Research Article Open Access
An Improved High Performance Liquid Chromatographic Method for Tryptophan Analysis in Rat Brain Administrated by Seaweed
Sana Mustafa1*, Wajiha Hashim1, Saima Khaliq2, Azizuddin1 and Rashid Ali Khan31Department of Chemistry, Federal Urdu University for Arts, Science and Technology, Karachi, Pakistan
2Department of Biochemistry, Federal Urdu University for Arts, Science and Technology, Karachi, Pakistan
3Pharmaceutical Research Center, PCSIR Laboratories Complex Karachi, Pakistan
- *Corresponding Author:
- Sana Mustafa
Federal Urdu University for Arts, Science and Technology
Gulshan-e-Iqbal Campus, Karachi-75300, Pakistan
Tel: 00923452957388
E-mail: sanachemana@yahoo.com
Received date: March 23, 2014; Accepted date: April 23, 2014; Published date: April 27, 2014
Citation: Mustafa S, Hashim W, Khaliq S, Azizuddin, Khan RA (2014) An Improved High Performance Liquid Chromatographic Method for Tryptophan Analysis in Rat Brain Administrated by Seaweed. J Anal Bioanal Tech 5:188. doi: 10.4172/2155-9872.1000188
Copyright: © 2014 Mustafa S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
IBrain functions can be affected by the dietary precursors of neurotransmitters. Tryptophan (TRP) is the precursor of serotonin (5- Hydroxytryptamine; 5-HT) neurotransmitter that plays an important role in sleeping, mood, eating behavior, consciousness and cognitive functions. Present study describes the estimation of tryptophan in rat brain by aqueous-reverse-phase high performance liquid chromatography. Estimation was carried out on a shim pack end-capped C-18 column using water as mobile phase (pH=9 adjusted by addition of 0.1 mM NaOH) at a flow rate of 1 ml min-1. Eluents were monitored at 273 nm by an ultra-violet detector. The method was linear (R2=0.9973) and the limit of detection (LOD) and limit of quantification (LOQ) were 4.3920 and 13.3091 ng ml-1, respectively. The developed HPLC method is simple convenient, reliable and economical for the estimation of tryptophan in rat brain. In the present study, Albino Wistar male rats were randomly divided into two groups control and seaweeds treated rats. Seaweeds (Jolyna Laminiriodes) were orally given to test rats at a dose of 200 mg kg-1 whereas control rats were given vehicle for 28 days. After 28 days of treatment, rats were decapitated and its brain samples were taken out for the estimation of TRP. Current study observed increased in TRP levels in the brain samples of seaweed treated rats as compared to control rats. Therefore present study suggested the use of seaweeds in the treatment of disorders caused by TRP depletion. By this newly developed method for the estimation of TRP, effect of various drugs on TRP levels in the brain could be studied.
Keywords
Tryptophan; Hormone; HPLC; Seaweed; Rat brain
Introduction
Amino acids are organic compounds of biological importance comprise of two fundamental functional groups i.e., amine and carboxylic acid, along with a side chain specific to each amino acid [1]. About 500 amino acids have been discovered, in which 20 amino acids are essentially required by the human body. Out of these 20 standard amino acids 9 essential amino acids cannot be synthesize by our body; they must be taken from diet and one of them is tryptophan (TRP). Among essential amino acids, TRP is of most considerable significance due to its diversity of biological tasks in human body [2]. It is a vital component of proteins and crucial in human diet for setting up a positive nitrogen balance [3]. It produced from proteins during digestion by the action of proteolytic enzymes. It is a heterocyclic compound with C11H12N2O2 formula and is present in small quantity in nearly all proteins. It takes part in nourishment of infants and in the biosynthesis of serotonin and niacin [4]. For the reason that, serotonin is synthesized from the dietary L-tryptophan made it crucial for brain functions and neuronal regulatory mechanisms. Brain serotonin level is greatly influenced by an unbalanced intake of TRP in diet [5].
In addition, TRP is an essential and regularly used preparatory material in the production of a variety of pharmaceuticals [6]. It has been used in the treatment of various diseases such as depression, schizophrenia, and hypertension [7]. Some of its derivatives are well known potent drugs [8]. It is extensively used in food industries to sustain the diet quality and added as a food fortifier and to balance the amino acid level. It can also be utilized to monitor the formation and dynamics of the proteins owing to its indole moiety [9].
