Case Report Open Access
An Attempt of Non-human Primate Modeling of Schizophrenia with Neonatal Challenges of Epidermal Growth Factor
Miwako Sakai1,2, Masafumi Kashiwahara3, Akiyoshi Kakita4 and Hiroyuki Nawa1*1 Department of Molecular Neurobiology, Brain Research Institute, Niigata University, Niigata 951-8585, Japan
2 Department of Psychiatry, Niigata University Graduate School of Medical and Dental Sciences, 951-8510, Niigata, Japan
3 Shin Nippon Biomedical Laboratories, Ltd., Miyanoura, Kagoshima 981-1394, Japan
4 Department of Pathology, Brain Research Institute, Niigata University, Niigata 951-8585, Japan
- *Corresponding Author:
- Hiroyuki Nawa
Department of Molecular Biology
Brain Research Institute Niigata University
Asahimachi-dori 1-757
Niigata 951-8585, Japan
E-mail: hnawa@bri.niigata-u.ac.jp
Received date: December 17, 2013; Accepted date: January 24, 2014; Published date: January 31, 2014
Citation: Sakai M, Kashiwahara M, Kakita A, Nawa H (2014) An Attempt of Nonhuman Primate Modeling of Schizophrenia with Neonatal Challenges of Epidermal Growth Factor. J Addict Res Ther 5:170. doi: 10.4172/2155-6105.1000170
Copyright: © 2014 Sakai M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Inflammatory cytokines are implicated in the neurodevelopmental hypothesis for schizophrenia. Based on this hypothesis, we studied the establsihment of rodent models for schizophrenia by subcutaneously challenging rat or mouse neonates with various cytokines. Because of the physical and behavioral difference between animal species; however, it is difficult to fully evaluate the appropriateness of rodent models. To overcome this problem, we attempted to establish a non-human primate model of schizophrenia. We subcutaneously injected epidermal growth factor (EGF: 0.3 mg/kg/day) to 3 male neonatal cynomolgus monkeys for a total of 7 or 9 days from 14 days of age. During the following 4 or 5 years, no abnormal behaviors were observed in these monkeys treated with EGF; however, at the age of 6 years, 1 monkey exhibited abnormal behavioral traits: stereotypic movement, vocalization, alert motion, and self injury. It was noteworthy that the monkey often showed agitation and clapped its hands over its eyes and bit his hands in periods of illumination, although not under darkened conditions. The behavioral abnormalities except vocalization were ameliorated by 10-day oral treatment with risperidone at 0.10 mg/kg/day. Although this study needs to be replicated, this case report suggests the possibility that hyper EGF signals at the perinatal stage of non-human primates result in post-pubertal behavioral/cognitive deficits, which possibly imitate some pathological features of human schizophrenia.
Keywords
Schizophrenia; EGF; Inflammatory cytokines; Cynomolgus monkey; ErbB; Hallucination; Risperidone
Abbreviations
EGF: Epidermal Growth Factor; RIS: Risperdal Solution; ANOVA: Analysis of Variance
Introduction
Schizophrenia is an intractable psychiatric disorder with unknown etiology, although various causes have been hypothesized. Among the hypotheses, the so-called “neuro-developmental hypothesis”, stressing the contribution of brain maldevelopment has recently gained much ground [1,2]. This hypothesis assumes that both genetic and environmental factors mutually interact and disrupt neural differentiation or maturation of the fetal or neonatal immature brain. The initial brain deficits presumably are latent but result in functional impairments when the full functions of the brain are required at postpubertal stages. Recent model studies have suggested that immune inflammatory reactions during pregnancy or delivery are most likely to be implicated in this hypothesis [3-5]. In particular, the studies pay great attention to inflammatory cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor alpha [6-8]. Similarly, we have been focusing on another type of cytokine, epidermal growth factor, which exerts an inflammatory action on microglia as well as neurotrophic function in midbrain dopaminergic neurons [9,10]. Our previous postmortem study indicates that the neurotrophic factor, EGF, is decreased and, conversely, that its receptor (ErbB1) expression is up-regulated in forebrain regions of patients with schizophrenia [11]. Moreover, the impaired EGF signal is also evident in the peripheral blood of drug-naïve patients [12]. These findings indicate that EGF/ ErbB1signals are indeed altered in both acute and chronic phases of schizophrenia. In agreement with the postmortem results, peripheral administration of EGF to neonatal rats or mice results in displays of post-pubertal abnormality in cognitive and behavioral traits such as acoustic startle response, prepulse inhibition, hyperlocomotion, social interaction, latent inhibition of learning and sensitivity to methamphetamine [13-16]. Since EGF signaling can be induced by brain injury or driven by infection with a number of viruses [17-20], it is considered that this cytokine might medicate the environmental factors/insults that are implicated in the above developmental hypothesis for schizophrenia. Although this rodent model appears to reflect several aspects of behavioral endophenotypes of schizophrenia, there are significant phenotypic gaps between these rodent behaviors and schizophrenia psychopathology. As this illness is diagnosed solely using the psychopathological criteria of DSM (Diagnostic and Statistical Manual of Mental Disorders) or ICD (International Statistical Classification of Diseases and Related Health Problems), in the critical sense, we have difficulty in evaluating the appropriateness of the rodent EGF model for schizophrenia.
