An Automated Microscope System to Analyze Cell Proliferation
Received: 10-Dec-2021 / Manuscript No. CMB-21-49080 / Editor assigned: 13-Dec-2021 / PreQC No. CMB-21-49080 (PQ) / Reviewed: 08-Jan-2022 / QC No. CMB-21-49080 / Revised: 13-Jan-2022 / Manuscript No. CMB-21-49080 (R) / Accepted Date: 13-Jan-2022 / Published Date: 20-Jan-2022 DOI: 10.4172/1165-158X.1000223
Abstract
Cell proliferation occurs via cell division under appropriate conditions, including a controlled cell cycle and cellular homeostasis, and the abrogation of cell proliferation results in pathological states such as cancer and senescence. Cell-based in vitro experiments were conducted in an optimal cell proliferation environment, and the analysis of cell proliferation is a critical tool for exploring cellular homeostasis and developing drugs to modulate cell growth. Although colorimetric assays are limited by the small culture area and the short tracing period and cell counting using a hemocytometer requires multiple plating for each time point to be analyzed, these two methods have been used widely for the analysis of cell proliferation. In this study, we aimed to develop a robust method to detect cell proliferation via an automated microscopic cell-number analysis (AMCA) system to overcome the limitations of traditional methods. In contrast colorimetric assays showed reduced sensitivity with increased cell density, AMCA showed constantly reliable detection. Also, the AMCA system exhibited a comparable cell proliferation trend with the direct whole-cell counting method. In conclusion, we suggest AMCA as an alternative method to analyzing cell proliferation with expanded spatial and culture period conditions and expect AMCA to provide an efficient drug developing experiment.
Keywords
Cell proliferation assay; WST-8 assay; Cell counting; Automated microscope
Introduction
Cell proliferation results from cell division, which is regulated by various cell cycle mechanisms [1]. A balanced cell cycle is an indicator of well-maintained cell homeostasis, and dysregulated or terminated cell cycles result in pathological states, including cancer and senescence [2]. Thus, the determination of cell proliferation and cell death are critical tools in drug development [3-5]. Under optimal the in vitro cell culture conditions, which include the supplementation of nutrition and growth factors, and appropriate temperature and pH, a regular cell cycle is maintained, resulting in cell proliferation. The analysis of cell number and proliferation offers a robust approach to explore cellular metabolic viability, and various methods have been developed to meet researchers’ needs.
Colorimetric assays, for determining the cell viability via converting the substrate by enzymatic activity during the cellular metabolic process, have been widely used owing to its accessibility. A water-soluble yellow tetrazolium salt (3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide, MTT) is converted to insoluble purple formazan crystals by the actionof NAD(P)H-dependent oxidoreductase in metabolically active cells [6]. The concentration of formazan is calculated from the optical density at 570 nm following dissolution using a solubilizing solution; the absorbance quantifies the amount of active cells. XTT (2,3-bis-(2-methoxy-4-nitro-5- sulfophenyl)-2H-tetrazolium-5-carboxanilide) and WST-8 are the second-generation colorimetric substrates that do not require cell lysis. As colorimetric changes indicate the level of cellular metabolic activity, the applications of these assays include the measurement of cell proliferation, cytotoxicity, and apoptosis [7-9]. Cell counting using a hemocytometer and trypan blue staining is a common method to obtain actual cell numbers; and presently, automated cell counting devices have been developed to support researchers. While colorimetric assays determine the relative values between groups that are plated in 96- well cell culture plates, cell counting yields the actual numbers of cells subjected to various experimental conditions or cultures. The adoption of appropriate methods to evaluate cell proliferation and viability is an essential component of successful cell-based research, such as drug development and genetic studies. The current study aimed to develop a straightforward and robust method to analyze cell proliferation and morphology as a screening tool by taking advantage of an automated microscope that obtains images within a designated field and counts cell numbers using phase difference.
