An Exploratory Study of the Overall Systemic and Oral Health Status in Older Patients with Alzheimer's Disease
Received: 10-Feb-2023 / Manuscript No. JADP-22-89274 / Editor assigned: 13-Feb-2023 / PreQC No. JADP-22-89274 (PQ) / Reviewed: 27-Feb-2023 / QC No. JADP-22-89274 / Revised: 06-Mar-2023 / Manuscript No. JADP-22-89274 (R) / Published Date: 13-Mar-2023 DOI: 10.4172/2161-0460.100564.
Abstract
Background: An exploratory study of the overall health conditions and physicochemical properties of saliva were performed in older patients with Alzheimer´s Disease (AD) who reside at long-term nursing homes. We also investigate sleep quality, functional capacity during the abilities of Activities of Daily Living (ADL), and mobility of these individuals.
Methods: We examine thirty-nine older adults with AD who resided at long-term private nursing homes. Oral and systemic health status was identified from medical-dental examinations and medical record database. Salivary parameters, including salivary flow rate, pH value, buffering capacity, and salivary cortisol levels (morning), were analyzed. Risk for Obstructive Sleep Apnea (OSA) and functional capacity during the Activities of Daily Living (ADL) were also assessed through specific questionnaires. An exploratory analysis was done, using descriptive and inferential statistics.
Results: The most of older patients were dentulous, showing residual dental root, periodontal diseases, and carie. The polypharmaceuticals did not substantially interfere on saliva production. No significant alterations in salivary flow and buffering capacity were detected. Risk of psychological-physical stress identified from salivary cortisol was controlled by medicines.
Conclusion: All older patients with high risk of OSA had cardiocirculatory disorders; additionally, the total and severe dependencies in ADL and mobility was strongly evidenced in institutionalized older patients with AD.
Keywords: Alzheimer disease; Oral health; Saliva; Sleep wake disorders; Obstructive sleep apnea
Introduction
Alzheimer ’s Disease (AD) is the most common type of pre-senile and senile dementia in older people, accounts for 50–75% of dementia cases. This neurodegenerative disorder is characterized by irreversible and progressive cognitive dysfunction, memory decline, disturbances of mood, and inability to recognize common objects, family members or friends (visual agnosia), to comprehend or formulate language (aphasia). These facts lead to loss of autonomy, daily functional impairment with associated severe neuropsychological symptoms (e.g., personality changes, delirium, and depression), immobility, severe weakness, and inanition [1-4].
Monitoring efforts to maintain the satisfactory general and oral health in these individuals have been still insufficient and limiting due to the shortage of specialized health professionals in service units, especially in nursing homes. Additionally, this disease becomes a significant financial burden on society, besides causing physical and psychological stress, social isolation, and financial difficulty to the family members. This dementia is considered a critical public health problem due to its high prevalence in worldwide [5]. Reports that the total healthcare costs for the treatment of AD have been estimated at $305 billion, increasing with to more than $1 trillion as the population ages [6].
The main neuropathological hallmarks of AD consist of extracellular fibrillar deposition of abundant Amyloid-β peptide (Aβ) senile plaques, neuropil threads (axonal and dendritic segments containing aggregated and hyperphosphorylated tau), and neurofibrillary helical tangles containing intraneuronal aggregates of hyperphosphorylated or misfolded tau proteins in brain regions. These lesions damage the central nervous system, causing neuronal death by apoptosis or necrosis by altering the plasticity of neurons and losses of neuropil and synaptic activities [2,3,6,8]. Likely, these alterations play a critical role in the cognitive impairment of this disease.
Various risk factors have been implicated in its pathogenesis, such as: Age, genetic factors (e.g.; mutations of the genes amyloid precursor protein, presenile 1 and 2, apolipoprotein E, and others), traumatic brain injury, associated co-morbidities (i.e.; cardiovascular diseases, congestive heart failure, obesity, hypercholesterolemia, immune system dysfunction, poorly controlled diabetes mellitus, and others), psychiatric factors (e.g.; depression, early stress), environmental factors (e.g.; exposure to metals as aluminium, copper, zinc; deficiencies of vitamin and calcium), mitochondrial dysfunction causing decline in cerebral metabolic rate, sedentary lifestyles (e.g.; alcohol use; lack of exercise and cognitive activity), and infections associated with gramnegative, anaerobic bacteria and viruses. [1-3,8].
Among the oral clinical manifestations in AD, some authors reported caries and periodontal disease, candidiasis, and hyposalivation. This last condition may provide irritation and inflammation of the oral mucosa or pathogen oral infections, besides dysarthria, dysgeusia, and dysphagia [1,3,9]. Furthermore, the salivary cortisol levels can identify psychological and physical stress, become a good biomarker to determine the real emotional conditions of older patients with AD, particularly the long-term nursing home residents [10]. It is still noteworthy that patients with DA and severe dysphagia are susceptible to develop aspiration pneumonia. According to Sato et al. (2014), the mortality from pneumonia is high and accounts for 70% of the causes of AD [10]. Given that, the use of assistive technologies in oral selfcare practices and routine exploratory clinical investigations must be recommended in health services to establish an appropriate planning and treatment, as well as to promote a better quality of life of this population [11].
Focusing in one of the sleep breathing disorders, the Obstructive Sleep Apnea (OSA) is one of the comorbidities that may aggravate the signs and symptoms of this neurodegenerative disease, increasing morbidity and mortality rates among people with AD [12]. The OSA causes a decrease of intracellular supply of O2, resulting in cellular hypoxia episodes and long-term tissue damages [10]. This fact can enhance the severity of AD and the intensity of preexistent comorbidities.
As AD progresses in severity, much effort has been devoted in mitigating the appearance of new comorbidities and improving the functional capacity during the Activities of Daily Living (ADL) through medical and dental approaches together, main at nursing homes.
Before outlining appropriate strategic plans for systemic and oral health care for institutionalized older patients with AD, it is important that the multi-professionals know the impact of this disease and its comorbidities on the patients’ daily living and understand its medical and dental implications. Certainly, these approaches could attenuate the enormous burden on society and public health due to the high costs associated with care and treatment of these dementia. Therefore, we perform an exploratory study of the overall health conditions and the physicochemical properties of saliva in institutionalized older patients with Alzheimer´s disease. We also investigate sleep quality, functional capacity during the abilities of ADL, and mobility of these individuals who reside at long-term nursing homes.
Methods
This study characterized the overall health conditions of institutionalized older patients with AD in the following aspects: Systemic disorders or diseases, pharmacological groups used by patients, general oral health, salivary parameter, sleep quality, functional capacity during the activities of daily living, and mobility. This investigation was approved by the Ethics Committees on Human Research of the Institute of Science and Technology of the São Paulo State University, IST-UNESP (CEPh/CAAE process number 82559818.3.0000.0077) and the Informed Consent Form (ICF) was signed by a legally responsible person.
Subjects
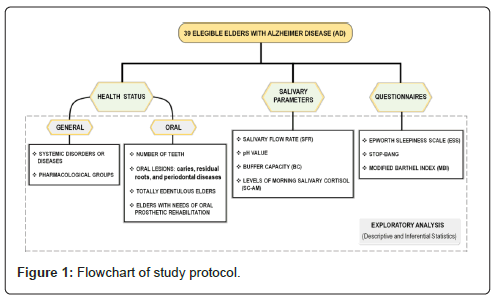
Thirty-nine older adults with Alzheimer Disease (AD), with agerange from 62 to 100 years old, were invited to participate in this study. These subjects showed varying degree of dementia and resided at long-term private nursing homes. The inclusion criteria were older patients who were diagnosed with AD, stages from mild to severe, by a neurologist using the International Classification of Diseases (ICD-10), Diagnostic and Statistical Manual of Mental Disorders (DSM-V), Mini- Mental State Examination (MMSE), and Clinical Dementia Rating scale (CDR). The exclusion criteria were when these residents refused to participate of the study or his/her legally responsible caregivers did not accept to sign the informed consent form (Figure 1).