Several attempts have been made to establish non-human primate models for schizophrenia on the basis of developmental hypothesis for schizophrenia [21-24]. We attempted to establish a schizophrenia model, applying the rodent procedure to cynomolgus monkey neonates [11]. However, the present study needed to wait the growth of monkey neonates into the adolescent stage and thus was time-consuming. After 6 years’ observation, finally, we found that 1 monkey exhibited signs of schizophrenia, recorded his abnormal behaviors with a video camera and analyzed for various parameters. We also examined the effect of risperidone, an antipsychotic drug, on the behavior of this schizophrenic monkey. The behavioral difference and similarity of this primate model to human schizophrenia are discussed.
Materials and Methods
Subjects
Male cynomolgus monkey neonates (Macaca fascicularis) were reared with dams in the animal facility of Shinn Nippon Biomedical Laboratories., Inc. (Kagoshima, Japan). These neonates were treated with EGF at the age of postnatal 2 weeks in 2002 (see below for details). The 3 EGF-treated young monkeys were weaned at the age of 6 months and housed individually thereafter. All the monkeys were housed in stainless steel cages of 680 mm (W)×620 mm (D)×770 mm(H) under temperature-controlled conditions, 26 ± 2°C, relative humidity 30- 60%, and a 12/12-hour light/dark cycle (ON, 6:00 am; OFF, 6:00 pm). Filtered water was given from an automatic supplier ad libitum. They were fed 108 g of commercial monkey chow (HF Primate 5K91 12G 5K9J, Purina Mills LCC) daily. Simultaneously with the food provision, we checked animal condition and made gross observations of behaviors between 9 am and 11 am every day. Two EGF-treated monkeys were euthanized and subjected to neurophathologic examinations at 4 years of age. Behavioral analyses were performed for the remaining 1 adult male cynomolgus monkey at 6 years of age. The experimental protocol was subjected to a prior review, and was approved by the Ethics Committee of Shin Nippon Biomedical Laboratories (Approval Number B72-41 issued on January 14, 2002). This study adhered strictly to the ethical code of practice outlined in the Guiding Principles for Animal Experiments Using Non-human Primates formulated by the Primate Society of Japan, 1986 [25].
Treatment with EGF and Risperidone
Three monkey neonates (410-484 g/body weight) were subcutaneously given human recombinant EGF (0.3 mg/kg; Higeta Shoyu Ltd., Noda, Japan) a total of 7 or 9 times from postnatal day 14. Administration was conducted as an injection to the back once a day. Two of the three neonates showed signs of sedation at the 7th administration; therefore, EGF treatment was suspended for 4 days until the animals recovered (the monkey examined at 6 years old received 9-day EGF treatment with a 4-day suspension).