Materials and Methods
Cell culture
The cells used in the current study, presented in Table 1, are maintained with Dulbecco’s Essential Media (DMEM) or RPMI1640 (Welgene, Gyeongsan-si, Gyeongsangbuk-do, Republic of Korea) containing 10% fetal bovine serum (FBS; Welgene) and penicillin/ streptomycin (100 U/mL and 100 μg/mL, respectively; Welgene) in a cell culture incubator at 37°C with 5% CO2.
Colorimetric assay for cell proliferation analysis
Cell proliferation was assessed by colorimetric cell viability assays using 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5- carboxanilide (XTT, Welgene) and WST-8 (BioMax, Seoul, Republic of Korea) in accordance with manufacturer’s instructions. Briefly, cells in 100 μL of culture media were plated in 96-well plate (Nunclon, Roskilde, Denmark) and maintained until the proliferation was to be analyzed. The cells were incubated for between 30 min and 2 h with XTT substrate (20 μL/well) or WST-8 substrate (10 μL/well), and the absorbance at 450 nm or 690 nm (for XTT only) was measured with a microplate reader (SpectraMax, Molecular Devices, Sunnyvale, CA). In the XTT assay, the cell density in each well was determined by subtracting the absorbance at 690 nm from 450 nm. Six replicates were analyzed for each sample. For the comparison of the WST-8 assay and automated cell count system, four sets of cells (2.5 × 103 cells/well) were plated in a 96-well plate and analyzed on Days 1 to 4. The absorbance of formazan was measured after 60-min substrate incubation.
Automated microscopic cell-number analysis (AMCA) system
The Lionheart Automated microscopic system (Biotek, Winoski, VT, USA) allows the evaluation of the number of cells using the phase difference between cell images without disrupting normal cell culture conditions. Cells (5 × 103 cells/well) were plated in a 6-well culture plate (SPL, Gyeonggi-do, Republic of Korea) and incubated for 4 days. The number of cells in 24 randomly designated fields were counted every day for 4 days using the provided software (Gen5 3.0 Image PRiME, Biotek), and the counts from 24 fields were summed.
Cell counting using a hemocytometer
Four sets of cells (5 × 103 cells/well, triplicate) were plated in 6-well plates to determine cell proliferation for 4 days. Then, the cells were rinsed with Dulbecco’s phosphate-buffered saline (Welgene), dissociated from the culture plate using TrypLE Express (Gibco, Grand Island, NY, USA), diluted in culture media, and stained with 0.4% trypan blue (Gibco). The number of trypan blue negatively stained cells was counted each day using a hemocytometer; triplicate measurements were performed.
Result
Colorimetric assay revealed cell-number–dependent absorption
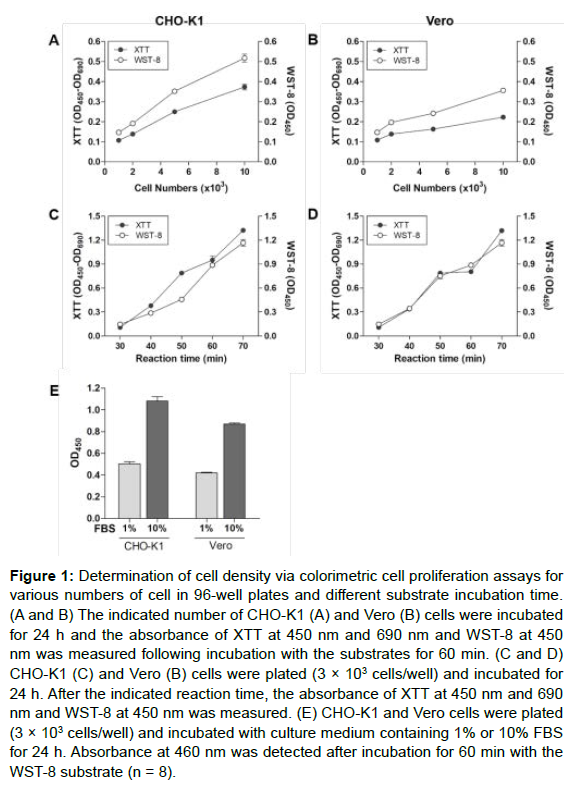
The determination of cell proliferation by colorimetric assay is generally conducted in a 96-well plate. To demonstrate the cell-number– dependent absorbance of colorimetric substrate in the XTT and WST-8 assays, cells (1 × 103 to 1 × 104) were first plated in 96-well culture plates, and the absorbance of XTT and WST-8 were measured at 450 nm and 690 nm after incubation for 24 h. As shown in Figures 1A and 1B, the absorbances of XTT and WST-8 increased in a cell-number– dependent matter in CHO-K1 and Vero cells. As the reaction time was increased further, the absorbance also increased in a time-dependent manner (Figures 1C and 1D).