The nutritional and biochemical significance emphasizes the need for reliable analytical methods for the determination of TRP in various matrixes. The analytical methods used for the determination of TRP content in biological media, in food or in pure form include titrimetry [10], capillary electrophoresis [11-13], voltammetry [3,14,15], chemiluminescence (CL) [16-18], amperometry [19,20], polarography [21], high-performance liquid chromatography (HPLC) [22-26], fluorescence spectroscopy and spectrophotometric method [27,28]. Among these methods, titrimetry is the easiest and straightforward, but it is insensitive and only the amino acids of samples with more than mM concentration can be precisely estimated [10]. Even though capillary electrophoresis has attained substantial attention due to its high resolution [13], but its operation is very complicated and it is critical to adopt good operational practices in order to uphold steady migration times and resolution [11,12]. Voltammetric methods are somewhat inexpensive and sensitive up to 1.7 mmol l-1 [14]. However, the qualitative and quantitative interpretation of results from electrochemical data is a tricky job, which is the major drawback of the voltammetric methods. Chemiluminescence methods also have lower detection limits in the femtomole [16-19,29], range [18,30], and vitamin C and protein in the samples may have negative interference in TRP analysis [16]. As far as the amperometry method is concerned, TRP sample first needed to be pretreated by passing to an anion-exchange column to separate from general amino acids. And thus, pretreatment made the amperometric procedure laborious, complicated and time consuming [19,20]. HPLC method is extensively used for the analysis of TRP; however, its analysis stays challenging due to its capability to hydrolyze by acids, mostly a precondition for HPLC analysis.
Therefore, it is usual to perform the time-consuming and sophisticated alkaline hydrolysis for the entire duration of the assay of TRP by HPLC [22-26]. Even though TRP have a luminescent chromophore, there is another fluorescing chromophore, tyrosine, in protein hydrolysates [30]. The spectrophotometric technique is normally adopted because of relatively cheap and easy instrumentation in contrast to other techniques. Unfortunately, the previously reported spectrophotometric methods [27,28,31], also experiences several weaknesses such as call for time taking heat pretreatment steps, complicated extraction of samples to minimize the interferences by other impurities and formation of diazotized complex. The object of the present study was to develop a quick, sensitive, economical, simple, safe and robust method that can be employed in routine analysis of TRP in biological samples. Moreover, the newly developed method was employed for monitoring the effect of seaweed (a natural herb) on brain TRP concentration level in rats.
Experimental
Materials and reagents
Methanol, hexane, EDTA, sodium metabisulphite, perchloric acid, cysteine, tryptophan, HCl, ortho-phosphoric acid, acetic acid, ammonium hydroxide and NaOH were purchased from Sigma chemicals Merk, Germany. Freshly prepared deionized filtered water was used to prepare the mobile phase. Filter paper of 0.45 micrometer for Millipore, UK was used. All reagents used were of analytical grade.
Instrumentation
Shimadzu HPLC model no. LC-20A, UV-Vis Spectrophotometer Shimadzu model 1800, Sonicator of Power Sonic model no. 603, filtration assembly of Sparmax with vacuum pump model no. TC- 2000, analytical balance of Dender model no. TP-214, high speed homogenizer of Germany Seiko, model no. MD-HMN-07, table top centrifuge 12,000 rpm multi-purpose model no. 1236 was used.
Prepartion of solutions
Standard preparation: Accurately weighed 0.0001 g of tryptophan and dissolved in deionized in order to prepare the standard solution of 4000 ng ml-1 in 25 ml volumetric flask. By the dilution of this primary stock standard solution of 4000 ng ml-1 secondary stock standard of 1000 ng ml-1 was prepared. The working standards were prepared by the dilution of these two standards.
Sample prepartion and extraction
Animals: Twelve Male Albino Wistar rats were purchased from Aga Khan University Hospital and used in the present study. They were placed individually in their home cages during the treatment. Standard rodent diet and tap water was available to them throughout the treatment procedure. All experiments in the present study were done according to the protocol approved by Local Animal Care Committee.
Drug administration
Male Albino Wistar rats weighing 150-180 g were divided into two groups: Control and test group (n=6). Jolyna Laminiriodes at a dose of 200 mg kg-1 were prepared in cooking oil and were given to test rats for 28 days whereas control animal received equal amount of vehicle (cooking oil) for 28 days. For oral administration of drugs small stainless steel feeding tube was used that was attached to 1 ml syringe. It was made sure that proper amount of dose was taken in by the rats. After 28 days of treatment procedure, rats were decapitated and brains taken out were dipped in chilled saline.
