Research Article Open Access
An Assessment of Seaweeds Diversity and Distribution at the Beach of Nathia Gali, Karachi, Pakistan
Rashida Qari*Institute of Marine Sciences, University of Karachi, Karachi, Pakistan
- *Corresponding Author:
- Rashida Qari
Director Institute of Marine Sciences,
University of Karachi, Karachi, 75270, Pakistan
Tel: 99261300-2378
E-mail: rqari2002@yahoo.com
Received date: April 27, 2017; Accepted date: May 13, 2017; Published date: May 20, 2017
Citation: Qari R (2017) An Assessment of Seaweeds Diversity and Distribution at the Beach of Nathia Gali, Karachi, Pakistan. J Marine Sci Res Dev 7: 228. doi: 10.4172/2155-9910.1000228
Copyright: © 2017 Qari R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
The seaweeds resources were sampled from exposed shore of Nathia Gali beach, Karachi coast at low tide. The present data of diversity and biomass of seaweeds provide a comparison of two different periods i.e., from 1989 to 1991 and 2012. A total of 60 taxa were recorded in the coastal area of Nathia Gali; 14 belonging to chlorophyta, 15 to Phaeophyta and 31 to Rhodophyta. The green seaweeds viz, Caulerpa racemosa, Codium iyengarii and Halimeda tuna; brown seaweeds, Cystoseira indica, Iyengaria stellata Padina pavonica and Sargassum boveanum and red seaweeds Gracilaria corticata and Scinaia fascicularis were found to be the dominant species of Nathia Gali. The total biomass 66056.18 g. m-2 was recorded during the study period and the highest biomass was recorded in January (16551.79 g m-2) and the lowest in July (1276.3 g. m-2). In winters, seaweeds biomass and growth increased due to low temperature of air and seawater with low intensity of light and high dissolved nutrients. The richness of seaweed resources in the studied area is due to the intertidal rocks available on the coast but nowadays, there is lot of disturbance due to anthropogenic activities such as discharge of domestic and industrial waste; tanneries effluents; rainfall and associated pollutant from runoff; shipping and agricultural sources. These disturbances play vital role in changing the ecosystem which effects flora and fauna of the coast.
Keywords
Diversity; Distribution; Seaweeds; Nathia Gali; Biomass; Community
Introduction
The floral components are represented by three groups of primary producers, the mangroves, sea grasses and the seaweed [1]. The macrobenthic algae are an important component of the marine ecosystem. They exhibit an enormous variety in size, color, form, and lifestyle and they are extremely rich in the sea. According to a very rough estimate there are more than 17,000 species of sea plants and 95% of them are algae [2]. To date, several investigations for the exploitation of seaweed resources in developed countries have been made about biomass and standing crop. More recently Faveri et al. [3] studied the temporal changes in the seaweeds flora in Southern Brazil and its potential causes. Hay and Villout [4] studied the seasonality of Undaria pinnatifida population in New Zealand waters. Jagtap [5] described the marine flora of Nicobar group of islands in Andaman Sea. Ballesteros [6] studied seasonal growth and production of deep-water population of Halimeda tuna in the Northwestern Mediterranean. Pedrini et al. [7] reported the marine algae of Trindade Island, Brazil. Basson et al. [8] also reported the benthic flora of Bahrain. Chock and Mathieson [9] quantified the standing crop and biomass variation of estuarine seaweed at Cedar point, Dover New Hampshire Maine, UK. Similar studies have also been conducted in other areas of the world such as, North Carolina [10], India [11], Dodger channel of Canada [12] and Central Puget Sound Washington, U.S.A. [13]. Josselyn and Mathieson [14] have reported the biomass variation of selected estuarine macrophytes at North temperate estuary, Falmouth Massachusetts, USA.
The seaweed resources are abundant around the rocky shores of Karachi. Many studies have been made on the standing crop and biomass of seaweeds like Shameel [15] served seaweed of from the coast of Lasbella, Pakistan. Saleem [16] and Saifullah [17] studied the distribution and biomass of marine algae along Karachi. Qari and Qasim [18,19] investigated the Seasonal change in the standing crop of intertidal seaweeds from the Buleji and Manora coast of Karachi. Recently Qari [20] studied Phytomass on natural bed of seaweed at Paradise Point, Karachi coast.
Pakistan is a developing country; the marine resources like seaweeds are under threat due to variety of pollutants. There are above 6,000 industries located in Karachi at different sites. According to an estimate all these industries contribute approximately 99% of the total industrial pollution beside the contamination from oil waste discharge [21,22]. Increasing concern on wrecking of floral resources deemed it necessary to study the seaweeds diversity and abundance for a better management of marine flora. At Nathia Gali of Karachi coast no any information on diversity of seaweeds although this coast has very luxuriant seaweed vegetation, therefore the present investigation deals with the seaweeds biomass, distribution, diversity of seaweeds species (Chlorophyta, Phaeophyta and Rhodophyta) of Nathia Gali coast Karachi, northern Arabian Sea.
Materials and Methods
The investigation was carried out at Nathia Gali that presents a very scenic point also known as Pacha, is situated at 24o50´ N, 66o 42.5´ E on the south western coast of Arabian Sea. It is not easily assessable; as a result it is still consist of undisturbed forms of flora and fauna. The jutting rocks lessen the force of the waves thus protecting the backwater beach and along with it the existing life. The beach starts with pale yellow coloured rocks at its front, the main boulder like forms, is exposed to the surface and slopes steep towards the sea. The sides of these rock formations are thickly covered with the biota especially rich in seaweed.
When the tide is low the back area is fully exposed while at high tides even the facing rock zone gets submerged with seawater.
Field surveys were undertaken to the selected sampling beach (Nathia Gali) of the Karachi coast over a period of four years from 1989 to 1991 and 2012 for the comparison of two data of different period. The seasonal distribution of seaweeds was studied by a wooden quadrate frame of one-meter square [23]. Each month fifty quadrates (ten feet apart) from low tidal mark to high tidal mark were randomly sampled in the intertidal belt. Phytomass falling inside each quadrate were harvested and studied for the composition and biomass. All seaweeds placed individually in prelabeled plastic bags and returned to the laboratory.
All collected seaweeds samples were carefully cleaned from mud debris and epiphytes, using seawater. For identification, the materials were sorted out on the basis of Anand [24,25], Round [26], Chapman and Chapman [27], Dawes [28], Morris [29], Dring [30]. The total and the differential biomass (wet weight) of each species was estimated after carefully scraping them and weighed with a fine degree of accuracy (0.001 g) and subsequently the biomass of each species was calculated and expressed in gram fresh weight per meter square (g. m-2). The average values of fifty quadrates were pooled and are used for comparing the condition in different seasons. The Shannon – Weiner diversity index of each monthly sample was calculated by using the formula on Excel program. Species richness and species evenness also calculated.