Figure 1: Determination of cell density via colorimetric cell proliferation assays for various numbers of cell in 96-well plates and different substrate incubation time. (A and B) The indicated number of CHO-K1 (A) and Vero (B) cells were incubated for 24 h and the absorbance of XTT at 450 nm and 690 nm and WST-8 at 450 nm was measured following incubation with the substrates for 60 min. (C and D) CHO-K1 (C) and Vero (B) cells were plated (3 × 103 cells/well) and incubated for 24 h. After the indicated reaction time, the absorbance of XTT at 450 nm and 690 nm and WST-8 at 450 nm was measured. (E) CHO-K1 and Vero cells were plated (3 × 103 cells/well) and incubated with culture medium containing 1% or 10% FBS for 24 h. Absorbance at 460 nm was detected after incubation for 60 min with the WST-8 substrate (n = 8).
Fetal bovine serum (FBS) is an essential substitute that sustains cell viability and proliferation by providing growth factors and nutrients. To identify whether the colorimetric analysis was an appropriate method to determine cell proliferation, CHO-K1 and Vero cells were incubated in 1% and 10% FBS, and the WST-8 assay was performed. The absorbance of CHO-K1 and Vero cells was higher in the 10% FBS-supplemented group than in the 1% FBS-supplemented group, which indicated greater proliferation in response to a higher concentration of FBS (Figure 1E). Thus, the colorimetric assay can be used as a tool for the analysis of cell proliferation.
Cell number determined by AMCA system appropriately represents cell density
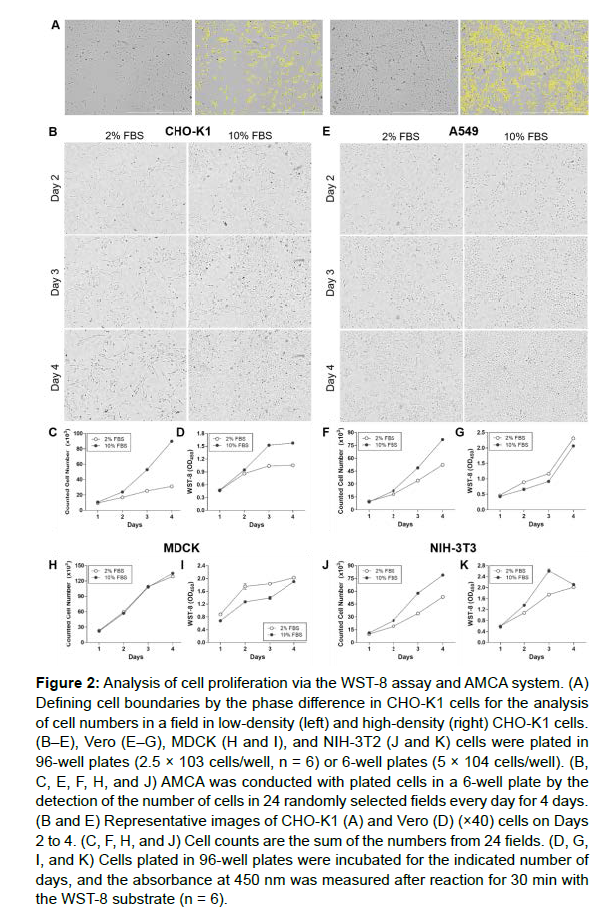
The cell counting using the AMCA system is based on the ability of the software to recognize the cell boundary via phase difference (Figure 2A). Cells were plated in 96-well or 6-well plates incubated for 4 days with culture medium containing 2% or 10% FBS to examine whether the results of AMCA followed a similar trend to the WST-8 assay. The AMCA of cell proliferation was assessed by the measurement of the number of cells in 24 identical fields every day for 4 days. In addition, cell density in 96-well plates was determined using the WST-8 assay.