Extraction of TRP from brain samples
Brain samples were then homogenized in 5-10 volumes of extraction medium. Extraction medium is prepared by 0.4 M perchloric acid containing 0.1% sodium metabisulphite, 0.1% EDTA and 0.01% cysteine. Samples were then centrifuged at 10000 × g in Eppendorf tubes for 15 minutes at low temperature. Supernatants were used for HPLC analysis.
Chromatographic conditions
The liquid chromatographic system consisted of a Shimadzu model LC-20A, pump with a LC-20 AT, variable wavelength UV detector. Pentium-IV PC loaded with LC-Solution Software. Analysis was conducted on an end-capped Shim pack C-18 (150 mm × 4.6 mm, 5 μm particle size) analytical aqueous-reverse-phase column with mobile phase of water (buffer) at pH 9 maintained by addition of 0.1 mM NaOH. The samples were introduced through a rheodyne injector valve with a 20 μl sample loop. Assays were performed at temperature of 40°C and at a flow rate 1 ml min-1. The eluents were monitored at 273 nm.
Method Development and Experimental Condition Optimization
Selection of stationary phase: HPLC is high ranking tool for analysis. Its advantages of short retention time method reliability and sensitivity. Therefore, primary objective of this study is to develop simple, economical, hasty and effective liquid chromatographic method to determine tryptophan in rat brain. In this way, a C-18 endcapped column (with dimension of 150 mm × 4.6 mm × 5 μm particle size) of silica was used for determination of TRP.
Selection of mobile phase: In solvent selection, different solvents including water in several compositions were tried. Acetonitrile due to its carcinogenicity and high coast were avoided. The pH of mobile phases was also varied by the addition of different acids and bases to obtain the best separated and resolved peak.
Mobile phase flow rate: The flow rate of pump A or/and pump B in between 0.5-2.0 ml min-1 were varied for selecting the suitable flow rate to carry the experimental work.
Selection of temperature: The temperature of column was varied linearly in between room temperature of 28°C and 40°C in order to obtain the optimum separation.
Selection of wavelength: In order to select the wavelength for tryptophan analysis, the standard tryptophan solution is prepared and scanned across the UV-Visible region (180-760 nm) and the cut-off point was determined for the analysis of TRP i.e., λmax.
Method validation
The proposed method was validated for various parameters given by ICH 2006 guidelines [32].
System suitability test
The system suitability was estimated on each working day of method validation. Initially, RP-18 column was conditioned with mobile phase to equilibrium, then ten replicate TRP standard solutions were injected and various parameters including capacity factors (k’), theoretical plates (N), tailing (T), separation factor (α), and resolution (Rss) were calculated.
Specificity
The specificity studies were carried by injecting and taking chromatogram of standard and spiked sample and solution, and the possible interferences of unwanted species were observed.
Linearity
Linearity of the method was found out from constructing the calibration curve between peak area and concentration of standard TRP at various concentration levels (1.0-250 ng ml-1). The regression parameters such as intercept, slope, and correlation coefficient were calculated.
Recovery
A concentrated solution of TRP with known concentration was prepared and shortly before extraction small volumes were added in a fraction of homogenized rat brain (n=3) such that final concentrations of tryptophan were 1, 5 and 10 ng ml-1. The untreated brain extracts fractions of these biological samples acted as reference and standards containing these different concentrations were run as calibrants. The chromatogram of all standards and reference extracts were taken under the standard conditions selected in proposed method. The percent recovery of the method was calculated for TRP in rat brain by applying the equation as given below.
% Recovery = C/X × 100
Where, C and X are the concentrations of tryptophan in spiked samples and in reference standard, respectively.
Precision
The precision of the method was analyzed in terms of intra-day and inter-day percent relative standard deviation (% RSD) at λmax of analyte. The tryptophan was analyzed at three concentration levels of 1, 5 and 10 ng ml-1 with triplicate injection on the initial day and three consecutive days under the same experimental conditions and % RSD of each analyte was calculated by using formula;
% RSD = (SD/ X’) × 100
Where, SD and X’ are the standard deviation and mean of analyte at specific concentration, respectively.
Detection and quantification limits
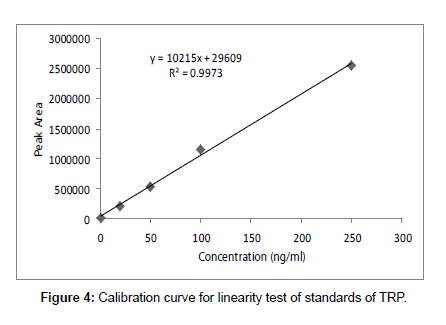
For this purpose pure tryptophan (standard) sample were manually spiked with 1.0-250 ng ml-1 concentrations of tryptophan. Calibration curve was prepared and slope of regression line and standard deviation of lowest concentration detectable and lowest concentration measured were taken into account (Figure 4).
