An Efficient Synthesis and Bio Efficacy Confirmation of Female Sex Pheromone of the Rice Moth, Corcyra Cephalonica Stainton
Received: 11-May-2018 / Accepted Date: 25-Jul-2018 / Published Date: 27-Jul-2018 DOI: 10.4172/2375-4338.1000196
Keywords: Rice moth; Sex pheromone; 6,10,14-Trimethyl-2-pentadecanol; Electroantennography (EAG); Wittig reaction; Hydrogenation; Oxidation
Introduction
Rice moth, Corcyra cephalonica Stainton (Lepidoptera: Pyralidae; Galleriinae) is a serious pest of stored paddy and other cereals. It nourishes well in humid climates and also attacks wheat, maize, sorghum, barley, millets, soybean and oilseeds like groundnuts. Its harm reaches a very unpleasant level in subtropical countries like India. The larva stage is mainly responsible for the damage. Young larva feeds on the broken grain or on the grains which already damaged by other insects. Besides polluting the grain with large quantities of frass and silken cocoons, webbing together the grains into large lumps occur and thus deflate the nutritional value of the grains. Using of insecticides to control storage pests led to contaminate the food grain which is not useful for human consumption. Insect pheromones play a major role in pest-control strategies and are considered to be potential tools in integrated pest management (IPM) [1], an eco-friendly and environmentally safe agricultural technique that is practiced worldwide.
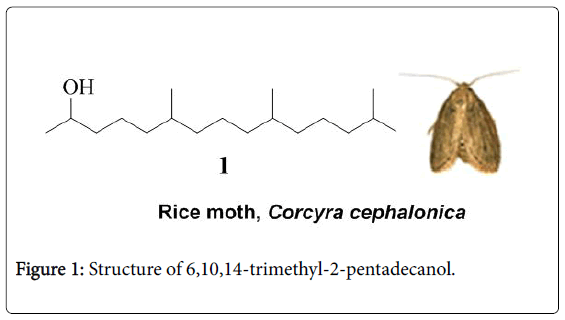
The female emitted sex pheromone of the rice moth was isolated and identified as 6,10,14-trimethyl-2-pentadecanol (compound 1) (Figure 1) by Hall and co-workers [2]. They synthesized 6,10,14- trimethyl-2-pentadecanol as a racemic mixture and found it to be bioactive. However, electrophysiological bioassay of synthetic pheromone compound revealed that it (3.5 ng) causes a lower EAG response (1.23 mV) from the male rice moths than that to an equal amount of the natural pheromone (2.31 mV). The lower EAG responses to the synthetic compound suggest that the natural material consists of only one or a few of the possible isomers. Mori et al. [3] synthesized four out of the eight possible stereoisomers of 6,10,14- trimethy1-2-pentadecanol [(2R,6R,10R)-, (2S,6R,10R)-, (2S,6S,10S)-, and (2R,6S,10S)-l] based on the hypothesis that the natural pheromone of the rice moth might be an isomer with two methyl groups at C6 and C10 in syn relative stereochemistry. Among these four synthetic stereoisomers of 6,10,14-trimethyl-2-pentadecanol, the (2R,6R,10R)-1 isomer alone was found to be at least twice as active as the diastereomeric mixture of 6,10,14-trimethyl-2-pentadecanol both by EAG and behavioral bioassay. The natural pheromone was also reported to be twice as active as the diastereomeric mixture of 6,10,14- trimethyl-2-pentadecanol and therefore, (2R,6R,10R)-1 was deduced to be the natural pheromone or at least the major component of the female blend.
The female rice moth sex pheromone has attracted the attention of synthetic chemists due to its significant biological activity and unique structural features comprising secondary alcohol and three methyl branches: six total syntheses have been published till date. Shafikov et al. [4] have reported a short synthetic route (4 steps) to chiral alcohol 1 starting from chlorophyll, a natural product. However, it suffers from the low abundance of starting material, poor yields (12%) and poor optical purity (26% ee). It is noteworthy that the racemic pheromone compound can be used for trapping the male rice moths in fields instead of expensive chiral pheromone compound, since the racemic compound itself showed excellent catching ability. The synthetic strategies to 6,10,14-trimethyl-2-pentadecanol reported to date suffer from one or more of the drawbacks including lengthy reaction sequences [5,6], poor yields [2,4,7] and expensive starting materials and reagents [5,6]. In continuation of our interest in the synthesis of pheromones [8-10], we describe herein an efficient synthesis of 6,10,14-trimethyl-2-pentadecanol and its biological evaluation studies.