When the EGF-treated monkey to be examined was 6 years old, it was intragastrically administered with risperidone (RIS; Risperdal Solution, Janssen Pharmaceuticals Inc, Tokyo, Japan) daily using a nasal catheter. The initial dose of risperidone was 0.05 mg/kg (0.05 mL/kg), for 10 days as the first set of medication. The second set of the medication was carried out with a risperidone dose of 0.1 mg/kg (0.1 mL/kg) for 10 days. The first and second sets of medication were separated by an interval of 20 days.
Behavioral assessment
The gross observations of behavioral features and for antipsychotic effects of risperidone on behaviors were made from three 1-hour videos recorded starting from 1:00 pm (1 hour before the risperidone treatments and on the following day: days 9, 10, and 11) in a way so as to minimize the influence of acute effects of risperidone treatment on behavioral scoring. The monkey for observation was transferred in its cage to an open room 1 day before video recording and placed next to a cage containing a naive male cynomolgus monkey of the same age. During video recording, no observer was present in the room. Videos were also recorded at 30, 21, and 10 days before risperidone treatment. Later we replayed the videos and counted the frequencies of stereotypic movement, alert behavior, vocalization, self injury and frenzied behavior. The behavioral categories of their definitions are as follows:
1) stereotypic movement: stereotypic, bipedal rotational movement
2) alert behaviors: standing on both legs, facing the front and looking around the vacant room for more than 5 sec
3) self injury and frenzied behavior (hallucination-like): the integrated behavioral sequence of batting both eyes with hands, gazing at the hand, biting the hand, and then frenzied behavior with leg flapping.
4) vocalization: any audible communication. A ‘Coo’ sound was most frequently detected.
Histopathological examination
Monkeys were deeply anesthetized with sodium pentobarbital (25 mg/kg, intravenous injection) and sacrificed with exsanguination. Brains were taken and fixed with 20% Formaline Neutral Buffer solution (Wako Pure Chemicals Industries Ltd., Osaka, JAPAN). The tissues were dehydrated with ethanol and embedded in paraffin wax. Serial sections (4 μm) were cut from paraffin block and processed for the Klüver-Barrera stain as well as for hematoxylin and eosin stain.
Statistical Analysis
Results are expressed as mean ± SEM. Mean and SEM were calculated with the results from 3 independent observations of the same subject. The effects of EGF and risperidone were initially analyzed using one-way analysis of variance (ANOVA) and subsequently subjected to Fisher’s LSD test for multiple comparisons. A P value less than 0.05 was regarded as statistically significant. Statistical analysis was performed using SPSS software (Japan IBM Inc., Tokyo, Japan). N values in parentheses represent the number of observations.
Results
Three cynomolgus monkey neonates (postnatal week 2) were subcutaneously treated with EGF. During EGF treatment, the skin around the injection sites showed mild rash and abnormal stool consistency (loose stool) was occasionally observed (total 1 or 4 observation points during the treatment period). In addition, these monkey neonates sometimes showed vomiting after drinking milk (1, 6 or 7 times) although they did not show decreased body weight: 410−484 g at the beginning or 409−582 g at the end of the EGF treatment. Gross behaviors and general health of the EGF-treated monkeys (i.e. food consumption, stool consistency, skin color) were all normal until the age of four years. Two of the EGF-treated monkeys were subjected to histological examination of the brain at the 4 years of age and did not show no apparent structural abnormalities (data not shown). The remaining 1 monkey was kept alive and observed thereafter.
At the age of 6 years, the remaining 1 monkey began to display abnormal behaviors. Since we assumed that those behavioral abnormalities might be relevant to schizophrenia-like behaviors, the monkey was orally treated with an antipsychotic drug, risperidone. Before and during risperidone treatment, we recorded the monkey on video 3 times for comparison with an age- and gender-matched naive monkey (6-year old male cynomolgus monkey: hereafter, “reference monkey”) in the same room. Several behavioral parameters such as stereotypic movement, alert behaviors, vocalization, and self injury/ frenzied behavior were quantified for this comparison.