Protocol of study
To understand the proposal methods, a flow diagram was done in order to illustrate the design of this exploratory study, investigating the overall health conditions, the salivary parameters, and the sleep quality in institutionalized older patients with AD.
Overall health conditions
The analysis of systemic health status was performed on each older patient with AD. Systemic disorders/diseases and pharmacological groups were identified from the medical record database and the information was only collected after ethical approval. In study, a novel protocol of screening intrabuccal clinical exam was created to verify the oral health status where the proposed indicators were number of teeth, Caries (Ca), Residual Dental Roots (RR), Periodontal Diseases (PD), total edentulous older patients, and older patients with needs of Oral Prosthetic Rehabilitation (OPR).
Analysis of saliva parameters
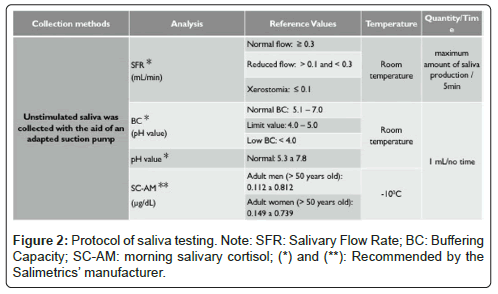
All subject´s preparation and salivary testing were accomplished from the methodology of Liu, et al [9]. Where were determined salivary flow rate (SFR; mL/min), pH value, buffering capacity (BC, pH value), and morning salivary cortisol levels (SC-AM, μg/dL) (Figure 2).
Questionnaires application
The sleep quality was investigated through the application of the Epworth Sleepiness Scale (ESS) and STOP-BANG questionnaires, identifying subjective daytime levels of sleepiness and risk for Obstructive Sleep Apnea (OSA), respectively. These two methods were used in the studies [13-15]. The measure of independence in basic activities of daily living (BADL) and mobility were evaluated using the Modified Barthel Index (MBI), being in accordance with the studies [16,17]. These questionnaires were answered by caregivers and/or health professionals from nursing homes.
Statistical analysis
The results of oral and systemic health status, physicochemical properties of saliva, sleep quality, functional capacity in BADL, dependence degrees, and mobility in older patients with AD were submitted to an exploratory analysis. The descriptive statistics consisted of mean and standard deviation of these data. The inferential statistic was used to estimate the Confidence Interval (CI) for prevalence in the following aspects: Edentulous older patients, older patients with needs of oral prosthetic rehabilitation, older patients with excessive daytime sleepiness, and older patients with risk for OSA. Another inferential statistic was to compare the means between the sexes, using the t-Student test of independent samples in each one of the four variables in analysis. The level of significance was set at p ≤ 0.05.
Results
Overall health status
The Tables 1 and 2 refer all systemic disorders/diseases and pharmacological groups found in older patients with AD. The demographic data showed 35.90% (14/39) older men with 68 to 94 (79.29 ± 8.39) years old and 64.10% (25/39) older women with 65 to 100 (83.24 ± 9.51) years old. Concerning the age ranges, we found the following data: 12.82% (5/39) to 60-70 years old, 28.20% (11/39) to 71- 80 years old, 33.34% (13/39) to 81-90 years old, and 25.64% (10/39) were 91-100 years old (Tables 1 and 2).
| Systemic disorders or diseases | Pharmacological groups | ||
|---|---|---|---|
| Mental disorders (MD) | Neurocognitive disorder | 1.1 Alzheimer's disease (AD) | 1.1.1 N-methyl-D-aspartate (NMDA) receptor antagonist (memantine); (A) |
| 1.1.2 Acetyl-cholinesterase inhibitors. AChEIs (donepezil. galantamine and rivastigmine); (B) | |||
| Other mental disorders (oMD) | 1.2 Anxiety and mood disorders | 1.2.1 Anxiolytic. anticonvulsant. psychotropic (benzodiazepines; valproic acid); (C) | |
| 1.3 Depression | 1.3.1 Antidepressants (tricyclic antidepressants; selective serotonin reuptake inhibitors); (D) | ||
| 1.4 Convulsive seizures | 1.4.1 Barbiturates; (E) | ||
| 1.5 Psychotic disorders (delusions and/or delirium) | 1.5.1 Antipsychotics; (F) | ||
| Sleep disorders (SD) | 2.1 Insomnia | 2.1.1 Sleep inducer. (G) | |
| Cardiocirculatory disorders (CcD) | 3.1 Coronary artery disease; Stroke; Thromboembolism | 3.1.1 Antithrombotics (antiplatelet agents; anticoagulants - coagulation cascade); (H) | |
| 3.2 Cardiac arrhythmia | 3.2.1 Antiarrhythmics; (I) | ||
| 3.3 Systemic Arterial Hypertension (SAH); Congestive Heart Failure (H) | 3.3.1 Antihypertensive (angiotensin-II receptor antagonists; calcium channels blocker; beta-1 selective blocker; angiotensin-converting enzyme inhibitors); (J) | ||
| 3.4 Edema and SAH | 3.4.1 Diuretics; (L) | ||
| 3.5 Chronic vascular diseases (varicose veins) | 3.5.1 Venoactive; (M) | ||
| 3.6 Hyperlipidemia | 3.6.1 Antilipemic; (N) | ||
| Endocrine disorders (ED) | 4.1 Diabetes mellitus - Type 2 | 4.1.1 Antidiabetics; (O) | |
| 4.2 Hyperlipidemia | 4.2.1 Thyroid hormone; (P) | ||
| Rheumatic diseases (RD) | 5.1 Inflammatory rheumatic disease (Gout) | 5.1.1 Anti-hyperuricemic; antigotous; (Q) | |
| Neoplasias (N) | 6.1 Post-treatment of breast carcinoma | 6.1.1 Antineoplastic; (R) | |
| Non-neoplastic proliferative lesions (nNPL) | 7.1 Benign prostatic hyperplasia | 7.1.1 Selective adrenergic alpha-1-blocker; (S) | |
| Gastrointestinal disorder (GD) | 8.1 Gastric reflux; Gastritis; and Esophagitis | 8.1.1 H2 receptor antagonists; hydrogen-potassium pump inhibitor; (T) | |
Note: These findings were obtained from of the medical record database of the health section at nursing homes.