Biological evaluation
Electroantennogram recording technique (EAG): Electroantennography (EAG), introduced by Schneider in 1957 [11], is an effective electrophysiological tool to evaluate and record the olfactory responses of an insect via their antennal receptors [10]. Insects perceive volatile organic compounds by means of olfactory receptors located on the antennae. EAG is essentially the record of the sum of many olfactory receptor potentials generated by a stimulus compound more or less simultaneously on the sensory epithelium of the antennal surface. Basically, it records the responses of many receptor neurons of the antenna to the presentation of a stimulus compound. The principle of EAG is to record voltage changes between the tip and the base of an antenna during stimulation by a volatile compound and the resulting EAG signal will be amplified and recorded with the help of data acquisition controller and software system (IDAC-2 EAG system from Syntech, The Netherlands) and displayed as electrical depolarization on the computer screen. The intensity of deflection is usually measured in milli Volts (mV). EAG recording experiments were conducted to confirm the bioactivity of synthesized pheromone compound 6,10,14-trimethyl-2-pentadecanol against 2-5 day old male Corcyra cephalonica in our laboratory utilizing established EAG facility.
Preparation of stimulus pipettes: EAGs were recorded directly by puffing the stimulus along with the humidified air on to the male moth antennal preparation. Stimulus cartridges were prepared by dispensing known amount (100 μg) of synthetic pheromone in n-hexane (50 μL) on to the filter paper. The solvent was allowed to evaporate for 30 sec before dispensing the puff.
Antennal preparation: The antenna of male moth was carefully excised along with the head and fixed between two probes of an electrode holder with the help of an electrically conducting gel. The antenna was continuously flushed with charcoal filtered and moistened air stream through a stainless tube (8 mm id ending 2 cm) before antennal preparation. For checking the olfactory response, stimulus pipette containing the test compound was inserted into the side hole of the flow tube carrying the air stream. Then by pressing the pedal, the air was allowed to pass through the stimulus pipette onto the antenna and the obtained signal was recorded. The same procedure is repeated with the control (Hexane) pipette. EAG responses were recorded from five male insects individually to synthetic pheromone. EAG response obtained with the test compound was always subtracted with the response from the solvent hexane which is also considered as control. Experiments were conducted in replicates with sequential puffs consisting air, 1-hexanol and three continuous puffs of synthesized pheromone, again air and 1-hexanol.
Preparation pheromone lures: Three blends were prepared based on the concentration of active ingredient to evaluate for its bio-efficacy. The synthesized 6,10,14-trimethyl-2-pentadecanol was dissolved in nhexane to make 80 mg and 100 mg/mL solutions. 100 μL of each solution (8 mg (blend-1) and 10 mg (blend-2) of pheromone) is impregnated on to the pretreated different colored (red, green, yellow and white) rubber septa dispensers after the addition of equal amount of Butylated Hydroxy Toulene (BHT). Blend-3 was prepared as a mixed blend by adding farnesol in 1:1 ratio to the active ingredient (4 mg 6,10,14-trimethyl-2-pentadecanol and 4 mg farnesol) with an intention to see an enhance trapping efficiency due to synergism of farnesol. However it did not provided the expected results.
Material and Methods
Synthesis of (2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienal (compound 5)
To a solution of farnesol (compound 4) (4 g, 18 mmol) in acetonitrile (40 mL) was added IBX (7.8 g, 27 mmol) slowly at room temperature. The flask was fitted with a condenser and refluxed under nitrogen atmosphere for 30 min. The reaction mixture was then allowed to cool to room temperature, precipitated solid was filtered through a pad of Celite and then the residue was washed with diethyl ether (2 mL × 50 mL). The filtrates were concentrated under reduced pressure and the residue was purified by column chromatography with use of 10% EtOAc/hexane (v/v) as the eluent to furnish the aldehyde 5 (3.76 g, 17.1 mmol) in 95% yield as a pale yellow liquid. IR (neat): 2965, 2921, 2856, 1674, 1444, 1379, 1191, 1119, 838 cm-1; 1H NMR (400 MHz, CDCl3) (S 1): δ 9.99 (d, J=8.0 Hz, 1H), 5.88 (dd, J=8.0, 1.1 Hz, 1H), 5.12-5.05 (m, 2H), 2.26-2.19 (m, 5H), 2.18-2.15 (m, 3H), 1.71-1.66 (m, 6H), 1.63-1.58 (m, 6H); MS (ESI): 243 [M + Na]+; GC MS: 220 [M]+, Rt=9.831 min (95.2% purity).