Diversity=H’ = - Σ pi. Ln pi
Species richness = S’ = S / √ N
Evenness = J = H’ / H max
Results and Discussion
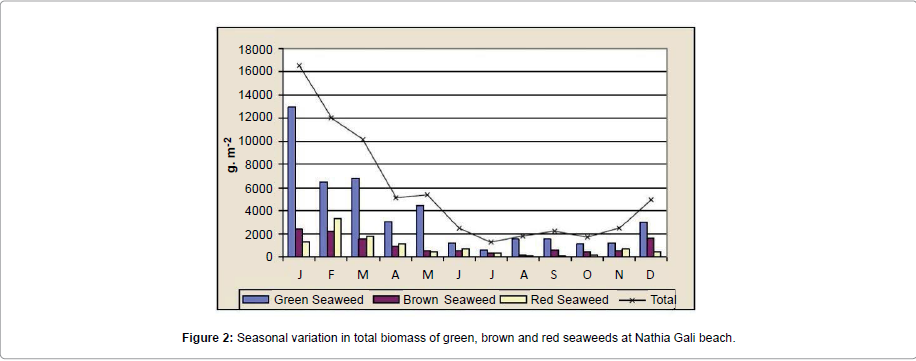
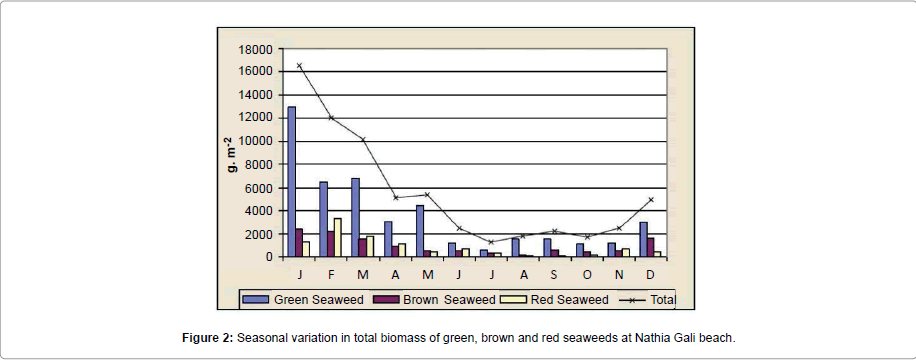
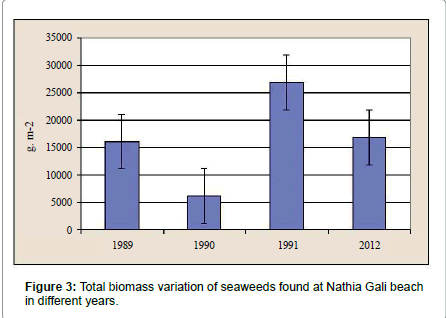
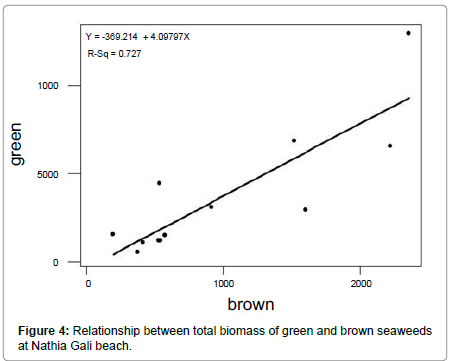
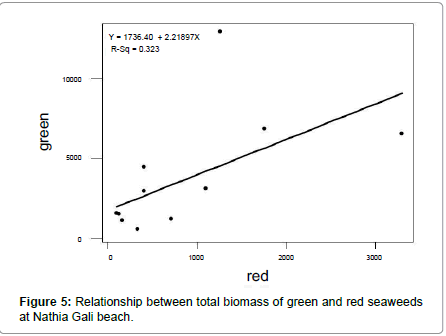
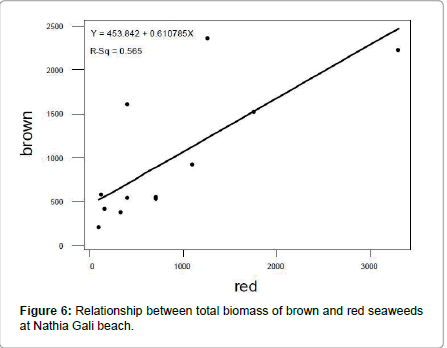
The rocky beach of Nathia Gali is very rich in seaweed. A total of sixty species of seaweeds (Fourteen green, fifteen brown and thirtyone red) were identified over the period of four years (January 1989 to December 1991 and 2012) (Tables 1-3). The total biomass 66056.18 g. m-2 was recorded during the study period and the highest biomass was recorded in January (16551.79 g. m-2) and the lowest (1276.3 g. m-2) was recorded in July (Figure 1). Figure 2 showed the highest contribution to the total biomass was observed by green seaweed 66.43 % followed by brown (17.85 %) and red (15.72 %). The highest total biomass value was recorded in the year 1991 (26921.1 g m-2) as compared to the values of other years of study period i.e., (1989: 16108.12 g. m-2, 1990: 6129.18 g. m-2 and 2012: 16897.78 g. m-2) (Figure 3). The greatest contribution of green seaweed was found in January (12929.67 g. m-2) whereas the lowest in July (566.73 g. m-2) (Figure 1). Green seaweeds were more abundant in the year 1989 (13822.96 g m-2) than the other three years 1990 (3587.64 g. m-2), 1991 (14331.01 g. m-2) and 2012 (12139.96 g. m-2) (Table 1 and Figure 2). The highest contribution of brown seaweed occurred in January (2360.66g. m-2) whereas the lowest in August from Nathia Gali (195.79 g. m-2) (Figure 1). The maximum biomass of brown year 1991 (Table 2 and Figure 2). The highest seaweed (4940.69 g. m-2) was observed in the contribution of total biomass of red seaweed occurred in February (3306.81 g. m-2) and the lowest contribution of total biomass of red seaweed was collected in August (96.38 g. m-2) (Figure 1). The maximum biomass of red seaweed (7649.4 g. m-2) was observed in the year 1991 (Table 3 and Figure 2). Seasonal variations in number of seaweed species collected at Nathia Gali of Karachi coast is given in Table 4. During the whole study period, all studied sixty seaweed species were collected 746 times. The highest number of seaweed species was recorded in March (101) and the lowest number of species (27) was recorded in August (Table 4). The highest contribution to the total number of seaweed species were by red seaweed (42.49 %) in comparison to brown (32.71 %) and green (24.8 %) seaweed. This indicates that red seaweed showed greater species diversity at Nathia Gali coast. There was positive significant corelationship was found in between total biomass of green and brown seaweeds (r2=0.852), green and red seaweeds (r2=0.568) and brown and red seaweeds (r2=0.751) (Figures 4-6). The completely randomized design with nested treatments analysis of variance (ANOVA) model was used to test the significant differences of biomass in seaweed between years and months. The results show that there were high significant variations were observed between years and months (F=12.52 and F=4.42 for years and months, respectively) in the total biomass of seaweeds. Significant variations were also observed between years and months in total biomass of green seaweeds (F=3.71 and F=5.69 for years and months, respectively) and brown seaweeds (F=6.77 and F=4.32 for years and months, respectively) but significant variations were observed only in between years for the total biomass of red seaweed (F=6.06) (Table 5).