Table 1: Overview of systemic disorders or diseases and pharmacological groups found in institutionalized older patients with AD (n=39).
| Gender | S. no | Age (years) | MD | SD | CcD | ED | RD | N | nNPL | GD | S Medicines per subjects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | oMD | |||||||||||||||||||||
| A | B | C | D | E | F | G | H | I | J | L | M | N | O | P | Q | R | S | T | ||||
| Men | 1 | 88 | 1 | 2 | 1 | 1 | 5 | |||||||||||||||
| 2 | 85 | 1 | 1 | 1 | 1 | 1 | 5 | |||||||||||||||
| 3 | 68 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||||||||||||
| 4 | 68 | 1 | 1 | 2 | 1 | 1 | 1 | 7 | ||||||||||||||
| 5 | 68 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | ||||||||||||
| 6 | 82 | 1 | 1 | 1 | 1 | 2 | 1 | 7 | ||||||||||||||
| 7 | 83 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | |||||||||||||
| 8 | 74 | 1 | 1 | 2 | 1 | 5 | ||||||||||||||||
| 9 | 94 | 1 | 1 | 1 | 1 | 4 | ||||||||||||||||
| 10 | 76 | 1 | 1 | |||||||||||||||||||
| 11 | 89 | 1 | 1 | 1 | 3 | |||||||||||||||||
| 12 | 73 | 1 | 2 | 2 | 1 | 1 | 1 | 8 | ||||||||||||||
| 13 | 83 | 1 | 2 | 1 | 1 | 1 | 6 | |||||||||||||||
| 14 | 79 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | ||||||||||||
| Women | 1 | 84 | 1 | 1 | 1 | 1 | 4 | |||||||||||||||
| 2 | 84 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | ||||||||||||
| 3 | 76 | 1 | 1 | 2 | 1 | 1 | 6 | |||||||||||||||
| 4 | 100 | 0 | ||||||||||||||||||||
| 5 | 82 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||||||||||||||
| 6 | 67 | 1 | 1 | 2 | ||||||||||||||||||
| 7 | 78 | 1 | 1 | 1 | 1 | 4 | ||||||||||||||||
| 8 | 91 | 1 | 1 | 2 | ||||||||||||||||||
| 9 | 90 | 1 | 1 | 2 | 1 | 1 | 6 | |||||||||||||||
| 10 | 83 | 1 | 1 | 1 | 3 | |||||||||||||||||
| 11 | 93 | 1 | 1 | 2 | ||||||||||||||||||
| 12 | 90 | 1 | 1 | |||||||||||||||||||
| 13 | 62 | 1 | 1 | 2 | ||||||||||||||||||
| 14 | 77 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | ||||||||||||
| 15 | 93 | 1 | 1 | 2 | ||||||||||||||||||
| 16 | 78 | 1 | 1 | 2 | 4 | |||||||||||||||||
| 17 | 89 | 1 | 1 | 2 | 1 | 1 | 6 | |||||||||||||||
| 18 | 76 | 1 | 1 | 1 | 3 | |||||||||||||||||
| 19 | 94 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||||||||||||
| 20 | 82 | 1 | 1 | 1 | 3 | |||||||||||||||||
| 21 | 91 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||||||||||||||
| 22 | 81 | 1 | 1 | 2 | ||||||||||||||||||
| 23 | 73 | 1 | 2 | 1 | 1 | 5 | ||||||||||||||||
| 24 | 71 | 1 | 2 | 3 | 1 | 1 | 8 | |||||||||||||||
| 25 | 96 | 1 | 1 | |||||||||||||||||||
| ∑Medicines | 16 | 19 | 8 | 16 | 2 | 24 | 12 | 2 | 29 | 6 | 5 | 9 | 9 | 11 | ||||||||
| 2 | 63 | 20 | 1 | 1 | 3 | 9 | ||||||||||||||||
Note: These findings were obtained from of the medical record database of the health section at nursing-homes. AD: Alzheimer Disease; MD: mental disorders; oMD: other Mental Disorders; SD: Sleep Disorders; CcD: Cardiocirculatory Disorders; ED: Endocrine Disorders; RD: Rheumatic disease; N: Neoplasia; nNPL: non-Neoplastic Proliferative Lesion; GD. Gastrointestinal Disorders.
Table 2: Primary and secondary systemic disorders/diseases related to the pharmacological groups found in each older patient with AD. according to the sex.
We consider AD as a primary systemic disorder/disease; whereas, the other comorbidities were identified as secondary systemic disorders/diseases. Our results showed as comorbidities: Other Mental Disorder (oMD; 82.05%; 32/39), cardiocirculatory disorder (69.23%; 27/39), endocrine disorder (43.59%; 17/39), gastrointestinal disorder (23.07%; 9/39), non-neoplastic proliferative lesion (7.69%; 3/39), sleep disorder (5.12%; 2/39), rheumatic disease (2.56%; 1/39), and neoplasia (2.56%; 1/39). The mean of secondary comorbidities (n=8) was of 2.36 (± 1.22) per subject with AD, being 3 (± 0.96) and 2 (± 1.22) in men and in women, respectively. There was a statistically significant difference between the sexes (t=2.63; gl=37; p=0.012 <0.05).
About the pharmacological therapies taken by the older patients (n=39), the types of drugs more used to treat the main systemic disorders or diseases were: 35 specific drugs to AD (28 patients); 29 antihypertensives (21 patients), 12 antithrombotic agent (10 patients), and 9 antilipemic agent (9 patients) to cardiocirculatory diseases; 24 antipsychotics (20 patients), 16 antidepressants (14 patients) and 8 benzodiazepines/valproic acid (7 patients) to oMD; and 9 antidiabetics drugs (9 patients) and 11 thyroid hormone (11 patients) to endocrine disorders. The mean of used drugs was of 4.72 (± 2.49) per patient, being 5.86 (± 2.14) in men and 4.08 (± 2.48) in women. There was a statistically significant difference between the sexes (t=2.25; gl=37; p=0.030 <0.05). In this study, the prevalence of polypharmacy (use of 5 or more medications) was of 53.85% (21/39 patients).
In the intrabuccal assessment, 17.94% (7/39) subjects showed satisfactory oral health, with any need of dental clinical procedures. Regarding the number of teeth, 22 subjects with AD had teeth, showing an average of 9.23 (± 9.83) teeth/subject. The mean of teeth/subject were 9.93 (± 11.17) and 8.84 (± 9.22) in men and in women, respectively. No statistically significant difference was found between the sexes (t=0.33; gl=37; p=0.745>0.05).
About oral lesions, 36.36% (8/22), 50% (11/22), and 50% (11/22) patients had caries, residual dental roots, and PD, respectively. Moreover, 17 subjects were full edentulous in the upper and lower arch, showing a prevalence of 43.59% [CI: (95%): 27.81 to 60.38%]. Among them, 23.52% edentulous patients (4/17; older men: patients 5, 12, and 13; older women: patient 19) had appropriate total dental prosthesis (denture) in the upper and/or lower arches; therefore, no further oral rehabilitation procedures were recommended. Finally, 48.72% (19/39) older patients [IC (95%): 32.42% a 65.21%] needed oral rehabilitation, especially dental prosthesis (Table 3).