Synthesis of ethyl-(2E,4E,8E)-5,9,13- trimethyltetradeca-2,4,8,12-tetraenoate (compound 3)
To a solution of aldehyde 5 (3.73 g, 16.9 mmol) in dichloromethane (56 mL) was added ethyl (triphenylphosphoranylidine) acetate (7.6 g, 21.9 mmol). The reaction mixture was stirred at room temperature for 4 h when TLC examination revealed the complete conversion of starting material. The solvent was evaporated under reduced pressure, diethyl ether (70 mL) was added to the residue and the reaction mixture cooled to 0°C. The precipitated solids were filtered and the filtrate was evaporated to dryness. The residue thus obtained was purified by column chromatography using 8% EtOAc/hexane (v/v) as the eluent to afford the ester 3 (4.5 g, 15.54 mmol) in 92% yield as a viscous oil. IR (neat): 2968, 2925, 1713, 1635, 1446, 1370, 1274, 1149, 1039, 979, 885 cm-1; 1H NMR (500 MHz, CDCl3) (S 2): δ 7.58 (dd, J=15.1, 11.5 Hz, 1H), 5.99 (d, J=11.5 Hz, 1H), 5.78 (d, J=15.1 Hz, 1H), 5.14-5.05 (m, 2H), 4.20 (q, J=7.1 Hz, 2H), 2.19-2.11 (m, 4H), 2.09-2.02 (m, 3H), 2.00-1.94 (m, 1H), 1.89 (s, 3H), 1.71-1.58 (m, 9H), 1.29 (t, J=7.1 Hz, 3H); MS (ESI): 313 [M + Na]+.
Synthesis of (2E,4E,8E)-5,9,13-trimethyltetradeca-2,4,8,12- tetraen-1-ol (compound 6)
To a solution of ethyl ester 3 (4.45 g, 15.34 mmol) in anhydrous dichloromethane (61 mL) cooled at -78°C was added DIBAL-H (1.4 M in toluene, 27.4 mL, 38.35 mmol) slowly under the nitrogen atmosphere. The reaction mixture was stirred at the same temperature for 30 min and then quenched by the slow addition of ice pieces. The precipitated solids were filtered through a pad of Celite and the solids were washed with hot ethyl acetate (2 mL × 50 mL). The combined filtrates were evaporated under reduced pressure and the residue was purified by column chromatography using 20% EtOAc/hexane (v/v) as the eluent to afford the allylic alcohol 6 (3.35 g, 13.5 mmol) in 88% yield as a viscous oil. IR (neat): 3381, 2965, 2922, 2857, 1677, 1627, 1447, 1379, 1089, 966, 830 cm-1; 1H NMR (400 MHz, CDCl3) (S 3): δ 6.52-6.42 (m, 1H), 5.88-5.82 (m, 1H), 5.78-5.67 (m, 1H), 5.16-5.06 (m, 2H), 4.22-4.14 (m, 2H), 2.22-2.15 (m, 1H), 2.15-2.02 (m, 6H), 2.01-1.94 (m, 1H), 1.80-1.75 (m, 3H), 1.70-1.58 (m, 9H); MS (ESI): 271 [M + Na]+; GC MS: 248 [M]+, Rt=12.691 min (98.1% purity).
Synthesis of 5,9,13-trimethyltetradecan-1-ol (compound 2)
To a solution of allylic alcohol 6 (3.32 g, 13.39 mmol) in methanol (45 mL) was added Pd/C (330 mg, 10% by wt). The reaction mixture was evacuated, subsequently filled with H2 and stirred for 20 h at atmospheric pressure. The solid catalyst was filtered through a small pad of Celite and catalyst was washed with ethyl acetate (30 mL). Evaporation of the combined filtrates in vacuo afforded a crude product that was purified by column chromatography with use of 15% EtOAc/hexane (v/v) as the eluent to furnish the hydroxy compound 2 (2.88 g, 11.25 mmol) in 84% yield as a viscous oil. IR (neat): 3334, 2926, 2862, 1461, 1375, 1054, 734 cm-1; 1H NMR (300 MHz, CDCl3) (S 4): δ 3.64 (t, J=6.6 Hz, 2H), 1.60-1.49 (m, 2H), 1.46-1.19 (m, 13H), 1.18-1.01 (m, 6H), 0.91-0.81 (m, 12H); MS (ESI): 279 [M + Na]+; GC MS: 255 [M-H]+, Rt=22.194 min (99.6% purity).