| S. No. | Name of species | Years | J | F | M | A | M | J | J | A | S | O | N | D | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bryopsisharveyana | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | 5.34 | _* | _* | _ | 5.34 |
| 1990 | _ | _ | _ | _* | _* | _ | 1.65 | _* | _* | _ | _ | _ | 1.65 | ||
| 1991 | _ | _ | _ | _ | _ | _ | _ | 0.82 | 5.41 | _* | _ | _ | 3.12 | ||
| 2012 | _ | _ | _ | 2.45 | _ | _* | _ | _ | _ | 2.13 | _* | _ | 2.29 | ||
| 2 | Caulerpamanorensis | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | 0.33 | _ | 0.33 | ||
| 1991 | _ | _ | _ | _ | _ | _ | _ | _ | _* | _ | _ | _ | |||
| 2012 | _ | _ | _ | _ | _ | _* | _ | _ | 0.55 | 0.76 | _* | _ | 0.66 | ||
| 3 | C. peltata | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | 11.65 | 11.65 |
| 1990 | _ | _ | 32.58 | _* | _* | _ | _ | _* | _* | _ | _ | _ | 32.58 | ||
| 1991 | _ | _ | 33.13 | 35 | _ | _ | _ | _ | _ | _* | 40 | _ | 36.04 | ||
| 2012 | _ | 31.88 | 26.69 | _ | 24.6 | _* | _ | 21 | _* | 26.03 | |||||
| 4 | C. racemosa | 1989 | 33.61 | 64.92 | _ | 11.57 | 0.85 | _ | _ | _* | 2.96 | _* | _* | 58.24 | 28.7 |
| 1990 | 225.2 | 224.32 | 105.21 | _* | _* | _ | _ | _* | _* | 22.96 | _ | _ | 144.4 | ||
| 1991 | 200 | 63.54 | 140 | 210 | _ | _ | _ | _ | _ | _* | 11.11 | 37 | 110.28 | ||
| 2012 | 87.8 | 76.6 | 65.44 | _ | 71 | _* | _ | 104 | 66.5 | 76 | _* | _ | 78.19 | ||
| 5 | C. scalpelliformis | 1989 | 20 | 28.7 | _ | 14.4 | 19 | _ | _ | _* | _ | _* | _* | 1.37 | 16.7 |
| 1990 | 65.67 | 14.22 | 17.68 | _* | _* | 9.65 | _* | _* | 7.81 | 11.41 | _ | 21.1 | |||
| 1991 | 74.43 | 19.3 | 20 | 60.6 | _ | 9 | _ | _ | _ | _* | _ | _ | 36.7 | ||
| 2012 | 61 | 22.56 | 34 | _ | 32 | _* | _ | _ | 32 | _ | _* | _ | 36.31 | ||
| 6 | C. taxifolia | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | 0.53 | _ | 0.53 | ||
| 1991 | _ | _ | _ | _ | _ | _ | _ | _ | _ | _* | _ | _ | _ | ||
| 2012 | _ | _ | _ | 0.44 | 0.45 | _* | _ | _ | _ | _ | _* | _ | 0.45 | ||
| 7 | Codiumiyengarii | 1989 | 5511 | 1341.4 | 2320 | 1630.8 | 1910 | 91.45 | _* | _* | _ | _* | _* | 650.8 | 1922.15 |
| 1990 | 380.1 | 739.15 | 832.85 | _* | _* | 192.2 | _ | _* | _* | 44.96 | 50.12 | _ | 373.23 | ||
| 1991 | 4327 | 1280 | 1250 | 1100 | 740 | 780 | _ | _ | _ | _* | 840 | 710 | 1378.33 | ||
| 2012 | 890 | 1032 | 911 | _ | 1320 | _* | _ | 875 | 1000 | 780 | _* | 1412 | 1027.5 | ||
| 8 | C. shameelii | 1989 | _ | _ | 163.2 | _ | _ | _ | _* | _* | _ | _* | _* | 216 | 189.6 |
| 1990 | 75.92 | 279.02 | 16.07 | _* | _* | _ | _ | _* | _* | _ | 172.2 | _ | 135.81 | ||
| 1991 | _ | 385 | 425 | _ | _ | _ | _ | _ | _ | _* | _ | 270 | 360 | ||
| 2012 | 340 | 112.6 | 178 | _ | 45 | _* | __ | _ | _ | _ | _* | 312 | 197.52 | ||
| 9 | Enteromorpha | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | 62.4 | _* | _* | _ | 62.4 |
| compressa | 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | |
| 1991 | _ | _ | _ | _ | _ | _ | 73.5 | 74.4 | 61.13 | _* | 64 | 16 | 57.81 | ||
| 2012 | _ | _ | _ | _ | _ | _* | 432 | 412.8 | 155 | 121.54 | _* | 64 | 237.07 | ||
| 10 | Halimeda tuna | 1989 | 5.3 | 1.5 | 9.1 | _ | 8 | 1.98 | _* | _* | _ | _* | _* | _ | 5.2 |
| 1990 | _ | _ | 3.03 | _* | _* | 6 | 14.63 | _* | _* | 24.38 | _ | _ | 12.01 | ||
| 1991 | 8 | 1.93 | 9.86 | 9.47 | 7.7 | 6.23 | 13.23 | _ | 2.81 | _* | 2.25 | _ | 6.83 | ||
| 2012 | 23 | 44.6 | 32 | 8.77 | _ | _* | _ | 33.42 | 13 | _ | _* | 11.6 | 23.77 | ||
| 11 | Udoteaindica | 1989 | _ | _ | _ | _ | _ | 9.22 | _* | _* | _ | _* | _* | _ | 9.22 |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | 1.56 | _ | _ | 1.56 | ||
| 1991 | _ | _ | _ | 1.17 | _ | 6.32 | 8.72 | _ | _ | _* | _ | _ | 5.4 | ||
| 2012 | _ | 1.23 | _ | 2.33 | _ | _* | _ | 6.75 | 5.87 | _ | _* | 5 | 4.24 | ||
| 12 | Ulvafasciata | 1989 | 3.3 | 360.2 | 63.6 | _ | _ | _ | _* | _* | 34.77 | _* | _ | 0.35 | 92.44 |
| 1990 | 3.28 | 10.26 | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | 6.77 | ||
| 1991 | _ | 52.5 | 165 | _ | _ | _ | _ | _ | _ | _* | _ | _ | 108.75 | ||
| 2012 | 5.87 | 9.56 | 12.76 | 9 | _* | 12.87 | 11 | _* | 11 | 10.29 | |||||
| 13 | U. lactuca | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | 2.57 | _ | 2.57 | ||
| 1991 | _ | _ | _ | _ | _ | _ | _ | _ | _ | _* | 22.14 | _ | 22.14 | ||
| 2012 | 1.42 | _ | _ | _ | _ | _* | _ | 11 | 13.6 | _ | _* | 11 | 9.26 | ||
| 14 | Valoniopsis | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ |
| pachynema | 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | 0.1 | _ | _ | 0.1 | |
| 1991 | 420 | 153.2 | _ | _ | _ | 63.2 | 23 | _ | _ | _* | _ | _ | 164.85 | ||
| 2012 | 168.8 | 171 | _ | _ | 231 | _* | _ | _ | 39.8 | _ | _* | _ | 152.64 |
Table 1: Seasonal variation in biomass (g m-2) of green seaweeds at Nathia Gali beach of Karachi.