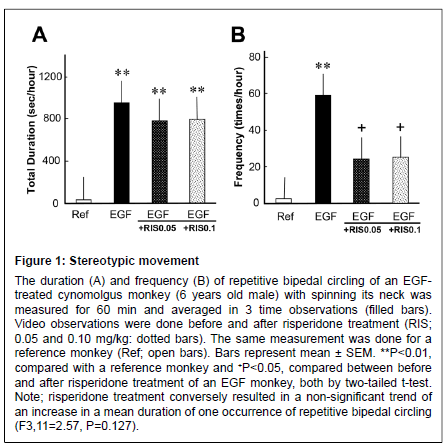
The EGF-treated monkey exhibited stereotypic, circular bipedal movement. The mean total duration and frequency of the stereotypical behavior was higher than for the reference monkey (Figure 1). The prolonged duration of stereotypical behavior was not affected by risperidone; however, its frequency was significantly suppressed (from an increased state) by risperidone treatment at 0.05 and 0.1 mg/kg, suggesting a suppressive effect of risperidone on this movement.
Figure 1: Stereotypic movement The duration (A) and frequency (B) of repetitive bipedal circling of an EGFtreated cynomolgus monkey (6 years old male) with spinning its neck was measured for 60 min and averaged in 3 time observations (filled bars). Video observations were done before and after risperidone treatment (RIS; 0.05 and 0.10 mg/kg: dotted bars). The same measurement was done for a reference monkey (Ref; open bars). Bars represent mean ± SEM. **P<0.01, compared with a reference monkey and +P<0.05, compared between before and after risperidone treatment of an EGF monkey, both by two-tailed t-test. Note; risperidone treatment conversely resulted in a non-significant trend of an increase in a mean duration of one occurrence of repetitive bipedal circling (F3,11=2.57, P=0.127).
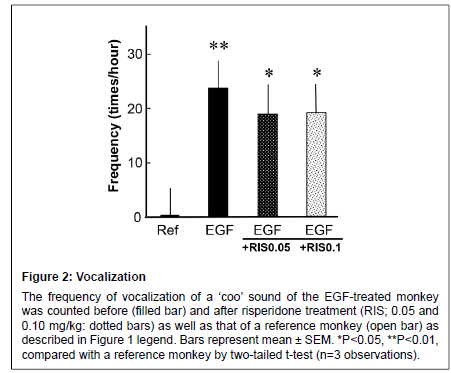
Figure 2: Vocalization
The frequency of vocalization of a ��?coo��? sound of the EGF-treated monkey was counted before (filled bar) and after risperidone treatment (RIS; 0.05 and 0.10 mg/kg: dotted bars) as well as that of a reference monkey (open bar) as described in Figure 1 legend. Bars represent mean ± SEM. *P<0.05, **P<0.01, compared with a reference monkey by two-tailed t-test (n=3 observations)
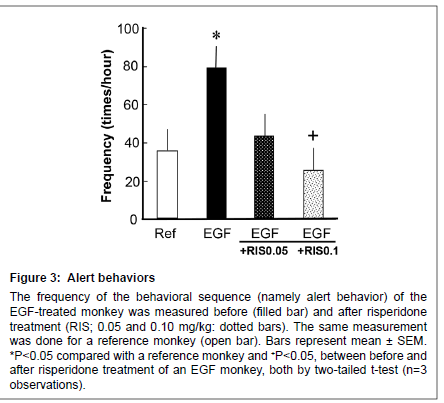
Figure 3: Alert behaviors
The frequency of the behavioral sequence (namely alert behavior) of the EGF-treated monkey was measured before (filled bar) and after risperidone treatment (RIS; 0.05 and 0.10 mg/kg: dotted bars). The same measurement was done for a reference monkey (open bar). Bars represent mean ± SEM. *P < 0.05 compared with a reference monkey and +P < 0 .05, between before and after risperidone treatment of an EGF monkey, both by two-tailed t-test (n=3 observations).