As it is not recommended to culture cells for more than 48 h after the addition of WST-8 substrate, owing to its toxicity, analysis was performed each day using independently plated cells. The cell count of CHO-K1 supplemented with 10% FBS-containing medium was higher than the cell count of CHO-K1 supplemented with 2% FBS-containing media (Figures 2B and 2C). Although WST-8 assay also exhibited enhanced absorbance of 10% FBS-supplemented cells compared to 2% FBS-supplemented cells (Figure 2D), the similar absorbance of Day 3 and Day 4 was not consistent with increased cell density (Figure 2B).
A549 cells grown in 2% FBS-supplemented medium showed delayed proliferation compared A549 cells grown in 10% FBS-supplemented medium (Figures 2E and 2F). However, the WST-8 assay revealed the increased proliferation of A549 cells grown in 2% FBS-supplemented medium compared with A549 cells grown in 10% FBS-supplemented medium (Figure 2G). Notably, the results for MDCK cells were similar to those for A549; that is, the WST-8 assay indicated the higher proliferation of MDCK cells grown in 2% FBS-supplemented medium compared with cells grown in 10% FBS-supplemented medium (Figure 2I), and the AMCA showed comparable proliferation in MDCK cells grown in 2% FBS-supplemented medium and 10% FBS-supplemented medium (Figure 2H). Enhanced proliferation of NIH-3T3 cells grown in 10% FBS-supplemented medium compared with 2% FBS-supplemented medium was found in the WST-8 assay and the AMCA (Figures 2J and 2K). However, on Day 4, the WST-8 assay indicated no difference in absorbance between NIH-3T3 cells grown in medium supplemented with 2% and 10% FBS. These results indicate the limitation of 96-well based cell proliferation assays and the benefit of AMCA as an analysis method for 96-well-scale cell culture.
Figure 2: Analysis of cell proliferation via the WST-8 assay and AMCA system. (A) Defining cell boundaries by the phase difference in CHO-K1 cells for the analysis of cell numbers in a field in low-density (left) and high-density (right) CHO-K1 cells. (B–E), Vero (E–G), MDCK (H and I), and NIH-3T2 (J and K) cells were plated in 96-well plates (2.5 × 103 cells/well, n = 6) or 6-well plates (5 × 104 cells/well). (B, C, E, F, H, and J) AMCA was conducted with plated cells in a 6-well plate by the detection of the number of cells in 24 randomly selected fields every day for 4 days. (B and E) Representative images of CHO-K1 (A) and Vero (D) (×40) cells on Days 2 to 4. (C, F, H, and J) Cell counts are the sum of the numbers from 24 fields. (D, G, I, and K) Cells plated in 96-well plates were incubated for the indicated number of days, and the absorbance at 450 nm was measured after reaction for 30 min with the WST-8 substrate (n = 6).