| S. No. | Name of species | Year | J | F | M | A | M | J | J | A | S | O | N | D | Mean |
| 1 | Colpomeniasinuosa | 1989 | 113.14 | 100 | 81.04 | _ | _ | _ | _* | _* | _ | _* | _* | 38.35 | 83.13 |
| 1990 | 41.07 | 156 | 7.95 | _* | _* | _ | _ | _* | _* | 4.8 | _ | _ | 52.56 | ||
| 1991 | 123.8 | 172 | 90.18 | _ | _ | _ | _ | _ | _* | _ | _ | 128.6 | |||
| 2012 | 97.6 | 112 | 104.8 | _ | _ | _* | _ | 26 | _ | 15 | _* | _ | 71.08 | ||
| 2 | Cystoseiraindica | 1989 | 5 | _ | 4.3 | 26 | _ | _ | _* | _* | 10.13 | _* | _* | _ | 11.36 |
| 1990 | 0.25 | _ | 4.01 | _* | _* | _ | _ | _* | _* | 22 | 10.4 | _ | 9.28 | ||
| 1991 | 6.5 | 38 | 58.58 | 53.9 | _ | 37.17 | _ | 13.67 | _ | _* | _ | _ | 34.67 | ||
| 2012 | 22 | 15 | _ | 28 | _ | _* | 21.5 | _ | 11.6 | _ | _* | _ | 19.58 | ||
| 3 | Dictyotadichotoma | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _ | _ | _ |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | 72 | 11.22 | _ | 41.83 | ||
| 1991 | 24.43 | 52 | _ | _ | _ | _ | _ | _ | 20.84 | _* | _ | 7.3 | 26.07 | ||
| 2012 | 2.11 | _ | 4.2 | _ | _ | _* | 4.43 | 3.22 | _ | _ | _* | _ | 3.49 | ||
| 4 | D. hauckiana | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | ||
| 1991 | _ | _ | _ | _ | _ | _ | _ | _ | _ | _* | 16 | _ | 16 | ||
| 2012 | 3 | 7.2 | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | 5.1 | ||
| 5 | Iyengariastellata | 1989 | 26.67 | 23 | 14.96 | _ | _ | _ | _* | _* | 110 | _* | _* | 102.4 | 55.45 |
| 1990 | 96 | 133 | 12.36 | _* | _* | _ | _ | _* | _* | 117 | _ | _ | 89.62 | ||
| 1991 | 88.32 | 250 | 172.7 | 126.7 | 90 | _ | _ | _ | 150 | _* | 188.85 | 425 | 186.45 | ||
| 2012 | 76.45 | 112 | 131.6 | 94.5 | 22 | _* | _ | _ | _ | _ | _* | 122 | 93.09 | ||
| 6 | Jolynalaminarioides | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | ||
| 1991 | _ | _ | _ | _ | _ | _ | _ | _ | 21.24 | _* | _ | _ | 21.24 | ||
| 2012 | _ | _ | _ | 16 | 25.11 | _* | _ | _ | _ | _ | _* | 24.6 | 21.9 | ||
| 7 | Lobophora | 1989 | _ | _ | 1.32 | _ | _ | _ | _* | _* | _* | _* | _ | 1.32 | |
| variegata | 1990 | 0.32 | _ | _ | _* | _* | _ | _ | _* | _* | 1.7 | _ | _ | 1.02 | |
| 1991 | _ | _ | 4.87 | 12.66 | _ | 9.5 | _ | _ | _ | _* | 0.8 | _ | 6.96 | ||
| 2012 | _ | 6.6 | 11.7 | _* | 7.21 | 3.4 | 3.2 | _* | _ | 6.42 | |||||
| 8 | Padinapavonica | 1989 | 1.8 | 1.4 | 15.46 | 55.3 | 2.08 | _ | _* | _* | 19.94 | _* | _* | 2.22 | 14.03 |
| 1990 | _ | 4.6 | 8.2 | _* | _* | _ | 44.7 | _* | _* | 16 | 30.82 | _ | 20.9 | ||
| 1991 | 6.54 | 18 | 25.05 | _ | 25 | 29.9 | 4.2 | _ | 14.81 | _* | 5.2 | 0.35 | 14.31 |
Table 2: Seasonal variation in biomass (g m-2) of brown seaweeds at Nathia Gali beach of Karachi.