Synthesis of 5,9,13-trimethyltetradecan-1-al (compound 7)
To a solution of hydroxy compound 2 (2.85 g, 11.13 mmol) in acetonitrile (28 mL) was added IBX (4.82 g, 16.7 mmol) slowly at room temperature. The flask was fitted with a condenser and refluxed under the nitrogen atmosphere until no starting material could be detected by TLC (ca. 30 min). The reaction mixture was then allowed to cool to room temperature, precipitated solid was filtered through a short pad of Celite and the residue was washed with diethyl ether (2 mL × 30 mL). The combined filtrates were concentrated under reduced pressure and the residue thus obtained was purified by column chromatography with use of 5% EtOAc/hexane (v/v) as the eluent to furnish the aldehyde 7 (2.57 g, 10.13 mmol) in 91% yield as a viscous liquid. IR (neat): 2954, 2926, 2866, 2713, 1728, 1462, 1380, 1172, 734 cm-1; 1H NMR (500 MHz, CDCl3) (S 5): δ 9.77 (t, J=1.8 Hz, 1H), 2.43-2.38 (m, 2H), 1.71-1.47 (m, 3H), 1.44-1.19 (m, 10H), 1.18-1.01 (m, 6H), 0.91-0.81 (m, 12H); GC MS: 254 [M]+, Rt=21.471 min (97.6% purity).
Synthesis of 6,10,14-trimethyl-2-pentadecanol (compound 1)
To a solution of aldehyde 7 (2.55 g, 10.04 mmol) in anhydrous diethyl ether (40 mL) cooled at 0°C was added methyllithium (1.6 M in diethyl ether, 12.6 mL, 20.08 mmol) drop wise over a period of 10 min under nitrogen atmosphere. The reaction mixture was allowed to warm to room temperature gradually and stirred further for a period of 3 h and then quenched with an aqueous saturated NH4Cl solution (15 mL). The two layers were separated and the aqueous layer was extracted with EtOAc (2 mL × 15 mL). The combined organic extracts were washed with brine and dried over Na2SO4. Evaporation of the solvent under reduced pressure afforded a crude product that was purified by column chromatography with use of 10% EtOAc/hexane (v/v) as the eluent to furnish the pheromone compound 1 (2.41 g, 8.93 mmol) in 89% yield as a viscous oil. IR (neat): 3348, 2956, 2926, 2865, 1462, 1375, 1118, 937, 735 cm-1; 1H NMR (400 MHz, CDCl3) (S 6): δ 3.84-3.76 (m, 1H), 1.63 (bs, 1H), 1.58-1.34 (m, 6H), 1.34-1.17 (m, 12H, covering a doublet at δ 1.19 (J=6.1 Hz)), 1.17-1.01 (m, 6H), 0.90-0.81 (m, 12H).
Results and Discussion
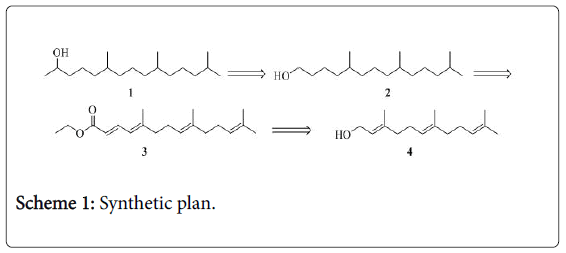
The retrosynthetic analysis is depicted in Scheme 1. The target molecule 1, 6,10,14-trimethyl-2-pentadecanol, was envisaged to be obtained from 5,9,13-trimethyltetradecane-1-ol 2, which in turn can be derived from ethyl ester 3. The ethyl ester 3 can be readily prepared from commercially available farnesol via oxidation followed by Wittig reaction.