The LOD and LOQ were determined were determined by using the following formulas;
LOD = 3.3 × SD/S
LOQ = 10 × SD/S
Where, SD and S are the standard deviation and slope of the calibration curve, respectively.
Robustness
In order to check the robustness of proposed method on analytical results the chromatographic parameters such as pH (±1) and flow rate (0.1 ml min-1) were deliberately varied. One parameter was changed in the experiments at a time and assays were carried out in triplicate.
Single point external standard method
In this study, we applied single point external addition method, standard solutions of TRP was not added in samples. The 20 μl of standard and each sample (control and test) were injected after filtration with 0.45 μm filter paper in HPLC column. Each sample was run for 15 min and the peak area of tryptophan in standard and sample at chromatogram were recorded. The concentrations of TRP were calculated in all twelve rat brain samples (6 controls and 6 tests) in order to find the effect of seaweeds administration to rat brain TRP level. The concentrations were calculated by using following formula;
Cu = Au/As × Cs
Where,
Cs = concentration of standard
Cu = concentration of unknown sample
Au = peak area of unknown sample
As = peak area of standard sample
Results and Discussion
The amino acid L-tryptophan is of particular scientific and medical attention, because it is not only a fundamental precursor of a large number of biologically active metabolites, such as the cerebral indolylamine serotonin, 5-hydroxytryptamine (5-HT), the pineal hormone melatonin and the many other metabolites of the hepatic kynurenine pathway that plays a vital role in brain function and related regulatory mechanisms [6] but also required for protein synthesis and hence growth. The 5-HT is engaged in mood, consciousness and sleep [33,34]. It is metabolized to a variety of other biologically active substances in vivo as melatonin, nicotinic acid and nicotinamide adenine dinucleotide (NAD) [35]. Indoleamine-2,3-dioxygenase (IDO) is an enzyme that is generated in different cells by interferongamma during inflammation. TRP catabolism increases due to hyper activation of IDO in many pathological conditions like viral infections, autoimmune disorders and malignant diseases. The net consequence of this action is the reduction in TRP concentration in the body. This depletion in turn influences 5-HT production in brain and sends the patient into depression [33-37]. Therefore, TRP depletion is commonly used to recognize the susceptibility of patients towards depression [38,39].
Hence, due to the great importance of this amino acid it is worthwhile to develop new, speedy and economical methods for both qualitative and quantitative analysis in various matrices.
Various methods have been developed for the analysis of TRP as described above in a number of matrixes; here we report a simple, safe, efficient and economical HPLC-UV method for the TRP determination in biological fluids with detection limits up to ppb level (ng ml-1). Some chromatographic parameters such as column type, mobile phase, wavelength and conditioning time were investigated to obtain a suitable peak for TRP within an acceptable time.
Optimization of chromatographic conditions
A number of parameters were varied for developing and optimizing chromatographic method for the estimation of TRP concentration in rat brain up to the ppb level.
In mobile phase selection, an isocratic mood was applied for elution instead of gradient to avoid re-equilibration. Initially, methanol was used as an important component of the mobile phase in different ratios with hexane (100%, 70: 30, 50: 50, 30: 70) and then with water (90: 10, 70: 30, 50: 50, 30: 70), but it was failed to estimate tryptophan in biological sample due to bad resolution, separation and long retention time. Instead of methanol acetonitrile was used, but due to its carcinogenicity and high coast we avoid acetonitrile and made an acetonitrile free method.