| 1 | Botryocladia | 1989 | _ | _ | _ | 7 | 3.92 | _ | _* | _* | 5.34 | _* | _* | _ | 5.5 | |
| leptopoda | 1990 | 5.8 | 1.42 | 75.12 | _* | _* | 41 | _ | _* | _* | _ | 69 | _ | 38.47 | ||
| 1991 | _ | 3.5 | 18 | 22 | 18 | 38.7 | _ | _ | _ | _* | 120.5 | _ | 36.86 | |||
| 2012 | 6.1 | 1.4 | 33.46 | _ | _ | _* | _ | _ | _ | _ | _* | 56.7 | 24.42 | |||
| 2 | Calliblepharis | 1989 | _ | _ | _ | 3 | _ | _ | _* | _* | _ | _* | _* | _ | 2.74 | |
| fimbriata | 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | ||
| 1991 | _ | 0.6 | _ | _ | _ | _* | _ | _ | 0.6 | |||||||
| 2012 | _ | _ | 4.45 | _ | 3.78 | _* | _ | _ | _ | 4 | _* | _ | 4.08 | |||
| 3 | Champiacompressa | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| 1990 | _ | _ | 11.9 | _* | _* | _ | _ | _* | _* | _ | _ | _ | 11.9 | |||
| 1991 | 27 | 16 | _ | _ | _ | _ | _ | _ | _ | _* | 0.92 | _ | 8.46 | |||
| 2012 | 18 | 20.2 | 16.87 | _ | _ | _* | _ | _ | _ | 4.65 | _* | 14.5 | 14.74 | |||
| 4 | C. plumosa | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | |||
| 1991 | _ | 4 | 9.17 | _ | _ | _ | _ | _ | _ | _* | _ | _ | 6.6 | |||
| 2012 | 5.7 | 3. 8 | 4 | _ | _ | _* | _ | _ | _ | _ | _* | 8 | 5.9 | |||
| 5 | C. salicornoides | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| 1990 | _ | 13.6 | 11.84 | _* | _* | _ | _ | _* | _* | _ | _ | _ | 12.72 | |||
| 1991 | _ | _ | _ | _ | _ | _ | _ | _ | _ | _* | _ | _ | _ | |||
| 2012 | _ | 10.3 | _ | _ | _ | _* | _ | _ | _ | _ | _* | 11.5 | 10.9 | |||
| 6 | Coelarthrum | 1989 | _ | _ | _ | ## | _ | _ | _* | _* | _ | _* | _* | _ | 168 | |
| murelli | 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | ||
| 1991 | _ | _ | 70.14 | ## | 48.5 | 49.6 | _ | _ | _ | _* | 26.75 | _ | 69.8 | |||
| 2012 | 38 | 57 | _ | _ | _ | _* | _ | _ | _ | _ | _* | 21.9 | 38.9 | |||
| 7 | Cystocloniumpurpureum | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| 1990 | 2.9 | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | 2.86 | |||
| 1991 | 14 | _ | _ | _ | _ | _ | _ | _ | _ | _* | 28.78 | _ | 21.46 | |||
| 2012 | 12 | 17 | 8 | _ | _ | _ | _* | _ | _ | _ | 4 | _* | 2.5 | 6 | ||
| 8 | Gelidiumpusillum | 1989 | 7.4 | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | 7.44 | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | |||
| 1991 | _ | _ | _ | _ | _ | 18.5 | _ | _ | _ | _* | _ | _ | 18.5 | |||
| 2012 | 15 | _ | _ | _ | _ | _* | _ | _ | _ | _ | _* | 2.5 | 8.55 | |||
| 9 | G. usmanghanii | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | 11.3 | 11.25 | |
| 1990 | 3.2 | _ | _ | _* | _* | _ | _* | _* | _ | _ | _ | 3.2 | ||||
| 1991 | _ | _ | _ | _ | _ | _ | 2.57 | 0.23 | _ | _* | _ | _ | 1.4 | |||
| 2012 | 4 | _ | _ | _ | _ | _* | _ | _ | _ | 2.12 | _* | 2 | 2.71 | |||
| 10 | Gracilariacorticata | 1989 | 13 | _ | _ | _ | 20 | _ | _* | _* | 13.5 | _* | _* | _ | 18.97 | |
| 1990 | 4.6 | 3.51 | _* | _* | 16.1 | 31.84 | _* | _* | _ | 7 | _ | 12.61 | ||||
| 1991 | 14 | 21.41 | 25.56 | 57 | _ | 40.8 | 32.15 | _ | _ | _* | 34.76 | 19.9 | 30.67 | |||
| 2012 | 17 | 12.5 | 11 | _ | _ | _* | 13.56 | _ | _ | 11.5 | _* | 10 | 12.59 | |||
| 11 | G. follifera | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | |||
| 1991 | 7.8 | _ | 20.1 | 60 | _ | 50.1 | _ | _ | _ | _* | _ | 23.82 | 32.4 | |||
| 2012 | _ | _ | _ | _ | _ | _* | _ | _ | _ | 3.5 | _* | _ | 3.5 | |||
| 12 | G. pygmea | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | 8.33 | _* | _* | _ | 8.33 | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | 6.6 | _ | 6.6 | |||
| 1991 | 48 | 46 | 40.8 | _ | _ | 25.9 | 40 | 26.8 | _ | _* | 5.86 | _ | 33.4 | |||
| 2012 | 4.5 | 3.8 | 6 | 11 | _ | _* | 12.6 | _ | _ | _ | _* | 4 | 6.98 | |||
| 13 | G. varrucosa | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | ||||
| 1991 | 65 | _ | 19.2 | _ | _ | 28.5 | _ | _ | _ | _* | 29.36 | _ | 35.6 | |||
| 2012 | _ | _ | _ | _ | _ | _* | _ | _ | _ | _ | _* | _ | _ | |||
| 14 | Halymenia | 1989 | _ | _ | _ | 23 | _ | _ | _* | _* | _ | _* | _* | _ | 23 | |
| porphyraeformis | 1990 | 24 | 100 | 52.8 | _* | _* | _ | _ | _* | _* | _ | 1.2 | _ | 44.6 | ||
| 1991 | _ | _ | 29.1 | 25 | 20 | 12.9 | _ | _ | _ | _* | 5.91 | _ | 18.5 | |||
| 2012 | 13 | 24 | 17.8 | 13 | 3 | _* | _ | _ | _ | 7 | _* | 4 | 11.6 | |||
| 15 | Hypneamusciformis | 1989 | _ | 8 | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | 8 | |
| 1990 | _ | _ | 2.1 | _* | _* | 2.3 | _ | _* | _* | _ | 17.95 | _ | 7.45 | |||
| 1991 | 290 | 245 | 169 | 80 | 34 | 10 | _ | _ | 0.78 | _* | _ | 40.11 | 109 | |||
| 2012 | 3.6 | 8.5 | _ | _ | 13 | _* | _ | _ | _ | 1.6 | _* | 7 | 6.74 | |||
| 16 | H. pannosa | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | |||
| 1991 | _ | 195 | _ | _ | 25 | _ | _ | 38.15 | 43.22 | _* | 30.2 | 29.94 | 36.2 | |||
| 2012 | _ | _ | _ | _ | 8 | _* | _ | _ | _ | _ | _* | 7.7 | ||||
| 17 | H. valentiae | 1989 | 50 | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | 50 | |
| 1990 | 55 | _* | _* | _ | _ | _* | _* | _ | _ | _ | 54.8 | |||||
| 1991 | 20 | 205 | _ | 82 | 14 | _ | _ | _ | _* | 13.72 | _ | 89.5 | ||||
| 2012 | 22 | 35 | _ | _ | _ | _* | 55 | 3 | _ | _ | _* | _ | 28.7 | |||
| 18 | Janiaadhaerens | 1989 | 5 | 1.62 | _ | _ | _ | _* | _* | _ | _* | _* | _ | 3.31 | ||
| 1990 | 0.3 | _ | 0.14 | _* | _* | 0.22 | _ | _* | _* | 2.86 | _ | _ | 0.9 | |||