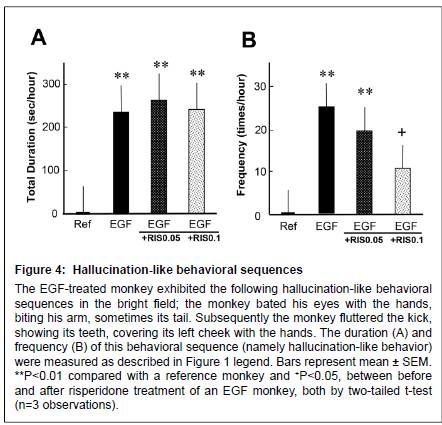
Figure 4: Alert behaviors
Hallucination-like behavioral sequences The EGF-treated monkey exhibited the following hallucination-like behavioral sequences in the bright field; the monkey bated his eyes with the hands, biting his arm, sometimes its tail. Subsequently the monkey fluttered the kick, showing its teeth, covering its left cheek with the hands. The duration (A) and frequency (B) of this behavioral sequence (namely hallucination-like behavior) were measured as described in Figure 1 legend. Bars represent mean ± SEM. **P < 0.01 compared with a reference monkey and +P < 0.05, between before and after risperidone treatment of an EGF monkey, both by two-tailed t-test (n=3 observations).
The EGF-treated monkey consistently exhibited the vocalization sign described above (“Coo” sound) while the reference monkey rarely produced such a sound (Figure 2). The risperidone treatment had no effects on the vocalization score of the EGF-treated monkey at either dose.
Both EGF-treated monkey and the reference monkey displayed alert behaviors against invisible targets (Figure 3); however, the EGFtreated monkey more often stood at the front, looking around the room (i.e. vigilance) in comparison with a reference monkey. The score for alert behavior was suppressed by 0.10 mg/kg risperidone (from an increased state) to the control level.
Following the alert behavior, the EGF-treated monkey often clapped its hands over both eyes, visually focused on the hands and bit them, and then showed frenzied behavior with leg flapping and struggling (Figure 4) (Supplemental Figure S1). The reference monkey did not display such behaviors at all. Ten-day treatment with risperidone at 0.1 mg/kg significantly reduced the frequency of the hallucinationlike behavior, but failed to affect the total duration of this behavioral sequence. It is noteworthy that the abnormal behaviors of the EGFtreated monkey were not detected by an infrared camera when the room light was turned off (data not shown). Accordingly, we assumed that the monkey was responding to illusionary stimuli, leading to the above-stated frenzied behaviors.
The EGF-treated monkey with the behavioral abnormality was subjected to histological examination of the brain at the 8 years of age. We failed to detect any histopathological alterations, including neuron loss, reactive astrocytosis, microglial proliferation and apparent structural changes in the neocortex, basal ganglia, and hippocampus (Supplemental Figure S2).
Discussion
Neonatal administration of EGF to rodents has been reported to induce several schizophrenia-associated behavioral endophenotypes [13-16]. Most of the behavioral impairments are sensitive to antipsychotics [13,26]. However, the strength and direction of the behavioral changes are markedly influenced by the genetic background of rodent strains [15]. In the present study, we tested the hypothesis that the EGF challenge influences primate behavioral traits or induces schizophrenia-related behavioral endophenotypes. With observation for a period of more than 6 years, we found the following severe and peculiar behaviors: stereotypic movement, alert behaviors, vocalization, and self injury/frenzied behavior, which had not been reported in the rodent EGF model. The present finding that these abnormal behaviors of the EGF–treated monkey occurred at the post-pubertal stage does not disaccord with the fact that psychopathologic abnormalities of schizophrenia often emerge after the adolescent stage of human, although this study needs to be replicated and data reproducibility needs to be evaluated with multiple animals.
One of the reasons why we sacrificed two EGF-treated monkeys at the age of four years was due to our wrong working hypothesis. We thought that the EGF-treated monkeys should display some behavioral deficits around the adolescent stage as the behavioral deficits of the EGF rodent models become apparent around that stage (i.e. postnatal 6-8 weeks) [11]. In human schizophrenia, patients often display several prodromes in behavioral and cognitive traits before and around the adolescence stage [27]. In this context, it was very unfortunate that we did not detect any sign of behavioral prodromes and lost two EGFtreated monkeys.