| Gender | Patients | Age (years) | Number of teeth | Dentate older patients | total edentulous older patients | Older patients with needs of OPR | ||
|---|---|---|---|---|---|---|---|---|
| Ca | RR | PD | ||||||
| Men | 1 | 88 | 21 | 🞻 | 🞻 | |||
| 2 | 85 | 0 | ● | ■ | ||||
| 3 | 68 | 23 | 🞻 | 🞻 | ||||
| 4 | 68 | 20 | 🞻 | 🞻 | 🞻 | |||
| 5 | 68 | 0 | ● | |||||
| 6 | 82 | 23 | 🞻 | 🞻 | ■ | |||
| 7 | 83 | 16 | 🞻 | |||||
| 8 | 74 | 0 | ● | ■ | ||||
| 9 | 94 | 8 | ■ | |||||
| 10 | 76 | 0 | ● | ■ | ||||
| 11 | 89 | 0 | ● | ■ | ||||
| 12 | 73 | 0 | ● | |||||
| 13 | 83 | 0 | ● | |||||
| 14 | 79 | 28 | ||||||
| Women | 1 | 84 | 11 | 🞻 | ||||
| 2 | 84 | 21 | 🞻 | 🞻 | ||||
| 3 | 76 | 0 | ● | ■ | ||||
| 4 | 100 | 18 | 🞻 | 🞻 | 🞻 | |||
| 5 | 82 | 8 | 🞻 | 🞻 | ■ | |||
| 6 | 67 | 19 | 🞻 | |||||
| 7 | 78 | 11 | 🞻 | |||||
| 8 | 91 | 7 | 🞻 | 🞻 | 🞻 | ■ | ||
| 9 | 90 | 0 | ● | ■ | ||||
| 10 | 83 | 0 | ● | ■ | ||||
| 11 | 93 | 0 | ● | ■ | ||||
| 12 | 90 | 0 | ● | ■ | ||||
| 13 | 62 | 3 | 🞻 | ■ | ||||
| 14 | 77 | 22 | 🞻 | 🞻 | ||||
| 15 | 93 | 0 | ● | ■ | ||||
| 16 | 78 | 27 | ||||||
| 17 | 89 | 0 | ● | ■ | ||||
| 18 | 76 | 13 | ■ | |||||
| 19 | 94 | 0 | ● | |||||
| 20 | 82 | 0 | ● | ■ | ||||
| 21 | 91 | 9 | 🞻 | |||||
| 22 | 81 | 8 | ||||||
| 23 | 73 | 20 | 🞻 | 🞻 | ||||
| 24 | 71 | 24 | 🞻 | |||||
| 25 | 96 | 0 | ● | ■ | ||||
Note: Ca: caries; RR: Residual dental roots; PD: Periodontal Diseases; OPR: Oral Prosthetic Rehabilitation; (á) Presence of Ca. RR and PD; empty space: Absence of oral lesions; dentate older patients and no needs of OPR; (●) total edentulous older patients; (■) needs of OPR.
Table 3: Overall oral health status in older patients with AD. according to the sex.
Salivary parameters
We evaluated salivary flow rate (SFR; mL/min) in 37 patients; and pH value, Buffering Capacity (BC, pH value), and morning salivary cortisol (SC-AM; μg/dL) in 34 patients. Two patients were excluded by death and insufficient collection of saliva samples to analysis because of hyposalivation associated with highly viscous saliva. The SFR testing showed 25 (67,56%), 7 (18.91%), and 5 (13.51%) patients with normal flow, reduced flow, and hyposalivation, respectively. The mean of SFR per individual was of 0.45 mL/min (± 0.43). Regarding the sexes, the mean of SFR was of 0.52 mL/ min (± 0.39) in men and 0.42 mL/min (± 0.51) in women, with no statistically significant difference (t=0.67; gl=35; p=0.550>0.05).
Regarding the salivary pH, 25 (64.10%) and 9 (23.08%) patients showed normal and high values, respectively; and the mean was of 7.36 (± 0.58). The BC testing showed 19 (55.88%) patients with normal values, 10 (29.41%) patients with limit value, and 5 (14.70%) patients with low values. The mean of BC was of 5.92 (± 1.28) in men and 5.11 (± 0.76) in women (t=0.50; gl=31; p=0.622>0.05). Thus, no significant difference was found between the sexes.
The SC-AM testing showed 32 patients with normal levels and 2 patients with low and high levels. The mean of SC-AM level was of 0.41 μg / dL (± 0.16) per individual, being 0.38 μg/dL (± 0.18) in men and 0.42 μg/dL (± 0.16) in women. No statistical difference was found between the sexes (t=0.68; gl=32; p=0.504>0.05) (Table 4).
| Gender | Patients | Age (year) | SFR | pH value | BC | SC-AM | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | 1 | 88 | 0.580 | N | 7.971 | 🠅 | 6.633 | N | 0.411 | N |
| 2 | 85 | 1.000 | N | 7.490 | N | 4.890 | ▲ | 0.331 | N | |
| 3 | 68 | 2.000 | N | 8.339 | 🠅 | 6.760 | N | 0.617 | N | |
| 4 | 68 | 0.300 | N | 7.794 | N | 6.877 | N | 0.213 | N | |
| 5 | 68 | (†) | (†) | (†) | (†) | |||||
| 6 | 82 | 0.290 | N | 8.600 | 🠅 | 5.622 | N | 0.622 | N | |
| 7 | 83 | 0.280 | N | 7.345 | N | 4.992 | ▲ | 0.190 | N | |
| 8 | 74 | 0.340 | N | 7.395 | N | 6.144 | N | 0.583 | N | |
| 9 | 94 | 0.240 | 🠇 | 7.325 | N | 6.010 | N | 0.270 | N | |
| 10 | 76 | 0.380 | N | 7.614 | N | 3.323 | 🠇 | 0.440 | N | |
| 11 | 89 | 0.270 | N | 7.823 | 🠅 | 4.151 | ▲ | 0.087 | 🠇 | |
| 12 | 73 | 0.260 | N | 7.022 | N | 3.100 | 🠇 | 0.254 | N | |
| 13 | 83 | (🞵🞵) | (🞵🞵) | (🞵🞵) | (🞵🞵) | |||||
| 14 | 79 | 0.300 | N | 6.788 | N | 5.000 | ▲ | 0.568 | N | |
| Women | 1 | 84 | 0.300 | N | 7.300 | N | 4.972 | ▲ | 0.435 | N |
| 2 | 84 | 0.300 | N | 6.740 | N | 3.567 | 🠇 | 0.430 | N | |
| 3 | 76 | 0.320 | N | 7.396 | N | 4.436 | ▲ | 0.352 | N | |
| 4 | 100 | 0.180 | 🠇 | 6.650 | N | 3.765 | 🠇 | 0.862 | 🠅 | |
| 5 | 82 | 0.220 | 🠇 | 7.714 | N | 5.786 | N | 0.350 | N | |
| 6 | 67 | 0.060 | ● | (🞵) | (🞵) | (🞵) | ||||
| 7 | 78 | 1.640 | N | 7.931 | 🠅 | 5.614 | N | 0.352 | N | |
| 8 | 91 | 0.440 | N | 6.491 | N | 6.012 | N | 0.224 | N | |
| 9 | 90 | 0.080 | ● | (🞵) | (🞵) | (🞵) | ||||
| 10 | 83 | 0.190 | 🠇 | 6.854 | N | 5.955 | N | 0.529 | N | |
| 11 | 93 | 0.760 | N | 7.935 | 🠅 | 5.857 | N | 0.278 | N | |
| 12 | 90 | 0.380 | N | 7.081 | N | 3.707 | 🠇 | 0.325 | N | |
| 13 | 62 | 0.230 | 🠇 | 6.129 | N | 4.636 | ▲ | 0.393 | N | |
| 14 | 77 | 0.200 | ” | 7.508 | N | 4.976 | ▲ | 0.361 | N | |
| 15 | 93 | 0.260 | N | 6.445 | N | 4.474 | ▲ | 0.240 | N | |
| 16 | 78 | 1.100 | N | 7.593 | N | 6.165 | N | 0.622 | N | |
| 17 | 89 | 0.250 | N | 8.091 | 🠅 | 5.805 | N | 0.550 | N | |
| 18 | 76 | 0.060 | ● | (🞵) | (🞵) | (🞵) | ||||
| 19 | 94 | 0.230 | 🠇 | 7.820 | 🠅 | 5.346 | N | 0.501 | N | |
| 20 | 82 | 0.620 | N | 7.125 | N | 5.273 | N | 0.218 | N | |
| 21 | 91 | 1.200 | N | 7.876 | 🠅 | 4.862 | ▲ | 0.631 | N | |
| 22 | 81 | 0.600 | N | 6.901 | N | 5.161 | N | 0.377 | N | |
| 23 | 73 | 0.060 | ● | 6.742 | N | 5.630 | N | 0.552 | N | |
| 24 | 71 | 0.090 | ● | 6.732 | N | 5.380 | N | 0.394 | N | |
| 25 | 96 | 0.620 | N | 7.721 | N | 5.146 | N | 0.319 | N | |
Note: SFR: Salivary Flow Rate; BC: Buffering Capacity; SC-AM: morning Salivary Cortisol; (N): Normal values or levels; (🠇 ): Reduced or low value; (▲): Limit value; (🠅): High value; (●): Hyposalivation; (†): Death; (🞵) Insufficient saliva sample; (🞵🞵) difficulty to collect saliva because of its high viscosity and hyposalivation; SFR (mL/min): Normal flow (≥ 0.3), reduces or low flow (>0.1;<0.3), and hyposalivation/xerostomia condition (≤ 0.1); pH value: Normal value (N;5.3 to 7.8), low value (<5.3), and high value (>7.8); BC: Normal value (5.1 to 7.0), limit value (4.0 to 5.0), and low value (< 4.0); and SC-AM (above 50 years old): Normal level range for men (0.112 a 0.812), low level for men (<1.112), and high level for men (>0.812); and normal level range for women (0.149 a 0.739), low level for women (<1.149), and high level for women (>0.739).