| 1991 | 4.1 | 6.87 | 6.12 | _ | 8.55 | 6.32 | _ | 0.74 | _* | 1.7 | _ | 4.91 | ||||
| 2012 | 2.1 | 1.1 | 1.2 | _ | _ | _* | _ | _ | 2.5 | _ | _* | _ | 1.73 | |||
| 19 | Laurenciaobtusa | 1989 | _ | _ | 2.2 | _ | _ | _* | _* | _ | _* | _* | _ | 2.2 | ||
| 1990 | 14 | 7.07 | _* | _* | 1.02 | _ | _* | _* | _ | 0.6 | _ | 5.77 | ||||
| 1991 | 11 | _ | 16 | 23.35 | _ | _ | 38 | _ | _ | _* | _ | _ | 22 | |||
| 2012 | _ | 3.6 | 7 | 4.5 | 4 | _* | 6 | _ | _ | 4 | _* | 4 | 4.73 | |||
| 20 | Melanothamnus | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| afaqhusainii | 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | 90.47 | _ | 90.5 | ||
| 1991 | _ | _ | _ | _ | _ | _ | _ | _ | _ | _* | _ | _ | _ | |||
| 2012 | _ | _ | _ | _ | _ | _* | _ | _ | _ | _ | _* | _ | _ | |||
| 21 | Osmundeapinnatifida | 1989 | 5.2 | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | 5.2 | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _* | 4.44 | _ | 4.44 | |||
| 1991 | _ | _ | _ | 1.81 | 5 | _ | _ | _ | 6.68 | _* | 5.41 | _ | 4.78 | |||
| 2012 | 4 | _ | _ | _ | _ | _* | _ | _ | _ | _ | _* | 5.4 | 4.7 | |||
| 22 | Plocamiumcartilagineum | 1989 | _ | _ | _ | 0.62 | _ | _ | _* | _* | _ | _* | _* | _ | 0.62 | |
| 1990 | _ | _ | 8.38 | _* | _* | _ | _ | _* | _* | _ | _ | _ | 8.38 | |||
| 1991 | _ | _ | _ | _ | _ | 30.2 | _ | _ | _ | _* | _ | _ | 30.21 | |||
| 2012 | _ | _ | _ | _ | _ | _* | _ | _ | _ | 8 | _* | 11 | 9.5 | |||
| 23 | Polysiphonianizamuddinii | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| 1990 | _ | 31 | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | 30.6 | |||
| 1991 | _ | _ | _ | 21 | 6 | _ | _ | _ | 18.7 | _* | _ | 6.3 | 13.1 | |||
| 2012 | 21 | _ | _ | 5.4 | _ | _* | _ | _ | _ | _ | _* | 6 | 10.8 | |||
| 24 | Sarcodiadichotoma | 1989 | _ | _ | _ | _ | 9 | _ | _* | _* | _ | _* | _* | _ | 8.5 | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | |||
| 1991 | _ | _ | _ | _ | 7 | 16.5 | _ | _ | _ | _* | _ | _ | 11.94 | |||
| 2012 | 3 | 4.1 | 6.32 | 5 | 2 | _* | 4.2 | _ | _ | 4 | _* | _ | 4.13 | |||
| 25 | Sarconema | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| filiforme | 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | ||
| 1991 | _ | _ | 22.4 | 15.5 | 14 | 9.85 | 18.4 | _ | _ | _* | 3.83 | _ | 14.02 | |||
| 2012 | 13 | 6.9 | 8.7 | _ | _ | _* | _ | _ | _ | 6.6 | _* | 2 | 7.34 | |||
| 26 | Scianiafascicularis | 1989 | _ | _ | 1.72 | 5.2 | _ | _ | _* | _* | 8.5 | _* | _* | _ | 5.14 | |
| 1990 | _ | 46 | 1.11 | _* | _* | 2.03 | 0.24 | _* | _* | _ | 0.64 | _ | 10.07 | |||
| 1991 | 61 | 132 | 44 | 40 | 34.5 | 30 | _ | _ | _* | 25.89 | 22.6 | 48.75 | ||||
| 2012 | 22 | 31 | 63 | 15.6 | 23 | _* | 12 | 7 | 2.5 | 3 | _* | 4.2 | 18.36 | |||
| 27 | S. hattei | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | 74.94 | 64.5 | _ | 69.72 | |||
| 1991 | _ | _ | _ | _ | _ | _ | _ | _ | _ | _* | 111.14 | _ | 111.14 | |||
| 2012 | 19 | _ | _ | _ | _ | _* | 26.8 | _ | _ | 7 | _* | 33.8 | 21.55 | |||
| 28 | S.saifullahii | 1989 | _ | _ | _ | _ | _ | _ | _* | _* | _ | _* | _* | _ | _ | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | |||
| 1991 | _ | _ | 320 | 170 | _ | 85.1 | _ | _ | _ | _* | _ | _ | 191.7 | |||
| 2012 | _ | _ | _ | _ | _ | _* | _ | 7.2 | _ | _ | _* | 8 | 7.6 | |||
| 29 | Sebdonia | 1989 | _ | _ | _ | 5.42 | _ | _ | _* | _* | _ | _* | _* | _ | 5.42 | |
| flabellata | 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | ||
| 1991 | _ | _ | 119 | _ | _ | _ | _ | _ | _ | _* | _ | _ | 119 | |||
| 2012 | _ | _ | _ | 22 | 15 | _* | _ | _ | _ | 12 | _* | _ | 16.17 | |||
| 30 | Solieriarobusta | 1989 | _ | _ | _ | 6 | _ | _* | _* | _ | _* | _* | _ | 6 | ||
| 1990 | _ | _ | 5.7 | _* | _* | _ | _ | _* | _* | _ | 2.43 | _ | 4.1 | |||
| 1991 | _ | 33 | 38.4 | _ | _ | 37.9 | _ | _ | _ | _* | _ | _ | 36.3 | |||
| 2012 | _ | _ | _ | _ | _ | _* | 4.4 | 7 | 8.12 | 4.3 | _* | 11 | 6.96 | |||
| 31 | Trichleocarpafragilis | 1989 | _ | _ | _ | _ | _ | 60 | _* | _* | _ | _* | _* | _ | 60 | |
| 1990 | _ | _ | _ | _* | _* | _ | _ | _* | _* | _ | _ | _ | _ | |||
| 1991 | 280 | 265 | 136 | 115 | _ | 77.9 | _ | _ | _ | _* | _ | 15 | 148.22 | |||
| 2012 | 32 | 24 | 6 | 11 | _ | _* | _ | 7 | 4 | 1.1 | _* | 4 | 11.09 | |||
Table 3: Seasonal variation in biomass (g m-2) of red seaweeds at Nathia Gali beach of Karachi.
| phylum | J | F | M | A | M | J | J | A | S | O | N | D | Total | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 23 | 26 | 23 | 14 | 14 | 11 | 7 | 9 | 17 | 12 | 12 | 17 | 185 | 24.8 | |
| Chlorophyta | ||||||||||||||
| 34 | 34 | 34 | 21 | 12 | 10 | 13 | 10 | 19 | 20 | 16 | 21 | 244 | 32.71 | |
| Phaeophyta | ||||||||||||||
| 41 | 36 | 44 | 31 | 22 | 26 | 17 | 8 | 14 | 21 | 27 | 30 | 317 | 42.49 | |
| Rhodophyta | ||||||||||||||
| 98 | 96 | 101 | 66 | 48 | 47 | 37 | 27 | 50 | 53 | 55 | 68 | 746 | 100 | |
| Total |
Table 4: Seasonal variation in number of seaweed species collected at Nathia Gali beach.
| Source | DF | F | P |
| Years | 3 | 12.52*** | 0.000 |
| Months | 11 | 4.42*** | 0.000 |
| Error | 32 | ||
| Total | 46 |
Table 5: Analysis of variance (ANOVA) for biomass of seaweeds at Natia Gali beach of Karachi. (DF is degree of freedom, F is the F-statistics and P is the probability level).