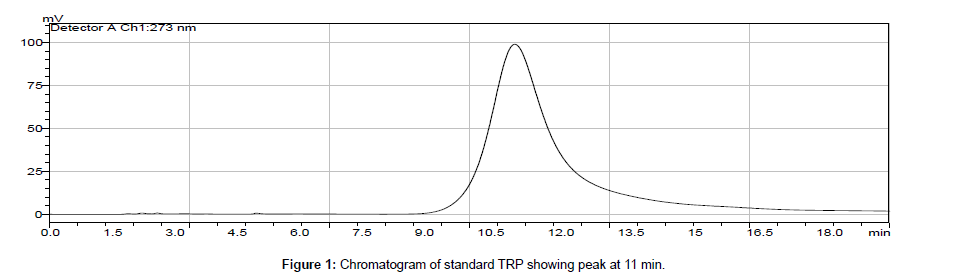
We tried deionized water at various pH as a mobile phase. Initially, we maintained mobile phase at acidic pH by drop wise adding hydrochloric acid, ortho-phosphoric acid and/or acetic acid but the retention time of the tryptophan peak was high as previous researches showed. Then we maintained basic pH of the mobile phase by adding ammonium hydroxide and/or sodium hydroxide and monitored the chromatogram. The deionized water only was also used as mobile phase. The best resolved peak with optimum retention time at 11 min of TRP was obtained by taking mobile phase of pH 9 which pH was previously maintained by addition of 0.1 mM NaOH. Therefore, the pH 9 mobile phase (deionized water with NaOH) was finally selected for further study. This experimental work was carried out by adjusting flow rate of pump A, 0.5 ml/min and 0.5 ml min-1 of pump B. The optimum temperature of column was choosed at 40°C. The standard tryptophan solution was prepared and scanned across the UVVisible region. The maximum absorbance of the TRP was observed at wavelength of 273 nm and it is considered for further study as detector wavelength. The concentration of standard, retention time and peak area are represented in Table 1.
| Standard | Concentration (ng ml-1) | Retention Time (min) | Peak Area |
|---|---|---|---|
| Tryptophan | 1.00 × 103 | 11 | 10577 × 103 |
Table 1: Peaks area, height and retention time of standards of TRP.
Method development
The proposed method is validated for the qualitative and quantitative analysis of TRP in biological samples at 273 nm according to the ICH guidelines for system suitability, specificity, linearity, accuracy, precision, limits of detection and quantification, and robustness [32].
System suitability
The system suitability was evaluated regularly (working day) and was determined to be in the approved limit. The efficiency of column was calculated by number of theoretical plates and tailing factor. The value of number of theoretical plates was greater than 7000 and tailing factor was less than 1 (Table 2 and 3).
| tR | K’ | N | T | Rs | α |
|---|---|---|---|---|---|
| 11 | 1.200 | 7744 | 0.940 | 1.950 | 6.777 |
Table 2: The chromatographic system suitability parameters for tryptophan analysis.
| Concentration of Standard TRP (ng ml-1) | Peak Area |
|---|---|
| 1.0 | 10577 |
| 20 | 211540 |
| 50 | 543101 |
| 100 | 1145200 |
| 250 | 2545500 |
Table 3: Concentration and peak area of standard solutions of TRP for preparing calibration curve for linearity.
Specificity
Specificity of the proposed method was demonstrated by means of blank serum spiked with samples. The chromatogram showed no interfering peak of serum at the TRP retention time which indicated the specificity of method.
Linearity
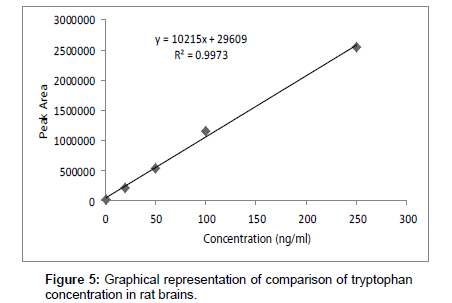
Linearity and regression analysis was performed using Microsoft Excel 2007 Software. The method showed excellent linearity in standards solutions at pH value of 9 over concentration range of 1.0- 250 ng ml-1 with correlation coefficient (r2) of 0.9973. The mean linear regression equation of standard curve is y=10215x + 29609
Accuracy/Recovery
Percent recovery values of TRP at three concentrations in rat brain extract were calculated. The accuracy of the newly developed method was represented by the value of % recovery and is found to be in the range of 92.43-101.49% as illustrated in Table 4. The spiked samples provide high level of recovery of TRP in rat brain samples.
| Standard | LOD | LOQ | Added concentration | Found | Recovery | Precision | |
|---|---|---|---|---|---|---|---|
| Intra-day | Inter-day | ||||||
| (ng ml-1) | (ng ml-1) | (ng ml-1) | (ng ml-1) | (%) | (% RSD) | ||
| TRP | 4.3920 | 13.3091 | 1.044 5.035 10.00 |
0.965 ± 0.13 5.11 ± 0.19 9.87 ± 0.15 |
92.43 101.49 98.70 |
1.99 1.42 0.82 |
2.01 1.98 0.98 |
Table 4: LOD, LOQ, % RSD and % recovery of the analytical method means ± SEM for TRP.
Precision
Precision was calculated as percent relative standard deviation (% RSD). The developed method provides good repeatability and reproducibility of the results with value of 0.82-1.99% for intra-day and 0.98-2.01% for inter-day precision as tabulated (Table 4).