Synthesis of sex pheromone component of female rice moth
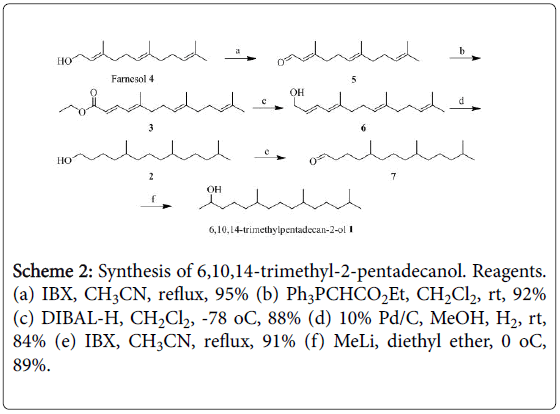
We began the synthesis from commercially available farnesol, which on oxidation with IBX in acetonitrile furnished aldehyde 5 [12,13]. Wittig olefination of aldehyde 5 with ethyl (triphenylphosphoranylidine) acetate in dichloromethane afforded the trans-ethyl ester 3 [14], which was chemoselectively reduced with DIBAL in dichloromethane to furnish allylic alcohol 6 [15]. Reduction of four olefinic bonds in allylic alcohol 6 with palladium over carbon under an atmosphere of hydrogen in MeOH yielded the primary alcohol 2 [16]. The primary hydroxy group in 2 was oxidized without incident with use of IBX in acetonitrile under reflux to yield aldehyde 7. Finally, the reaction of aldehyde 7 with methyl lithium in diethyl ether [12] produced the desired racemic pheromone compound 1 (Scheme 2). The synthesized compound 1 had physical characteristics that were in excellent agreement with those reported in the literature [2].
Bio efficacy confirmation of 6,10,14-trimethyl-2- pentadecanol, the female rice moth produced sex pheromone, in the laboratory
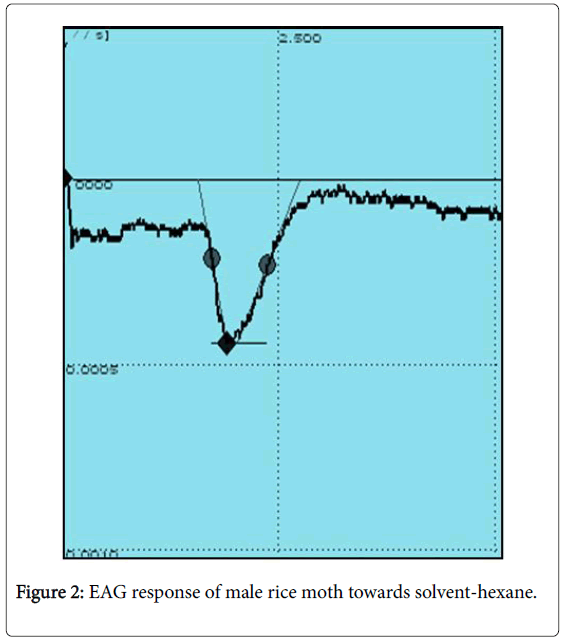
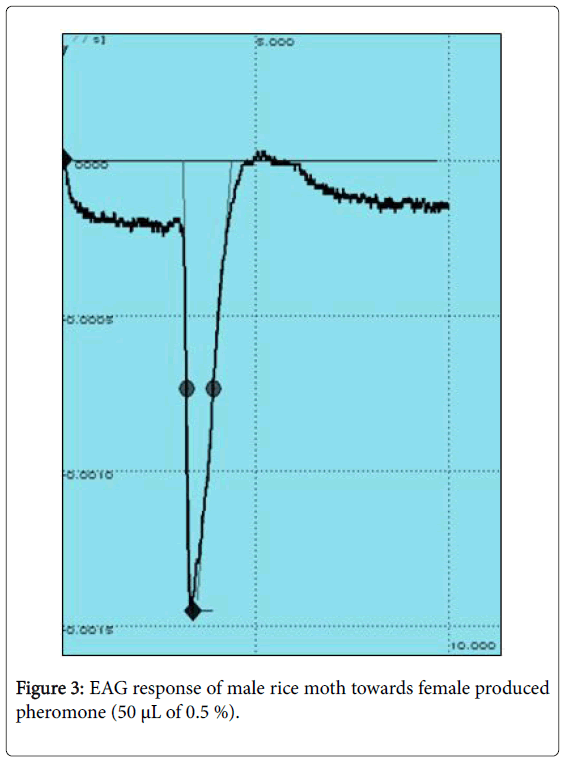
EAG recording experiments were conducted to confirm the bioactivity of synthesized pheromone compound 6,10,14-trimethyl-2- pentadecanol against 2-5 day old male Corcyra cephalonica in our laboratory utilizing established EAG facility. The synthesized 6,10,14- trimethyl-2-pentadecanol elicited a significant EAG responses ranging from 1.28 mV to 1.40 mV from the male antenna of C. cephalonica (Any response measured from 0.5 mV and above is considered as good response). The representative EAG profiles caused due to solvent (nhexane alone) and the synthesized pheromone are represented in Figures 2 and 3.