Hypothetically applying the human diagnostic criteria to these symptoms (i.e., stereotypical movement, alert behaviors, vocalization, and self injury/frenzied behavior), we assumed that the EGF-treated monkey models a non-human primate version of autism, front cortical epilepsy, or schizophrenia. When the EGF-treated monkey was exposed to an unfamiliar monkey, however, the EGF-treated monkey responded to it socially and aggressively, but did not exhibit social withdrawal or social neglect (data not shown). Thus, we considered that there was no communication deficit as would be shown in a case of autism. It was also considered unlikely that the EGF-treated monkey suffered from front cortical epilepsy, because the bouts of self-injury/frenzied behavior were fully suppressed when the room was darkened. Having excluded the other options, we diagnosed this EGF-treated monkey with a possibly schizophrenia-related disease.
Amphetamine, methamphetamine and phencyclidine are known to induce schizophrenia-like psychotic symptoms in humans and are used to produce non-human primate models for schizophrenia [28-30]. Macaque monkeys treated with amphetamine or methamphetamine display various stereotypic movements and vocalization [28-30]. These monkeys also exhibit strong alert responses for illusionary stimuli, as well as showing target-less searching and grasping air [31-33]. Castner et al. described these abnormal behaviors as “hallucination-like” [34]. Macaque monkeys challenged with phencyclidine show decreased locomotor activity [35-39] and fear/anger expressions toward observerincensed stimuli [40]. The present EGF-treated monkey appeared to exhibit a similar set of behavioral abnormalities: stereotypic movement, alert behaviors, vocalization and self injury. Therefore, the EGF-treated monkey seemed to replicate the above-stated psychostimulant-induced primate models for schizophrenia.
It is noteworthy that the self-injury and frenzied behavior started with clapping hands over both eyes and was light-sensitive, and thus presumably dependent on a perceived visual cue(s). The EGFtreated monkey behaved as if it had experienced disturbing binocular hallucinatory sensations or visual hallucinations. There has been no report describing the severe and abnormal behavior of clapping hands over eyes followed by frenzied behavior with leg flapping in macaque monkeys treated with amphetamine, methamphetamine, or phencyclidine [28-40].
However, vocalization and self injury can be triggered by maternal separation in monkeys at the infantile stage and by acute severe stress loading as well [41-43]. In this context, we do not exclude the possibility that the higher frequencies of stereotypic movement, alert behaviors, vocalization, and self injury were incidental findings in the present EGF-treated monkey or resulted from chronic cage rearing. In conclusion, we argue that this study needs to be replicated, but we hope that the present case report will stimulate interest in non-human primate modeling of schizophrenia with the use of cytokines.
Acknowledgements
We are grateful to Drs M Takada and K Nakamura of Institute of Primate Research, Kyoto University, Drs I Fujita and T Minami of Osaka University, and Dr Y Yoshikawa of Tokyo University for their comments on the behavioral profile of the EGF-treated monkey. This study was supported in part by the Grant-in-Aid for Scientific Research on Innovative Areas (Micro-endophenotypes) from MEXT, Core Research for Evolutional Science and Technology from the JST Corporation, a grant for Promotion of Niigata University Research Projects. A preliminary observation was reported in the International Schizophrenia Congress [44].
References
- Hsiao EY, Patterson PH (2012) Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol 72: 1317-1326.
- Meyer U (2013) Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 42: 20-34.
- Kunugi H, Nanko S, Takei N, Saito K, Hayashi N, et al. (1995) Schizophrenia following in utero exposure to the 1957 influenza epidemics in Japan. Am J Psychiatry 152: 450-452.
- Sham PC, O'Callaghan E, Takei N, Murray GK, Hare EH, et al. (1992) Schizophrenia following pre-natal exposure to influenza epidemics between 1939 and 1960. Br J Psychiatry 160: 461-466.
- Takei N, Lewis S, Jones P, Harvey I, Murray RM (1996) Prenatal exposure to influenza and increased cerebrospinal fluid spaces in schizophrenia. Schizophr Bull 22: 521-534.
- Barak V, Barak Y, Levine J, Nisman B, Roisman I (1995) Changes in interleukin-1 beta and soluble interleukin-2 receptor levels in CSF and serum of schizophrenic patients. J Basic Clin Physiol Pharmacol 6: 61-69.