Limit of detection and limit of quantification
To assure the validity of results obtained, limit of detection, the lowest amount of analyte that the system can detect and limit of quantification, the lowest amount of analyte that the system quantified were determined by the standard deviation of area under the curve and mean at low concentrations.
The standard deviation of the response can be calculated using the standard deviation of y-intercepts of regression lines. The LOD and LOQ of the method are 4.3920 and 13.3091 ng ml-1, respectively (Table 4).
Robustness
The pH and flow rate were deliberately changed and successive effects in chromatograms were monitored. These variations had a minor effect in operating conditions. Theoretical plates (N) ranged in between 6000-9000 and tailing factor was less than 2. The data mentioned in Table 5 signified that there were not many variations in theoretical plates and tailing factor that proved the robustness of planned method.
| Parameters | N | T | Rs | |
|---|---|---|---|---|
| pH | 8 9 10 |
6993 7534 8578 |
1.51 1.35 1.41 |
0.99 1.76 1.89 |
| Flow rate | 0.9 1.0 1.1 |
7859 7759 6998 |
0.95 1.23 1.11 |
1.53 1.49 1.68 |
Table 5: Robustness data for tryptophan analysis.
The linearity, the value of limit of detection and limit of quantification represented that the method is capable of quantifying concentration of TRP in ng ml-1 hence; we developed a ppb level, robust and economical method using end-capped C-18 HPLC column.
Tryptophan level in rat brain administrated by seaweeds and saline
The newly developed HPLC method was subsequently applied to observe the effect of seaweed (Jolyna Laminiriodes) on TRP levels. It was observed in the present study that seaweeds administration for 28 days significantly increased brain TRP levels in seaweeds treated rats as compared to control rats.
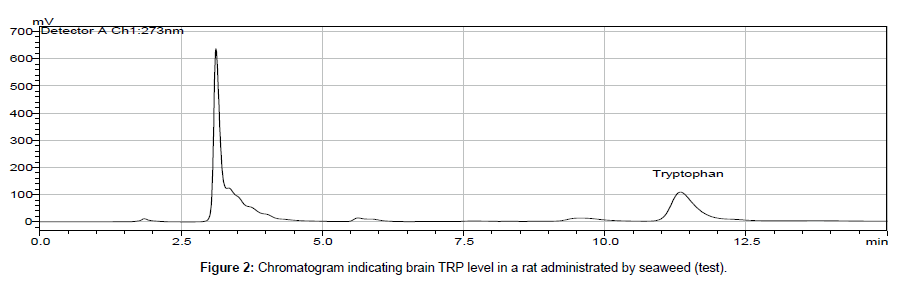
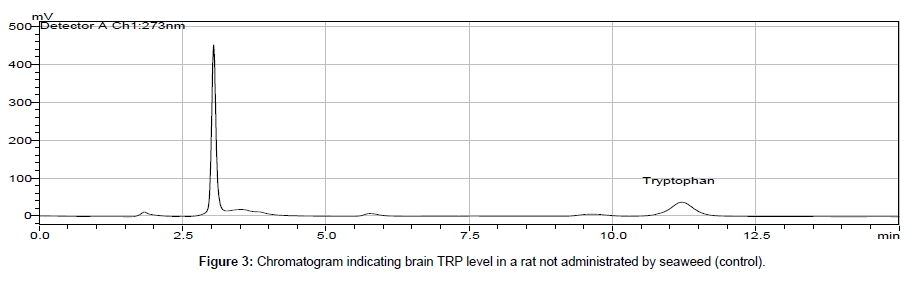
In biological samples (rat brain), 5-hydroxy tryptophan was always presented and it might interfere in determination of tryptophan (TRP). Hence, under optimized conditions, the peak of TRP was identified at 11 min retention time and by comparing chromatogram of standard TRP of 1.0 × 103 ng ml-1 (peak area = 10577 × 103) (Figure 1) with the chromatogram obtained from biological samples (Figures 2 and 3), the concentration in brain extracts is quantified (Table 6).
| Serial Number | Sample Code | TRP in biological samples (ng ml-1) | Serial Number | Sample Code | TRP in biological samples (ng ml-1) |
|---|---|---|---|---|---|
| 1 | Control 1 | 120.7 | 7 | Test 1 | 36.3 |
| 2 | Control 2 | 77.8 | 8 | Test 2 | 27.6 |
| 3 | Control 3 | 137.5 | 9 | Test 3 | 421.4 |
| 4 | Control 4 | 65.9 | 10 | Test 4 | 64.4 |
| 5 | Control 5 | 94.4 | 11 | Test 5 | 70.5 |
| 6 | Control 6 | 13.3 | 12 | Test 6 | 87.1 |
| Average | Control | 84.95 | Average | Test | 117.89 |
Table 6: Concentration of TRP in rat brain (Standard TRP = 1.0 × 103 ng ml-1).