Table 4: Results of the saliva testing in subjects with AD. according to the sex.
Questionnaire applications
The results of sleep quality, functional capacity to daily personal self-cares and mobility are demonstrated (Tables 5 and 6).
| Patients | Ages (year) | ESS | STOP-BANG | |||
|---|---|---|---|---|---|---|
| Scores ∑1-8 | Scores ∑1-8 | |||||
| Men | 1 | 88 | 7 | 3 | 🞻 | |
| 2 | 85 | 24 | 🞻 | 5 | 🞻 | |
| 3 | 68 | 24 | 🞻 | 5 | 🞻 | |
| 4 | 68 | 11 | 🞻 | 4 | 🞻 | |
| 5 | 68 | 8 | 4 | 🞻 | ||
| 6 | 82 | 24 | 🞻 | 5 | 🞻 | |
| 7 | 83 | 21 | 🞻 | 4 | 🞻 | |
| 8 | 74 | 24 | 🞻 | 4 | 🞻 | |
| 9 | 94 | 5 | 5 | 🞻 | ||
| 10 | 76 | 18 | 🞻 | 2 | ||
| 11 | 89 | 24 | 🞻 | 5 | 🞻 | |
| 12 | 73 | 5 | 5 | 🞻 | ||
| 13 | 83 | 3 | 2 | |||
| 14 | 79 | 18 | 🞻 | 5 | 🞻 | |
| Women | 1 | 84 | 5 | 1 | ||
| 2 | 84 | 17 | 🞻 | 2 | ||
| 3 | 76 | 16 | 🞻 | 1 | ||
| 4 | 100 | 24 | 🞻 | 1 | ||
| 5 | 82 | 13 | 🞻 | 2 | ||
| 6 | 67 | 24 | 🞻 | 3 | 🞻 | |
| 7 | 78 | 5 | 3 | 🞻 | ||
| 8 | 91 | 18 | 🞻 | 2 | ||
| 9 | 90 | 8 | 2 | |||
| 10 | 83 | 3 | 1 | |||
| 11 | 93 | 5 | 2 | |||
| 12 | 90 | 7 | 2 | |||
| 13 | 65 | 19 | 🞻 | 1 | ||
| 14 | 77 | 3 | 1 | |||
| 15 | 93 | 14 | 🞻 | 2 | ||
| 16 | 78 | 6 | 4 | 🞻 | ||
| 17 | 89 | 20 | 🞻 | 2 | ||
| 18 | 76 | 7 | 2 | |||
| 19 | 94 | 8 | 2 | |||
| 20 | 82 | 15 | 🞻 | 1 | ||
| 21 | 91 | 23 | 🞻 | 3 | 🞻 | |
| 22 | 81 | 2 | 1 | |||
| 23 | 73 | 6 | 2 | |||
| 24 | 71 | 11 | 🞻 | 2 | ||
| 25 | 96 | 6 | 2 | |||
Note: (🞻) Risk; (empty space) no risk; ESS: Epworth Sleepiness Scale. value ≥ 10 (excessive and disordered daytime sleepiness and high risk for OSA: Obstructive Sleep Apnea; STOP-BANG: Valor ≥ 3 (great risk for OSA).
Table 5: Results of the sleep quality in older patients with AD, according to the sex.
| Patients | Age (years) | Score of items | ∑1-10 Scores | Level of dependence in BADL | Mobility | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||||
| Men | 1 | 88 | 8 | 4 | 8 | 4 | 10 | 8 | 5 | 15 | 5 | 15 | 82 | 🞻🞻 | ⃝ | |
| 2 | 85 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 🞻🞻🞻🞻 | ● | ||
| 3 | 68 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 🞻🞻🞻🞻 | ● | ||
| 4 | 68 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 🞻🞻🞻🞻 | ● | ||
| 5 | 68 | 10 | 3 | 8 | 3 | 8 | 5 | 5 | 3 | 2 | 8 | 55 | 🞻🞻🞻 | ⃤ | ||
| 6 | 82 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 🞻🞻🞻🞻 | ▲ | ||
| 7 | 83 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 10 | 15 | 50 | 🞻🞻🞻 | ⃝ | ||
| 8 | 74 | 10 | 4 | 10 | 4 | 8 | 8 | 10 | 15 | 10 | 15 | 94 | 🞻 | ⃝ | ||
| 9 | 94 | 8 | 3 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 14 | 🞻🞻🞻🞻 | ● | ||
| 10 | 76 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 2 | 3 | 13 | 🞻🞻🞻🞻 | ⃤ | ||
| 11 | 89 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 🞻🞻🞻🞻 | ▲ | ||
| 12 | 73 | 8 | 4 | 8 | 3 | 8 | 8 | 5 | 8 | 2 | 3 | 57 | 🞻🞻🞻 | ⃤ | ||
| 13 | 83 | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 15 | 10 | 15 | 51 | 🞻🞻🞻 | ● | ||
| 14 | 79 | 5 | 0 | 2 | 0 | 2 | 8 | 0 | 3 | 8 | 12 | 40 | 🞻🞻 | ⃝ | ||
| Women | 1 | 84 | 10 | 4 | 8 | 5 | 10 | 10 | 10 | 15 | 10 | 15 | 97 | 🞻 | ⃝ | |
| 2 | 84 | 10 | 3 | 10 | 4 | 5 | 8 | 2 | 15 | 2 | 12 | 71 | 🞻🞻 | ⃝ | ||
| 3 | 76 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 🞻🞻🞻🞻 | ⃤ | ||
| 4 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 🞻🞻🞻🞻 | ▲ | ||
| 5 | 82 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 5 | 12 | 30 | 🞻🞻🞻 | ⃝ | ||
| 6 | 67 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 🞻🞻🞻🞻 | ▲ | ||
| 7 | 78 | 10 | 0 | 8 | 0 | 10 | 10 | 5 | 15 | 10 | 15 | 83 | 🞻🞻 | ⃝ | ||
| 8 | 91 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 15 | 🞻🞻🞻🞻 | ⃤ | ||
| 9 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 🞻🞻🞻🞻 | ● | ||
| 10 | 83 | 10 | 0 | 0 | 0 | 0 | 0 | 8 | 15 | 10 | 15 | 58 | 🞻🞻🞻 | ⃝ | ||
| 11 | 93 | 10 | 4 | 10 | 3 | 10 | 10 | 10 | 15 | 10 | 15 | 97 | 🞻 | ⃝ | ||
| 12 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 10 | 15 | 40 | 🞻🞻🞻 | ⃝ | ||
| 13 | 62 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 🞻🞻🞻🞻 | ● | ||
| 14 | 77 | 8 | 4 | 5 | 1 | 8 | 8 | 5 | 15 | 10 | 15 | 79 | 🞻🞻 | ⃝ | ||
| 15 | 93 | 8 | 4 | 8 | 3 | 8 | 8 | 5 | 8 | 5 | 8 | 65 | 🞻🞻 | ⃤ | ||
| 16 | 78 | 8 | 5 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 8 | 28 | 🞻🞻🞻 | ⃤ | ||
| 17 | 89 | 5 | 0 | 5 | 0 | 2 | 2 | 0 | 15 | 10 | 15 | 54 | 🞻🞻🞻 | ⃝ | ||
| 18 | 76 | 8 | 0 | 2 | 0 | 0 | 2 | 2 | 15 | 10 | 15 | 54 | 🞻🞻🞻 | ⃝ | ||
| 19 | 94 | 10 | 3 | 5 | 3 | 8 | 0 | 5 | 15 | 5 | 15 | 69 | 🞻🞻 | ⃝ | ||
| 20 | 82 | 10 | 0 | 2 | 0 | 0 | 0 | 5 | 15 | 10 | 15 | 57 | 🞻🞻🞻 | ⃝ | ||
| 21 | 91 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 🞻🞻🞻🞻 | ⃤ | ||
| 22 | 81 | 10 | 3 | 8 | 3 | 8 | 0 | 10 | 15 | 10 | 15 | 82 | 🞻🞻 | ⃝ | ||
| 23 | 73 | 10 | 0 | 2 | 1 | 0 | 0 | 2 | 15 | 10 | 15 | 55 | 🞻🞻🞻 | ⃝ | ||
| 24 | 71 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 🞻🞻🞻🞻 | ● | ||
| 25 | 96 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 🞻🞻🞻🞻 | ● | ||