Efficacy of synthesized 6,10,14-trimethyl-2-pentadecanol in experimental mini rice storage warehouse at Agricultural Research Station of ANGRAU at Ananthapur, A. P., India
Electrophysiological (EAG) bioassay provides information only about activation of receptors. It does not provide information about the type of insect behavior the compound elicits; which could be as an attractant, repellent or another type of behavioral response. Volatiles proved positive in EAG experiments are typically taken on to further behavioral assays involving olfactometers/wind tunnels as the chemical identity and electrophysiological activity of a compound are of little value if no behavioral response associated with it is demonstrated. Since behavioral assays involving olfactory cues are often tedious and time-consuming, it is decided to evaluate the synthetic rice moth pheromone for its efficacy in the experimental warehouse of Agricultural Research Station of ANGRAU at Ananthapur, A.P.
Agriculture Research Station of ANGARAU at Ananthapur, India. The results of male moth catches recorded during evaluation of pheromone lures were displayed in Tables 1 and 2. These results revealed that the blend-1 and blend-2 have certainly showed excellent trapping ability irrespective of the color of the dispenser. And also It was observed that the blend-2 was attracted significantly more male moths than blend-1. However addition of farnesol in blend-3 depleted the trapping efficiency although we expected the synergistic effect of farnesol may improve the catching efficiency. It is concluded that the synthetic pheromone blend in combination of antioxidant BHT is good enough for better trapping.
| S No | Days/ Weeks | Number of male moths trapped | ||
|---|---|---|---|---|
| Blend-1 | Blend-2 | Blend-3 | ||
| 1 | 1 | 7 | 11 | 5 |
| 2 | 3 | 28 | 37 | 21 |
| 3 | 5 | 51 | 64 | 29 |
| 4 | 1 Week | 76 | 81 | 41 |
| 5 | 10 Days | 95 | 112 | 59 |
| 6 | 2 Weeks | 134 | 145 | 73 |
| 7 | 3 Weeks | 199 | 211 | 101 |
| 8 | 1 Month | 253 | 266 | 121 |
| 9 | 6 Weeks | 345 | 386 | 165 |
| 10 | 2 Months | 449 | 493 | 189 |
*Note: Blend 1: 8 mg of synthetic pheromone in hexane with equal amount of BHT; Blend 2:10 mg of synthetic pheromone in hexane with equal amount of BHT; Blend 3:8 mg of synthetic pheromone and farnesol (1:1 ratio) in hexane with equal amount of BHT
Table 1: Male moth catches in different traps baited with C. cephalonica pheromone green rubber septa lures using different blends over a period of two months.
| Colour of dispenser | Number of male moths trapped | ||
|---|---|---|---|
| Blend-1 | Blend-2 | Blend-3 | |
| Red | 478 | 514 | 196 |
| Green | 449 | 493 | 189 |
| Yellow | 395 | 458 | 221 |
| White | 497 | 557 | 203 |
Table 2: Male moth catches in different traps baited with C. cephalonica pheromone different colors lures using different blends over a period of two months.
Conclusion
In summary, we have designed an efficient synthetic route to 6,10,14-tri-methyl-2-pentadecanol 1 employing Wittig reaction, hydrogenation, and oxidation as key steps. In contrast to many earlier syntheses, our synthetic strategy afforded high yields and would be useful for the synthesis of 1 in kilogram scale. The synthesized 6,10,14- trimethyl-2-pentadecanol is found to be active in both EAG response from the male moth and attracting male moths in the bio-evaluation field studies at warehouse.
Acknowledgements
A.S. and R. A. K thank CSIR-IICT, JSY thank CSIR and DST for the award of CSIR-Bhatnagar and J C Bose Fellowships respectively. We also thank ANGRAU at Ananthapur, A. P., India for providing mini rice storage warehouse.