Cell counts of the designated fields are sufficient to represent the total cell count
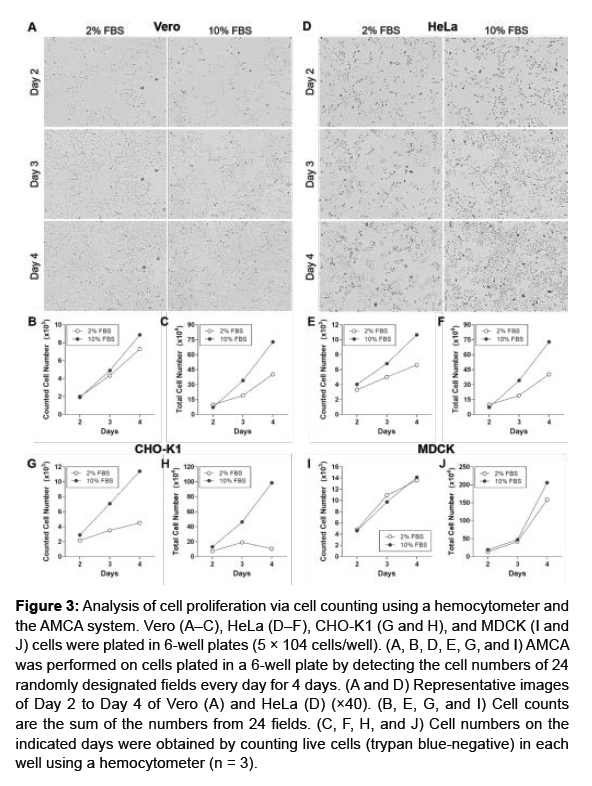
To demonstrate whether the sum of cell counts from designated fields was sufficiently representative of the total cell count. The cells were plated in 6-well plates and the cell numbers were determined by the AMCA system or counting the number of cells using hemocytometer. As shown in Figure 3C, the total count of Vero cells, determined using a hemocytometer, was higher when cells were grown in medium supplemented with 10% FBS than 2% FBS. Consistent with the total cell counts, AMCA also revealed the higher proliferation rate with 10% FBS supplementation than 2% FBS (Figures 3A and 3B). Further, HeLa (Figures 3D-3F), CHO-K1 (Figures 3G and 3H), and MDCK (Figures 3I and 3J) cells also exhibited greater levels of proliferation when grown in 10% FBS-supplemented medium compared with 2% FBS-supplemented medium measured by both methods. These results suggest that the assessment of cell growth using AMCA may be able to replace the need for total cell counts.
Figure 3: Analysis of cell proliferation via cell counting using a hemocytometer and the AMCA system. Vero (A–C), HeLa (D–F), CHO-K1 (G and H), and MDCK (I and J) cells were plated in 6-well plates (5 × 104 cells/well). (A, B, D, E, G, and I) AMCA was performed on cells plated in a 6-well plate by detecting the cell numbers of 24 randomly designated fields every day for 4 days. (A and D) Representative images of Day 2 to Day 4 of Vero (A) and HeLa (D) (×40). (B, E, G, and I) Cell counts are the sum of the numbers from 24 fields. (C, F, H, and J) Cell numbers on the indicated days were obtained by counting live cells (trypan blue-negative) in each well using a hemocytometer (n = 3).
Discussion
Although the purpose of analyzing cell proliferation varies, a considerable number of studies have used colorimetric assays, such as the MTT, XTT, and WST-8 assays, owing to their accessibility and sensitivity [10]. Colorimetric assays analyze the enzymatic activity of viable cells plated in 96-well plates via the detection of the formazan product. Unlike the MTT assay, which requires a solubilization step, the XTT and WST-8 assays detect the absorbance of the formazan product in live cells. However, owing to the toxicity of the substrate, the period over which proliferation can be tracked is limited to 2 days. The current study aimed to develop a long-term analysis method for cell proliferation without disturbing cellular homeostasis, and took advantage of an automated microscope system to recognize the cell boundary using phase difference (Figure 2A). The AMCA system allows the analysis of identical fields for each detection and provides images that indicate the increase in cell-number (Figures 2B, 2E, 3A, and 3D). Culturing cells in 96-well plates results in a higher possibility of inhibiting cell growth owing to the limited area (Figure 2D and 2K). As automated microscopic analysis can be performed on a larger area, such as a 6-well plate or a 100-mm culture dish, the analysis of proliferation over a longer time is possible compared with 96-well plate-based colorimetric assays. Thus, the advantage of extended tracking of cell proliferation may provide the flexible application of the AMCA system in cell culture-based in vitro drug efficacy experiments.