Note: (🞻) Mild dependence; (🞻🞻) Moderate dependence; (🞻🞻🞻) Severe dependence; (🞻🞻🞻🞻) Total dependence; (⃝) Walk alone; (⃤) Walk with assistance; (●) Wheelchair user; (▲) Bedridden person; <20 points (total dependence); 21-60 points (severe dependence); 61-90 points (moderate dependence); 91-99 (mild dependence); and 100 points (independence).
Table 6: Results of the modified barthel index in each older patient with AD, according to the sex.
Considering the daytime symptoms of OSA, 21 patients [53.85% - CI (95%): 37.18% to 69.91%] and 16 patients [41.03% - CI (95%): 25.57% to 57.90 %] had excessive daytime sleepiness and high risk for OSA, respectively. Moreover, both sleep abnormalities were found in 10 patients (25.64%); whereas, 27 (69.23%) patients had one of the sleep abnormalities. About sexes, 9 men (64.29%) and 12 women (48%) had excessive daytime sleepiness; while 12 men (85.71%) and 4 women (16%) had high risk of OSA. Eight older men (57.14%; patients 2,3,4,6,78,11, and 14) and 2 older women (8%; patients 6 and 21) showed risk for OSA in both tests (ESS and STOP-BANG).
Regarding the performance of BADL, the oldest patients manifested high levels of dependence on his/her basic functional capacity, implicating in loss of autonomy and functional independence in his/her daily living. Sixteen (41.03%), 12 (30.77%), 8 (20.51%), and 3 (7.69%) older volunteers showed total, severe, moderate, and mild dependence in her/his BADL, respectively. No volunteer showed full autonomy and functional independence. Emphasizing the sexes, 7 (50%) older men and 9 (36%) older women were totally dependents in personal performance of his/her BADL, as well as 4 (28.6%) older men and 8 (32%) older women had severe dependence. Finally, we verify the following mobility features per sex: 5 older men and 14 older women to walk alone, 3 older men and 5 older women to walk with assistance, 4 older men and 4 older women to wheelchair user, and 2 older men and 2 older women to bedridden person.
Discussion
In our study, the prevalence of older patients was women, agreeing with the findings of [4,18]. Certainly, this fact occurs because of higher life expectancy in older women than in older men (World Health Organization). Also, we also identify that the women had earlier AD than men, as well as mental and cardiocirculatory disorders and diabetes mellitus were more found in men. The main comorbidities were oMD, cardiocirculatory disorders, and endocrine disorders, agreeing with studies of [1,2,19]. We infer that the AD severity may aggravate these disorders due to dependence and loss of autonomy to self-medications. Besides, 43.5% of patients had three or more systemic illnesses, being statistically more found in men. Therefore, the men’s general health was much unsatisfactory, strongly affecting their good quality of life.
The use of multiple medicines is common in older patients with multimorbidity. Polypharmacy has been defined as the use of five or more medicines per patient [20]. Continuous multiple drugs have been administered by our patients. The mean was of 4.72 medicines per individual, being 5.86 medicines for men and 4.08 medicines for women. Briefly, 53.85% of patients ingested five or more medications, indicating cases of polypharmacy in older patients. Among them, 52.38% and 47.62% were men and women, respectively, reinforcing the high prevalence of illnesses in older men. Until now, the medicines used in AD have stabilized its signs/symptoms and/or delayed its progression.
As an alternative symptomatic treatment available for AD, 19, 16 and 7 patients used AChEIs (donepezil, galantamine, and rivastigmine), NMDA receptor antagonist (memantine), or both the medicines, respectively. These medicines temporarily helped in the cognitive-performance, delayed loss of functional capacity and dependency in BADL and improved the behavioral symptoms. AChEIs inhibits the acetylcholine breakdown in the synapse, favoring cholinergic transmission and modulating the cognitive functions [21,22]. Other drugs largely used were antihypertensive, antipsychotics, and antidepressants agents. Studies describe that long-time use of antipsychotics may trigger repetitive involuntary movements and/ or spasms of masticatory muscles, called tardive dyskinesia. This side effect can limit oral functions and injure the use of removable oral prostheses [1,23,24].
Regarding to the study's limitations, some nursing homes had unsatisfactory and precarious healthcare services and sectors. This condition difficulted the patient´s handling and the appropriate intrabuccal clinical procedures, mainly in patients with high severity of dementia and total or severe functional dependence. So, we standardize a protocol of screening intrabuccal clinical exam, so that all the patients could be similarly evaluated. Given that, overall oral health status was properly homogenized in this population. As a strength, all the nursing home administrators fully supported the development of this exploratory study in assessing the residents’ health quality and status. They awakened a great interest in making a real self-acknowledgement of the benefits of the provided social-health services.