Seaweeds found in the present study were categorized into four groups based on their frequency of occurrence during the study period [31]. Species found mostly all year round i.e., nine to twelve months named as perennials and species found in the major portion of the year i.e., from six to eight months called pseudoperennials. Seasonal annuals were those species found in limited period of occurrence i.e., from three to five month and species restricted to only one, two or three months named as rare.
The detail account of biomass for each species is given below alphabetically as presented in Tables 1-3 for each group of seaweeds.
Chlorophyceae (green seaweed)
Bryopsis harveyana was rare and was found in different months. The total biomass of this seaweed ranged from 0.82-5.41 g. m-2. Caulerpa manorensis was also rare and was found only in November 1990 (0.33 g. m-2) and September (0.55 g. m-2) and October 2012 (0.76 g. m-2). C. peltata species was also rarely collected and the biomass ranged from 11.65-40 g. m-2. C. racemosa species classified as pseudoperennial and the maximum biomass were recorded in January (225.2 g. m-2) and February (224.32 g. m-2) of 1990 and minimum value of biomass was recorded in May (0.85 g. m-2) 1989. C. scalpelliformis species was seasonal annual (1.37-74.43 g. m-2). C. taxifolia was rare with biomass 0.53 g. m-2 in November of 1990 and April (0.44 g. m- 2) and May (0.45 g. m-2) of 2012. Codium iyengarii species was pseudoperennial, found abundantly compared to all other green species. The maximum values were recorded in the month of January (5511.4 g. m-2) and minimum value was recorded in October (44.96 g. m-2). C. shameelii (16.07-425 g. m-2) and Enteromorpha compressa species (16-432 g. m-2) were also rare. Halimeda tuna species was seasonal annual in 1989 and 1990 but in 1991 and 2012 this species was pseudoperennial. A maximum biomass value was observed in February 2012 (44.6 g. m-2) whereas minimum value was recorded in February 1989 (1.5 g. m-2). Udotea indica was rare and found only in low quantity (1.17-9.22 g m-2). Ulva fasciata was seasonal annual and the biomass ranged from 0.35-360.2 g. m-2. U. lactuca and Valoniopsis pachynema were also rare and found with very low range in a whole study period (1.42-22.14 g. m-2 and 0.1-420 g. m-2 respectively) (Table 1).
Phaeophyceae (brown seaweed)
Colpomenia sinuosa species was seasonal annuals (4.79-171 g. m-2) and abundantly found during December to January. Cystoseira indica species was seasonal annual (0.25-58.58 g. m-2). Dictyopteris dichotoma species was seasonal annual (7.3-72.43 g. m-2) whereas the other species of the same genus D. hauckiana was rare collected only in 1991 in the month of November (16 g. m-2) and in 2012 in the months of January (3 g. m-2) and February (7.2 g. m-2). Iyengaria stellata species was pseudoperennial in 1991 and seasonal annual in other three years (1989, 1990 and 2012). The highest biomass was collected in December 1991 (425 g. m-2) and lowest was recorded in March 1990 (12.36 g. m-2 g. m-2). Jolyna laminarioides (16-25.11 g. m-2) and Lobophora variegate species (0.32-12.66 g. m-2) were also rare. Among Phaeophyceae (brown seaweed) Padina pavonica was perennial and was collected all year round except in the year of 1990 it was seasonal annual. Highest peak of abundance was found in April 1989 (55.3 g. m-2) and lowest biomass was recorded in December 1991 (0.35 g. m-2). P. tetrastromatica species was rare (0.8-17.33 g. m-2). Sargassum boveanum species was Pseudoperennial. The biomass was high in June 1989 (242.34 g. m-2), July 1990 (110 g/m2), and January 1991 (344 g. m-2) and in April
2012 (256 g. m-2). S. lanceolatum species was rare in 1989 and 1990, pseudoperennial in 1991and perennial in 2012. The maximum value was obtained in June 1990 (47.93 g. m-2) and minimum was recorded in September 1989 (4.8 g. m-2). S. tenerrimum was rare (4-153.6 g. m-2) in the whole study period whereas S. wightii species was not present in 1989 and rare in 1990 and in 1991 and 2012 it was pseudoperennial. Its maximum value was obtained in January 1991(104.5 g. m-2) and minimum was recorded in October 1990 (6.3 g. m-2) 2012 (6 g. m-2). Spatoglossum variabile was rare (2.45-16.44 g. m-2) and Stoechospermum marginatum (1.5-160 g. m-2) was seasonal annual (Table 2).