The most straightforward approach to cell proliferation is to count the number of cells after the collection of all the cells in the culture plate. Traditionally, counting the number of trypan blue-stained cells with hemocytometer is used and automated cell counting instruments have been developed to minimize human labor and maximize accuracy. However, counting all the cells in culture plates require that culture is stopped in the culture plate and multiple plating is needed to trace each time point that accompanies an experimental condition. Unlike direct cell counting, the AMCA system allows continuous monitoring of the number of cells without disturbing the cell culture conditions. Determining the cell proliferation via the AMCA system successfully reproduced the trends in cell growth from the total cell count (Figure 3).
Thus, we suggest that the AMCA system is a reliable method for the long-term analysis of cell proliferation required for drug development and cell biology investigations.
Acknowledgement
This work was supported by the Material Parts Global Investment Linkage Technology Development (R&D)-Global Open Technology Development Program (20006871, Serum substitutes, serum-free• protein-free media, trypsin, etc. development of biomaterial technology for the culture of animal cells) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
References
- Lubischer JL (2007) The Cell Cycle, Principles of Control, David O. Morgan. Integr Comp Biol 47:794-795.
- Polymenis M, Kennedy BK (2017) Unbalanced Growth, Senescence and Aging. Adv Exp Med Biol 1002:189-208.
- Shapiro GI, Harper JW (1999) Anticancer drug targets: cell cycle and checkpoint control. J Clin Invest 104:1645-1653.
- Méry B, Guy J-B, Vallard A, Espenel S, Ardail D, et al. (2017) In Vitro Cell Death Determination for Drug Discovery: A Landscape Review of Real Issues. J Cell Death 10.
- Gordon JL, Brown MA, Reynolds MM (2018) Cell-Based Methods for Determination of Efficacy for Candidate Therapeutics in the Clinical Management of Cancer. Diseases 6:85.
- Berridge MV, Tan AS (1993) Characterization of the Cellular Reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction. Arch Biochem Biophys 303:474-482.
- Rakhshan R, Atashi HA, Hoseinian M, Jafari A, Haghighi A, et al. (2021) The Synergistic Cytotoxic and Apoptotic Effect of Resveratrol and Naringenin on Y79 Retinoblastoma Cell Line. Anticancer Agents Med Chem 21:2243-2249.
- Güngör T, Ozleyen A, Yılmaz YB, Siyah P, Ay M, et al. (2021) New nimesulide derivatives with amide/sulfonamide moieties: Selective COX-2 inhibition and antitumor effects. Eur J Med Chem 221:113566.
- Ayoup MS, Abu-Serie MM, Awad LF, Teleb M, Ragab HM, et al. (2021) Halting colorectal cancer metastasis via novel dual nanomolar MMP-9/MAO-A quinoxaline-based inhibitors; design, synthesis, and evaluation. Eur J Med Chem 222:113558.
- Carreño EA, Alberto AVP, de Souza CAM, de Mello HL, Henriques-Pons A, et al. (2021) Considerations and Technical Pitfalls in the Employment of the MTT Assay to Evaluate Photosensitizers for Photodynamic Therapy. Appl Sci 11:2603.
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Citation: Kwon JI, Park S, Cha YH, Park SY (2022) An Automated Microscope System to Analyze Cell Proliferation. Cell Mol Biol, 68: 223. DOI: 10.4172/1165-158X.1000223
Copyright: © 2022 Kwon JI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2208
- [From(publication date): 0-2022 - Apr 18, 2025]
- Breakdown by view type
- HTML page views: 1735
- PDF downloads: 473