After the intrabuccal clinical exam, the obtained data were inserted into the medical-dental records of the health sections at the nursing homes. Subsequently, these records were forwarded to the dental specialists to perform more accurate examinations (e.g., Periodontal Screening and Recording, PSR; Decayed, Missing, and Filled Teeth (DMFT) Community Periodontal Index (CPI) and others and treatment in these individuals. The screening data showed higher prevalence in dentate subjects than edentate subjects, agreeing with studies [18,25]. Residual dental roots, PD, and caries were the oral lesions more frequent. Thus, dental treatment and oral rehabilitation were recommended in almost all the subjects. However, use of dental prostheses must be cautious for dependent patients and monitored by caregivers to avoid adverse complications. Additionally, other authors described masticatory muscle weakness in patients with AD [9,26]. Probably, these findings occur due to poor oral hygiene caused by apraxia and apathy in AD, altered physicochemical properties of saliva, and oral-motor muscle hypotonia. Considering the influence of PD in cardiopathies and endocrine diseases (diabetes mellitus/ hypothyroidism), we verify that this correlation was well-evidenced in our results. Then, we suggest that the PD could affect the AD progression. Thus, a continuing control treatment of PD must be recommended in healthcare services in nursing home.
With concern to the salivary parameters, we evidence that the mean of flow rate was normal in both sexes; however, some subjects showed reduced flow and hyposalivation. Probably, this occurred due to the synaptic dysfunction and/or alteration of neuronal activity in neurotransmitter synthesis of autonomic nervous system, which is responsible for salivary secretion, affecting mainly major salivary glands. We still conjecture that the long-time use of antihypertensives, anxiolytics, antidepressant, diuretics, benzodiazepines/valproic acid agents contributed to decrease of saliva secretion, agreeing with the studies [9,27]. Despite their deleterious effects, hyposalivation was found in 5 subjects, especially in older women. Probably, the cholinesterase inhibitor agents in many patients raised the acetylcholine levels, maintaining normal saliva production.
About pH value, high pH was identified in 3 men and 2 women with PD. Researchers have reported that increased pH may strongly influence on salivary calculus formation and, indirectly, on the development of PD the correlation between caries and BC was not detected once 75% of subjects had caries and normal values of BC [3].
For salivary cortisol, we identified 2 subjects with high and low SCAM levels in saliva. The high levels of SC-AM were found in a woman, indicating a great risk of physical and/or psychological stress. We infer that this biomarker showed altered because of harmful conditions of this patient, such as: Age of 100 years old, bedridden person, no use of medicines, presence of caries, residual dental roots and PD, reduced SFR making it difficult in chewing/swallow and facilitating the action of pathogens on the oral cavity, and total dependence in her functional capacity to personal self-care. Given these facts, we deduce that this woman experienced pain and suffering in the absence of medical and dental clinical practices and other transdisciplinary approaches. In contrast, the low SC-AM level was found in an older man who used antipsychotic medicines. Antipsychotic agents may inhibit cortisol release [28]. Therefore, we recommend the SC examination for patients with neuropsychomotor and/or neurodegenerative disorders in routine clinical practices, especially in the health services of nursing home where the communication between patients and professionals in health is impaired by their disability and saliva samples are easily collected and noninvasive.
With respect to sleep quality, 69.2% of older patients showed positive rates to ESS and STOP-BANG questionnaires, being 25.6% of subjects with high risk for OSA and excessive daytime sleepiness. The older men (80%) were more affected than the older women (20%). The men with high risk for OSA had 100% of oMD and cardiocirculatory disorders, 50% of gastrointestinal disorders and 40% of endocrine disorders, particularly diabetes mellitus. These findings were in consonance with the studies [29,30]. When the OSA is not appropriately treated in older patients with dementia, cognitive and functional losses can be exacerbated, compromising largely his/her life quality. The negative impact of OSA in AD has been more evident. OSA is strongly associated with fragmentation in sleep architecture, intermittent hypoxia and oxidative stress, cardiovascular comorbidities and hemodynamic alterations. These conditions together could increase the risk to AD and also accelerate the progression of this disease. So, the treatment of OSA in this population may contribute to AD prevention and/or progression control [31-33].
Regarding the functional capacity evaluation, the prevalence of total and severe dependencies was more found in older men (78.6%) than in older women (68%), concomitantly corresponding to the elevated quantity of illnesses in older men. Among them, the oMD and cardiocirculatory disorders were the most frequent in all dependence degrees and in both sexes. Teeth losses caused by caries and PD were well evidenced in all patients, especially in patients with total and severe dependences which were compromised the performance of his/her BADL. Residual dental roots were also identified, mainly in women with all functional dependencies. So, the oral hygienist inserted into the transdisciplinary team become necessary to promote a continuous and appropriate oral prophylaxis in older patients with AD. We still demonstrate that the severity of functional dependence occurred, especially in older men, due to the high number and longtime appearance of comorbidities associated with AD. Therefore, we reinforce that a high severity of AD may be related to bad general and oral health.
With AD progression, the older patients become more dependent, resulting in major limitations in their self-care for prevention and maintenance of general and oral health. Therefore, it is extremely important that family members and/or caregivers are continually guided to control the evolution of preexisting illness and to avoid the appearance of new comorbidities by physicians and dentists. Transdisciplinary approach is also applied to active his/her cognitive skills, including games, music therapy, physical exercises, and others. This surely allow a good adaptation to several limitations which are caused by chronic and progressive exacerbation of AD. Dentists have great difficulty to assist this public-target due to the physical, behavioral, and mental impairments. Oral dysfunctions as sucking habits and involuntary oral and perioral movements are frequent, compromising an appropriate oral rehabilitation. Thus, therapeutic approaches depend on the evolution stages of AD. In initial stage, the dentist must remove all focus infections and to restore the functions of oral and maxillofacial complex. In severe stage, oral health must be preserved through clinical procedures using intravenous sedation or general anesthesia in a hospital environment.
Considering that AD is recognized by as a global public health priority and the exploratory findings detected in this study, we recommend the management of AD progression, the control of its comorbidities, and the application of the best clinical practices with transdisciplinary approaches, in together, in healthcare services for older patients with AD who lives at long-term private or public nursing-home [5]. Certainly, this may trigger the following benefits, such as: Combination of knowledge, skills, and attitude from the interaction among the health multiprofessionals; development of novel innovative therapeutic protocols and approaches; suffering mitigation for residents; lower financial expenses for family; and increased life expectancy for this population.
Conclusion
Other Mental Disorders (oMD) and cardiocirculatory disorders were the most common comorbidities in older patients with AD, especially in men. Many patients were dentulous, showing residual dental roots, periodontal diseases, and caries. The polypharmaceuticals did not substantially interfere on saliva production, probably, because of the use de cholinesterase inhibitors. No significant alterations in salivary flow and buffering capacity were detected, and the risk of psychological and/or physical stress identified from salivary cortisol was controlled by medicines indicated to treat oMD. All older patients with high risk of OSA had cardiocirculatory disorders, while the total and severe dependencies in BADL and mobility was more evidenced in institutionalized older patients with AD.
References
- Friedlander AH, Norman DC, Mahler ME, Norman KM, Yagiela JA (2006). Alzheimer's disease: Psychopathology, medical management and dental implications. J Am Dent Assoc 137(9):1240-51.
[Crossref] [Google Scholar] [PubMed]
- Lane CA, Hardy J, Schott JM (2018). Alzheimer's disease. Eur J Neurol 25(1):59-70.