Table 6 and Figure 5 shows the effects of Jolyna Laminiriodes (seaweeds) on brain TRP levels in brain samples. The significant difference between test and control tryptophan concentration is clear. Seaweeds treated rats exhibited significantly higher concentration of tryptophan (117.89 ng ml-1) as compared to control rats (84.93 ng ml-1).
Therefore, it is said that the seaweed (Jolyna Laminiriodes)) is one of among the natural products that could enhance tryptophan level in brain and could use as a drug.
Conclusion
In conclusion, a rapid, cheap, conventional and acetonitrile free HPLC method is developed to monitor the level of tryptophan rat brain samples. Present study also suggested the use of seaweed to increase the tryptophan concentration in tryptophan depletion disorder.
References
- Wagner I, Musso H (1983) New naturally occurring amino acids. Angew Chem Int Ed 22: 816-828.
- Moffat AC, Jackson JV, Moss MS, Widdop B (1986) Clarke’s isolation and identification of drugs. The Pharmaceutical Press, London, UK, 1056.
- Fiorucci AR, Cavalheiro ET (2002) The use of carbon paste electrode in the direct voltammetric determination of tryptophan in pharmaceutical formulations. J Pharm Biomed Anal 28: 909-915.
- Nelson DL, Cox MM (2000) Lehninger principles of biochemistry. Worth Publishers, 41 Madison Avenue, New York, USA, 834.
- Markus CR, Olivier B, de Haan EH (2002) Whey protein rich in alpha-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. Am J Clin Nutr 75: 1051-1056.
- Liang YD, Song JF (2005) Flow-injection chemiluminescence determination of tryptophan through its peroxidation and epoxidation by peroxynitrous acid. J Pharm Biomed Anal 38: 100-106.
- Budavari S, O’Neil MJ, Smith A and Heckleman PE (1996) Merck Index, Merck Research Laboratories, Whitehouse Station, New Jersey, USA, 1669.
- Sanger GJ (2008) 5-hydroxytryptamine and the gastrointestinal tract: where next? Trends Pharmacol Sci 29: 465-471.
- Van Praag HM, Lemus C (1986) Monoamine precursors in the treatment of psychiatric disorders. In: Wurtman RJ, Wurtman JJ, (eds). Nutrition and the brain. Raven Press, New York, USA, 89-139.
- El-Brashy AM, Al Ghannam SM (1996) Titrimetric determinations of some amino acids. Microchem J 53: 420-427.
- Altria KD, Harkin P, Hindson MG (1996) Quantitative determination of tryptophan enantiomers by capillary electrophoresis. J Chromatogr B Biomed Appl 686: 103-110.
- Altria KD, Kelly MA, Clark BJ (1996) The use of a short-end injection procedure to achieve improved performance in capillary electrophoresis. Chromatographia 43: 153-158.
- She Z, Sun Z, Wu L, Wu K, Sun S, et al. (2002) Rapid method for the determination of amino acids in serum by capillary electrophoresis. J Chromatogr A 979: 227-232.
- Wang H, Sui H, Zhang A, Liu R (1996) Adsorptive stripping voltammetric determination of tryptophan at an electrochemically pretreated carbon paste electrode on solid paraffin as a binder. Anal Commun 33: 275-277.
- Moreno L, Merkoci A, Alegret S, Hernandez Cassou S, Saurina J (2004) Analysis of amino acids in complex samples by using voltammetry and multivariate calibration methods. Anal Chim Acta 507: 247-253.
- Alwarthan AA (1995) Chemiluminescent determination of tryptophan in a flow injection system. Anal Chim Acta 317: 233-237.
- Chen GN, Lin RE, Zhao ZF, Duan JP, Zhang L (1997) Electrogenerated chemiluminescence for determination of indole and tryptophan. Anal Chim Acta 341: 251-256.
- Hanaoka S, Lin JM, Yamada M (2000) Chemiluminescence behavior of the decomposition of hydrogen peroxide catalyzed by copper (II)-amino acid complexes and its application to the determination of tryptophan and phenylalanine. Anal Chim Acta 409: 65-73.