- Katila H, Appelberg B, Hurme M, Rimón R (1994) Plasma levels of interleukin-1 beta and interleukin-6 in schizophrenia, other psychoses, and affective disorders. Schizophr Res 12: 29-34.
- Monteleone P, Fabrazzo M, Tortorella A, Maj M (1997) Plasma levels of interleukin-6 and tumor necrosis factor alpha in chronic schizophrenia: effects of clozapine treatment. Psychiatry Res 71: 11-17.
- Nawa H, Takahashi M, Patterson PH (2000) Cytokine and growth factor involvement in schizophrenia--support for the developmental model. Mol Psychiatry 5: 594-603.
- Iwakura Y, Zheng Y, Sibilia M, Abe Y, Piao YS, et al. (2011) Qualitative and quantitative re-evaluation of epidermal growth factor-ErbB1 action on developing midbrain dopaminergic neurons in vivo and in vitro: target-derived neurotrophic signaling (Part 1). J Neurochem 118: 45-56.
- Futamura T, Kakita A, Tohmi M, Sotoyama H, Takahashi H, et al. (2003) Neonatal perturbation of neurotrophic signaling results in abnormal sensorimotor gating and social interaction in adults: implication for epidermal growth factor in cognitive development. Mol Psychiatry 8: 19-29.
- Ikeda Y, Yahata N, Ito I, Nagano M, Toyota T, et al. (2008) Low serum levels of brain-derived neurotrophic factor and epidermal growth factor in patients with chronic schizophrenia. Schizophr Res 101: 58-66.
- Futamura T, Toyooka K, Iritani S, Niizato K, Nakamura R, et al. (2002) Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol Psychiatry 7: 673-682.
- Mizuno M, Sotoyama H, Narita E, Kawamura H, Namba H, et al. (2007) A cyclooxygenase-2 inhibitor ameliorates behavioral impairments induced by striatal administration of epidermal growth factor. J Neurosci 27: 10116-10127.
- Tohmi M, Tsuda N, Mizuno M, Takei N, Frankland PW, et al. (2005) Distinct influences of neonatal epidermal growth factor challenge on adult neurobehavioral traits in four mouse strains. Behav Genet 35: 615-629.
- Mizuno M, Malta RS Jr, Nagano T, Nawa H (2004) Conditioned place preference and locomotor sensitization after repeated administration of cocaine or methamphetamine in rats treated with epidermal growth factor during the neonatal period. Ann N Y Acad Sci 1025: 612-618.
- Kawahara N, Mishima K, Higashiyama S, Taniguchi N, Tamura A, et al. (1999) The gene for heparin-binding epidermal growth factor-like growth factor is stress-inducible: its role in cerebral ischemia. J Cereb Blood Flow Metab 19: 307-320.
- Jin K, Mao XO, Sun Y, Xie L, Jin L, et al. (2002) Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci 22: 5365-5373.
- Johnston JB, McFadden G (2003) Poxvirus immunomodulatory strategies: current perspectives. J Virol 77: 6093-6100.
- Zhang B, Srirangam A, Potter DA, Roman A (2005) HPV16 E5 protein disrupts the c-Cbl-EGFR interaction and EGFR ubiquitination in human foreskin keratinocytes. Oncogene 24: 2585-2588.
- Bauman MD1, Iosif AM2, Smith SE3, Bregere C3, Amaral DG4, et al. (2014) Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry 75: 332-341.
- Gil-da-Costa R, Stoner GR, Fung R, Albright TD (2013) Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proc Natl Acad Sci U S A 110: 15425-15430.
- Beauregard M, Bachevalier J (1996) Neonatal insult to the hippocampal region and schizophrenia: a review and a putative animal model. Can J Psychiatry 41: 446-456.
- Willette AA, Lubach GR, Knickmeyer RC, Short SJ, Styner M, et al. (2011) Brain enlargement and increased behavioral and cytokine reactivity in infant monkeys following acute prenatal endotoxemia. Behav Brain Res 219: 108-115.