[Crossref] [Google Scholar] [PubMed]
- Gomes MF (2021). Basic Sciences and Clinical Implications of Alzheimer disease. J Clin and Commun Med 3(2):286-287.
[Crossref]
- Aragón F, Zea-Sevilla MA, Montero J, Sancho P, Corral R, et al (2018). Oral health in Alzheimer’s disease: A multicenter case-control study. Clin Oral Investig 22:3061-70.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. World report on ageing and health. World Health Organization; 2015 Oct 22.
- Wong W (2020). Economic burden of Alzheimer disease and managed care considerations. Am J Manag Care 26(8 Suppl):S177-83.
[Crossref] [Google Scholar] [PubMed]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1(1):a006189.
[Crossref] [Google Scholar] [PubMed]
- Armstrong RA (2019). Risk factors for Alzheimer’s disease. Folia Neuropathol 57(2):87-105.
[Crossref] [Google Scholar] [PubMed]
- Liu B, Dion MR, Jurasic MM, Gibson G, Jones JA (2012). Xerostomia and salivary hypofunction in vulnerable elders: Prevalence and etiology. Oral Surg Oral Med Oral Pathol Oral Radiol 114(1):52-60.
[Crossref] [Google Scholar] [PubMed]
- Sato E, Hirano H, Watanabe Y, Edahiro A, Sato K, et al (2014). Detecting signs of dysphagia in patients with A lzheimer's disease with oral feeding in daily life. Geriatr Gerontol Int 14(3):549-55.
[Crossref] [Google Scholar] [PubMed]
- Affoo RH, Foley N, Garrick R, Siqueira WL, Martin RE (2015). Meta‐analysis of salivary flow rates in young and older adults. J Am Geriatr Soc 63(10):2142-51.
[Crossref] [Google Scholar] [PubMed]
- Andrade AG, Bubu OM, Varga AW, Osorio RS (2018). The relationship between obstructive sleep apnea and Alzheimer’s disease. J Alzheimers Dis 64(s1):S255-70.
[Crossref] [Google Scholar] [PubMed]
- Gomes MF, Giannasi LC, Fillietaz‐Bacigalupo E, de Mancilha GP, de Carvalho Silva GR, et al (2020). Evaluation of the masticatory biomechanical function in Down syndrome and its influence on sleep disorders, body adiposity and salivary parameters. J Oral Rehabil 47(8):1007-22.
[Crossref] [Google Scholar] [PubMed]
- Fonseca LB, Silveira EA, Lima NM, Rabahi MF (2016). STOP-Bang questionnaire: Translation to Portuguese and cross-cultural adaptation for use in Brazil. J Bras Pneumol 42:266-72.
[Crossref] [Google Scholar] [PubMed]
- Campbell AJ, Neill AM, Scott DA (2018). Clinical reproducibility of the epworth sleepiness scale for patients with suspected sleep apnea. J Clin Sleep Med 14(5):791-5.
[Crossref] [Google Scholar] [PubMed]
- Duarte RL, Fonseca LB, Magalhães-da-Silveira FJ, Silveira EA, Rabahi MF (2017). Validation of the STOP-Bang questionnaire as a means of screening for obstructive sleep apnea in adults in Brazil. J Bras Pneumol 43:456-63.
[Crossref] [Google Scholar] [PubMed]
- Minosso JS, Amendola F, Alvarenga MR, Oliveira MA (2010). Validation of the barthel index in elderly patients attended in outpatient clinics, in Brazil. ACTA Paul. Enferm. 23:218-23.
- Ohura T, Hase K, Nakajima Y, Nakayama T (2017). Validity and reliability of a performance evaluation tool based on the modified barthel index for stroke patients. BMC Med Res Methodol 17(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Machado MC, Lopes GH, Marchini L (2012). Oral health of Alzheimer's patients in São José dos Campos, Brazil. Geriatr Gerontol Int 12(2):265-70.
[Crossref] [Google Scholar] [PubMed]
- Leelakanok N, D'Cunha RR (2019). Association between polypharmacy and dementia–A systematic review and metaanalysis. Aging Ment Health 23(8):932-41.
[Crossref] [Google Scholar] [PubMed]
- Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE (2017). What is polypharmacy? A systematic review of definitions. BMC Geriatr 17:1-0.
[Crossref] [Google Scholar] [PubMed]
- Dong XX, Wang Y, Qin ZH (2009). Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 30(4):379-87.
[Crossref] [Google Scholar] [PubMed]
- Matsunaga S, Kishi T, Iwata N (2015). Memantine monotherapy for Alzheimer’s disease: A systematic review and meta-analysis. PloS One 10(4):e0123289.
[Crossref] [Google Scholar] [PubMed]
- Kocaelli H, Yaltirik M, Yargic LI, Özbas H (2002). Alzheimer's disease and dental management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93(5):521-4.
[Crossref] [Google Scholar] [PubMed]
- Forlenza OV, Loureiro JC, Pais MV, Stella F (2017). Recent advances in the management of neuropsychiatric symptoms in dementia. Curr Opin Psychiatry 30(2):151-8.
[Crossref] [Google Scholar] [PubMed]
- Campos CH, Ribeiro GR, Rodrigues Garcia RC (2016). Oral health‐related quality of life in mild Alzheimer: Patient versus caregiver perceptions. Spec Care Dentist 36(5):271-6.
[Crossref] [Google Scholar] [PubMed]
- Campos CH, Ribeiro GR, Stella F, Rodrigues Garcia RC (2017). Mandibular movements and bite force in A lzheimer's disease before and after new denture insertion. J Oral Rehabil 44(3):178-86.
[Crossref] [Google Scholar] [PubMed]
- Leal SC, Bittar J, Portugal A, Falcao DP, Faber J, et al (2010). Medication in elderly people: Its influence on salivary pattern, signs and symptoms of dry mouth. Gerodontology 27(2):129-33.
[Crossref] [Google Scholar] [PubMed]
- Baliga S, Muglikar S, Kale R (2013). Salivary pH: A diagnostic biomarker. J Indian Soc Periodontol 17(4):461.
[Crossref] [Google Scholar] [PubMed]
- Walker E, Mittal V, Tessner K (2008). Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol 4:189-216.
[Crossref] [Google Scholar] [PubMed]
- Ing AJ, Ngu MC, Breslin AB (2000). Obstructive sleep apnea and gastroesophageal reflux. The American journal of medicine. Mar 6;108(4):120-5.
[Crossref] [Google Scholar] [PubMed]
- Dincer HE, O’Neill W (2006). Deleterious effects of sleep-disordered breathing on the heart and vascular system. Respiration 73(1):124-130.
[Crossref] [Google Scholar] [PubMed]
- Andrade MR, Salazar SL, de Sá LF, Portela M, Ferreira-Pereira A et al (2015). Role of saliva in the caries experience and calculus formation of young patients undergoing hemodialysis. Clin Oral Investig 19:1973-1980.
[Crossref] [Google Scholar] [PubMed]
Citation: Gomes MF, de Abreu MFF, Giannasi LC, Babinskas D, Koga-Ito CY, et al. (2023). An Exploratory Study of the Overall Systemic and Oral Health Status in Older Patients with Alzheimer's Disease. J Alzheimers Dis Parkinsonism.13:564. DOI: 10.4172/2161-0460.100564.
Copyright: © 2023 Gomes MF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1710
- [From(publication date): 0-2023 - Jul 11, 2025]
- Breakdown by view type
- HTML page views: 1415
- PDF downloads: 295