- Simoniam AL, Rainina EI, Fitzpatrik P, Wild JR (1997) A tryptophan-2-monooxygenase based amperometric biosensor for L-tryptophan determination: use of a competitive inhibitor as a tool for selectivity increase. Biosens Bioelectron 12: 363-371.
- Hanko VP, Rohrer JS (2002) Direct determination of tryptophan using high-performance anion-exchange chromatography with integrated pulsed amperometric detection. Anal Biochem 308: 204-209.
- Levina II, Chechekin GV, Arzamastsev AP, Gaevskii AV, Degterev EV, et al. (1997) Indirect polarographic determination of tryptophan, tryptamine and serotonin in aqueous organic solutions. Pharmaceutical Chemistry Journal 3150-3151.
- Alegría A, Barberá R, Farré R, Ferrerés M, Lagarda MJ, et al. (1996) Isocratic high-performance liquid chromatographic determination of tryptophan in infant formulas. J Chromatogr A 721: 83-88.
- Herve C, Beyne P, Jamault H, Delacoux E (1996) Determination of tryptophan and its kynurenine pathway metabolites in human serum by high-performance liquid chromatography with simultaneous ultraviolet and fluorimetric detection. J Chromatogr 675: 157-161.
- Mattivi F, Vrhovsek U, Versini G (1999) Determination of indole-3-acetic acid, tryptophan and other indoles in must and wine by high-performance liquid chromatography with fluorescence detection. J Chromatogr A 855: 227-235.
- Yust MM, Pedroche J, GironCalle J, Vioque J, Millan F, et al. (2004) Determination of tryptophan by high performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection. Food Chem 85: 317-320.
- Ravindran G, Bryden WL (2005) Tryptophan determination in proteins and feedstuffs by ion exchange chromatography. Food Chem 89: 309-314.
- Tummuru MK, Sastry CSP (1985) Spectrophotometric determination of tryptophan with N,N-dimethyl-p-phenylenedia-mine and chloramine-T. J Royal Inst Chem 57: 109-110.
- Molnár-Perl I, Pintér-Szakács M (1989) Spectrophotometric determination of tryptophan in intact proteins by the acid ninhydrin method. Anal Biochem 177: 16-19.
- Moreno FA, Gelenberg AJ, Heninger GR, Potter RL, McKnight KM, et al. (1999) Tryptophan depletion and depressive vulnerability. Biol Psychiatry 46: 498-505.
- Reynolds DM (2003) Rapid and direct determination of tryptophan in water using synchronous fluorescence spectroscopy. Water Res 37: 3055-3060.
- Ren J, Zhao M, Wang J, Cui C, Yang B (2007) Spectrophotometric method for determination of tryptophan in protein hydrolysates. Food Technol Biotechnol 45: 360-366.
- International Conference on Hormonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (2005) Validation of Analytical Procedures: Text and Methodology Q2 (R1). Complementary Guideline on Methodology incorporated in November 2005, UK, London.
- Greco AV, Mingrone G, Favuzzi A, Bertuzzi A, Gandolfi A, et al. (2000) Subclinical hepatic encephalopathy: role of tryptophan binding to albumin and the competition with indole-3-acetic acid. J Investig Med 48: 274-280.
- Laich A, Neurauter G, Widner B, Fuchs D (2002) More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem 48: 579-581.
- Kawai K, Ishikawa H, Ohashi K, Itoh Y, Teradaira R (2007) Rapid simple and simultaneous measurement of kynurenine and tryptophan in plasma by column switching HPLC method. Clin Chem 1304: 415-419.
- Maneglier B, Rogez-Kreuz C, Cordonnier P, Therond P, Advenier C, et al. (2004) Simultaneous measurement of kynurenine and tryptophan in human plasma and supernatants of cultured human cells by HPLC with coulometric detection. Clin Chem 50: 2166-2168.
- Sheehy C, Murphy E, Barry M (2006) Depression in rheumatoid arthritis--underscoring the problem. Rheumatology (Oxford) 45: 1325-1327.
- van Donkelaar EL, Kelly PA, Dawson N, Blokland A, Prickaerts J, et al. (2010) Acute tryptophan depletion potentiates 3,4-methylenedioxymethamphetamine-induced cerebrovascular hyperperfusion in adult male Wistar rats. J Neurosci Res 88: 1557-1568.
- Molnár-Perl I (1999) Advances in the analysis of tryptophan and its related compounds by chromatography. Adv Exp Med Biol 467: 801-816.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15135
- [From(publication date):
May-2014 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 10494
- PDF downloads : 4641