- Guiding principles for animal experiments using nonhuman primates (in Japanese), Primate Res. 2 (1986), pp. 111-113. Primate Society of Japan.
- Sotoyama H, Zheng Y, Iwakura Y, Mizuno M, Aizawa M, et al. Pallidal hyperdopaminergic innervation underlying D2 receptor-dependent behavioral deficits in the schizophrenia animal model established by EGF. PLoS One 6: e25831.
- Olsen KA, Rosenbaum B (2006) Prospective investigations of the prodromal state of schizophrenia: review of studies. Acta Psychiatr Scand 113: 247-272.
- Ridley RM, Baker HF, Owen F, Cross AJ, Crow TJ (1982) Behavioural and biochemical effects of chronic amphetamine treatment in the vervet monkey. Psychopharmacology (Berl) 78: 245-251.
- Machiyama Y (1992) Chronic methamphetamine intoxication model of schizophrenia in animals. Schizophr Bull 18: 107-113.
- Sams-Dodd F, Newman JD (1997) Effects of administration regime on the psychotomimetic properties of d-amphetamine in the squirrel monkey (Saimiri sciureus). Pharmacol Biochem Behav 56: 471-480.
- Castner SA, Goldman-Rakic PS (1999) Long-lasting psychotomimetic consequences of repeated low-dose amphetamine exposure in rhesus monkeys. Neuropsychopharmacology 20: 10-28.
- Ellinwood EH Jr, Sudilovsky A, Nelson LM (1973) Evolving behavior in the clinical and experimental amphetamine (model) psychosis. Am J Psychiatry 130: 1088-1093.
- Snyder SH (1973) Amphetamine psychosis: a "model" schizophrenia mediated by catecholamines. Am J Psychiatry 130: 61-67.
- Castner SA, Goldman-Rakic PS (2003) Amphetamine sensitization of hallucinatory-like behaviors is dependent on prefrontal cortex in nonhuman primates. Biol Psychiatry 54: 105-110.
- Liddle PF, Friston KJ, Frith CD, Frackowiak RS (1992) Cerebral blood flow and mental processes in schizophrenia. J R Soc Med 85: 224-227.
- Linn GS, O'Keeffe RT, Lifshitz K, Schroeder C, Javitt DC (2007) Behavioral effects of orally administered glycine in socially housed monkeys chronically treated with phencyclidine. Psychopharmacology (Berl) 192: 27-38.
- Mao CV, Hori E, Maior RS, Ono T, Nishijo H (2008) A primate model of schizophrenia using chronic PCP treatment. Rev Neurosci 19: 83-89.
- Linn GS, O'Keeffe RT, Schroeder CE, Lifshitz K, Javitt DC (1999) Behavioral effects of chronic phencyclidine in monkeys. Neuroreport 10: 2789-2793.
- Linn GS, Negi SS, Gerum SV, Javitt DC (2003) Reversal of phencyclidine-induced prepulse inhibition deficits by clozapine in monkeys. Psychopharmacology (Berl) 169: 234-239.
- Jentsch JD, Roth RH, Taylor JR (2000) Object retrieval/detour deficits in monkeys produced by prior subchronic phencyclidine administration: evidence for cognitive impulsivity. Biol Psychiatry 48: 415-424.
- Lewis JK, McKinney WT, Young LD, Kraemer GW (1976) Mother-infant separation in rhesus monkeys as a model of human depression. A reconsideration. Arch Gen Psychiatry 33: 699-705.
- Minami T (1996) Locomotive stereotyped behavior in cynomolgus macaques, Macaca fascicularis. Percept Mot Skills 83: 935-938.
- Porsolt RD, Roux S, Jalfre M (1984) Effects of imipramine on separation-induced vocalizations in young rhesus monkeys. Pharmacol Biochem Behav 20: 979-981.
- Nawa H, Sakai M, Someya T (2009) A novel schizophrenia model established by injecting an inflammatory cytokine to cynomolgus monkey neonate. Schizo Bull 35: S252.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 15338
- [From(publication date):
February-2014 - Jul 17, 2024] - Breakdown by view type
- HTML page views : 10896
- PDF downloads : 4442