References
- Pickett JA, Wadhams LJ, Woodcock CM (1986) Chemical ecology and pest management: Some recent insights. Int J Tropic Insect Sci 10: 741–750.
- Hall DR, Cork A, Lester R, Nesbitt BF, Zagatti P (1987) Sex pheromones of rice moth, Corcyra cephalonica Stainton. J Chem Ecol 13: 1575–1589.
- Mori K, Harada H, Zagatti P, Cork A, Hall DR (1991) Pheromone synthesis, CXXVI. Synthesis and biological activity of four stereoisomers of 6,10,14-trimethyl-2-pentadecanol, the female-produced sex pheromone of rice moth (Corcyra cephalonica). Liebigs Annalen der Chemie 3: 259–267.
- Shafikov RV, Spivak AY, Odinokov VN (2011) Chemoenzymatic synthesis of (2R,6R,10R)-6,10,14-trimethylpentadecan-2-ol, sex pheromone of rice moth (Corcyra cephalonica), and of its (2S,6R,10R)-diastereomer. Russian J Org Chem 47: 290-294.
- Nakamura Y, Mori K (2000) New Syntheses of the Rice Moth and Stink Bug Pheromones by Employing (2R, 6S)-7-Acetoxy-2,6-dimethyl-1-heptanol as a Building Block. Biosci Biotechnol Biochem 64: 1713-1721.
- Naoshima Y, Munakata Y, Yoshida S, Funai A (1991) Synthesis of chiral alcohol and ester pheromones through enzyme-catalysed hydrolysis using Pseudomonas fluorescens lipase: preparation of (2R,6S,10S)-6,10–14-Trimethylpentadecan-2-ol and the propionate of (2R,8R)-8-methyldecan-2-ol. J Chem Soc 3: 549-553.
- Rane SS, Chadha MS, Mamdapur VR (1993) Synthesis of racemic 6,10,14-trimethylpentadecan-2-ol, a pheromone of rice moth and 5,9,13-trimethyltetradecanoic acid, a component of marine sponge from a common intermediate. Bioorg Medi Chem 1: 399-401.
- Yadav J, Reddy P, Ather H, Kumar A, Prasad A, et al. (2012) Stereoselective Synthesis of (4S,6S)-6-Hydroxy-4-undecanolide: A Pheromone of the Giant White Butterfly Idea leuconoe. Synthesis 2012: 579-84.
- Yadav J, Chandravathi C, Thrimurtulu N, Prasad A, Ghamdi A (2013) A Practical Total Synthesis of Both E- and Z-Isomers of Optically Pure (S)-14-Methylhexadec-8-enal (Trogodermal). Synthesis 45: 1513-1518.
- Rao CN, George A, Prasad AR, Rehman HU, Kumar RA, et al. (2017) Field Testing of Indigenously Synthesized Sex Pheromone for the Management of Phyllocnistis citrella Stainton under Central Indian Conditions. Current Sci 113: 2298-2304.
- Schneider D (1957) Electrophysiological investigations of chemo and mechanoreceptors of the antennae of the silk moth Bombyx mori L. J Comp Physiol 40: 8-41.
- Swapnil N, Kumar AS, Babu NJ, Yadav JS (2017) Synthetic Approach Towards the Synthesis of Antimalarial Gomphostenin. Asian J Org Chem 6: 1091-1098.
- Yadav JS, Venkatesh M, Kumar AS, Reddy PAN, Reddy BVS, et al. (2014) A Stereoselective Total Synthesis of Decarestrictine I. Helvetica Chimica Acta 97: 830-838.
- Yadav JS, Chandravathi C, Thrimurtulu N, Prasad A, Ghamdi A (2013) A Practical Total Synthesis of Both E- and Z-Isomers of Optically Pure (S)-14-Methylhexadec-8-enal (Trogodermal). Synthesis 45: 1513-1518.
- Raghavan S, Krishnaiah V (2010) An Efficient Stereoselective Synthesis of Penaresidin A from (E)-2-Protected Amino-3,4-unsaturated Sulfoxide. The J Org Chem 75: 748-761.
- Yadav JS, Reddy PAN, Kumar AS, Prasad AR, Reddy BVS, et al. (2014) Stereoselective total synthesis of 4-((3S,5R)-3,5-dihydroxynonadecyl)phenol. Tetrahedron Letters 55: 1395-1397.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5163
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 4236
- PDF downloads: 927