezele MO, Augunbiade S (2013) phytochemical constituents and medicinal properties of different extracts of Anacardium occidentale and Psidium guajava. J Bio Pharma Sci 3: 20-23.
- Piaxao N, Peresrelo R, Marques JC, Camara JS (2017) Relationship between antioxidant and total phenolic content in red, rose and white wines. Food Chem 105: 204-214.
- Karou D, Dicko MH, Simpore J, Traore AS (2005) Antioxidant and antibacterial activity of polyphenols from ethnomedicinal plants of Burkina Faso. Afr J Biotech 4: 823-828.
- Shahidi F, McDonald J, Chandrasekara A, Zhong Y (2008) Phytochemicals of foods, beverages and fruit vinegars: Chemistry and health effect. Assian Pacific J Clin Nutri 17: 380-382.
- Kahkonen MP, Hopia AI, Vuorela HJ (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agric and Food Chem 47: 3954-962.
- Juan MY, Chou CC (2010) Enhancement of antioxidant activity, total phenol and total flavonoid content of black soyabeans by solid state fermentation by Bacillus sutilis BCRC 14715. Food Microb 27: 586-591.
- Shahidi F, McDonald J, Chandrasekara A, Zhong Y (1994) Phytochemicals of foods, beverages and fruit vinegars: Chemistry and health effects. Asia Pacific J Clin Nutri 17: 380-382.
- Kamtekar S, Vrushali K, Vijaya P (2014) Estimation of phenolic content, flavonoid content, antioxidant and α-amylase inhibitory activity of Marketed Polyherbal Formulation. J App Pharma Sci 4: 61-65.
Citation: Mborige B (2021) An In vitro Study to Assess Alpha Amylase Inhibition and Antioxidant Activities of The Ethanol and Acetone Extracts of Anacardium occidentale Linn (Cashew) Stem Bark. Biochem Physiol 10: 293. DOI: 10.4172/2168-9652.1000296
Copyright: © 2021 Mborige B. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1761
- [From(publication date): 0-2021 - Apr 17, 2025]
- Breakdown by view type
- HTML page views: 1034
- PDF downloads: 727