Rhodophyceae (red seaweed)
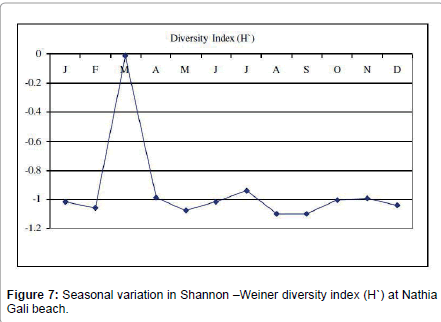
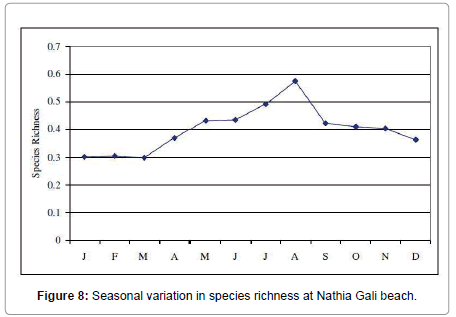
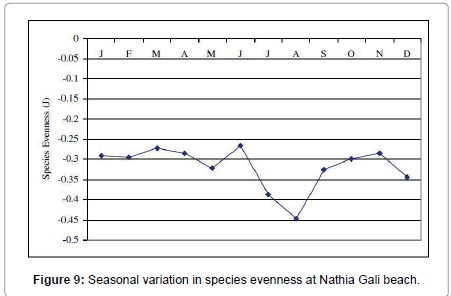
Botryocladia leptopoda species was found as seasonal annual (1.4 -120.5 g. m-2). Calliblepharis fimbriata (0.6- 0.45 g. m-2) and all Champia species were found as rare i.e., Champia compressa (0.92-26.9 g. m-2), C. plumose (3.8-9.17 g m-2) and C. salicornioides (10.3-13.6 g. m-2). Coelarthrum muelleri was seasonal annual (26.75-154 g. m-2) in 1991 and in 1989 and 2012 it was rare whereas in 1990 it was not collected. Cystoclonium purpureum (2.5-28.78 g. m-2), Gelidium pusillum (2.5- 18.5 g. m-2) and G. usmanghanii (0.23-11.25 g. m-2) species were found rare. Gracilaria corticata was seasonal annual in 1989, 1990 and 2012 whereas in 1991 it was Pseudoperennial. The highest biomass was recorded in April 1991 (57 g. m-2) and lowest biomass was recorded in March 1990 (3.51 g. m-2). G. foliifera species was present only in 1991 (7.84-60 g. m-2) and October 2012 (3.5 g. m-2). G. pygmaea species was rare in 1989 (8.33 g. m-2) and 1990 (6.6 g. m-2), Pseudoperennial in 1991 (5.86-48.42 g. m-2) and seasonal annual in 2012 (3.8-12.6 g. m-2). G. verrucosa species was present only in 1991 as seasonal annual (19.24- 65.2 g. m-2). Halymenia porphyraeformis species was seasonal annual (1.2-100.2 g. m-2). Hypnea musciformis was rare in 1989 (8 g. m-2) and 1990 (2.1-17.95 g. m-2), pseudoperennial in 1991 (0.78-290 g. m-2) and seasonal annual in 2012 (1.6-13 g. m-2). H. pannosa was seasonal annual (7.7-195 g. m-2). H. valentiae species was also seasonal annual in 1991 (13.72-205 g. m-2) and 2012 (3-55 g. m-2) whereas in 1989 and 1990 it was rare. Jania adhaerens (0.14-8.55 g. m-2) and Laurencia obtuse (0.6-38 g. m-2) were seasonal annual. Melanothamnus afaqhusainii (90.47 g. m-2), Osmundea pinnatifida (1.81-6.68 g. m-2), Plocamium cartilagineum (0.62-30.21 g. m-2) and Polysiphonia nizamuddinii (5.4- 30.6 g. m-2) were rare. Sarcodia dichotoma was rare in 1989 (8.5 g. m-2) and 1991 (7.37-16.5 g. m-2), pseudoperennial in 2012 (3-6.32 g. m-2) and in 1990 it was absent. Sarconema filiforme was seasonal annual found only in 1991 (3.83-22.37 g. m-2) and 2012 (2-12.5 g. m-2). Scinaia fascicularis species was seasonal annual in 1989 (1.72-8.5 g. m-2) and 1990 (0.24-46.35 g. m-2), pseudoperennial in 1991 (22.6-132.21 g. m-2) and perennial in 2012 (2.5-63 g. m-2). The highest biomass was recorded in March 1991 (132.21 g. m-2) and lowest biomass was recorded in July 1990 (0.24 g. m-2). S. hatei was rare found only in 1990 (64.5-74.94 g. m-2), 1991 (111.14 g. m-2) and 2012 (7-33.8 g. m-2). S. saifullahii (7.2- 320 g. m-2), Sebdenia flabellate (5.42-119 g. m-2), Solieria robusta (2.43- 38.4 g. m-2) and Trichleocarpa fragilis (1.1-280 g. m-2) species were rare (Table 3). The total biodiversity index of seaweeds at Nathia Gali beach was -11.36641 ranged from -0.01083 to -1.10039. Figure 7 showed the monthly variation in Shannon-Weiner diversity index (H’) at Nathia Gali during the study period January 1989-December 1991 and 2012. The peak value was found in the month of March (-0.01083) while the lowest value was recorded in the month of September (-1.10039). The values of species richness S’ varied from 0.298 to 0.577. The highest value (0.577) was observed in the month of August and lowest value (0.298) was recorded in March (Figure 8). The values of species evenness (J) ranged from -0.272 to -0.447. The maximum values of species evenness were observed in the months of August (-0.447) and minimum values in the month of June (-0.266) (Figure 9). The diversity, distribution and abundance of biomass varied from season to season and interannual variation were also observed in three years sampling consecutive like another coast of Karachi, Paradise Point [20]. The green seaweeds were the major contributors in biomass of intertidal seaweed whereas red seaweeds have greater diversity at the coast, showed maximum number of species. Aliya and Shameel [32] also observed the abundance of green seaweed and they occupy a large area of the Karachi coast with variation in the distribution of type and species. The green seaweed Caulerpa racemosa, Codium iyengarii and Halimeda tuna, brown seaweed, Cystoseira indica, Iyengaria stellata Padina pavonica and Sargassum boveanum, red seaweed Gracilaria corticata and Scinaia fascicularis were found to be the dominant species of Nathia Gali in winter (December to January). This period is dominated with northeast monsoon wind season in the winter over the North Arabian Sea [33]. The richness of seaweed resources is due to the intertidal rocks available on the coast of Nathia Gali. In winter period due to low temperature of air and seawater with low intensity and small duration and high dissolve nutrients seaweeds biomass and growth increased when compared to other seasons [21,24]. Chauhan [34] has also found abundant growth of seaweed in winter. The lowest value of biomass was recorded in May to August and this period is associated with the southwest monsoon season. In this period most of the seaweeds were either absent from the rocky shores or an occasional species corresponding to low biomass were present because in this season sea becomes turbulent under the influence of strong winds and wave action and especially at the continental shelf likely inhibits the growth of drifted seaweed. Chock and Mathieson [9] from New England and Gaur et al. [11] from India found maximum values in October. Saifullah [17], Qari and Qasim [18,19] and Qari et al. [20] have observed the paucity of sublittoral seaweeds during May to August period. Satheesh and Wesley [35] reported the high seaweed abundance during pre-monsoon and low in post monsoon season at Kudankulam coastal waters, South-Eastern coast of India.
Seaweeds are important raw material in polysaccharides production. Most of them have very important economic value [36]. Michalak et al. [37] and Zia et al. [38-40] found that seaweeds could help to reduce the use of chemical fertilizer and improve physicochemical properties of the soil and to increase the plant growth. Gavino and Trono [38] reported that the seaweeds and their products are the third most important fishery export. From the present study, it is apparent that many economically and medicinally important species of seaweeds such as Gracilaria corticate, Hypnea musciformis, H. pannosa, Sargassum boveanum, Ulva fasciata etc., are richly found at this coast [39-42] but nowadays there is lot of disturbance due to anthropogenic activities such as discharge of untreated and partially treated domestic and industrial waste, coolant waters of industrial and atomic nuclear power plants and harbour activities such as dredging and cargo handling, the dumping of ship waste and other coastal activities. Therefore for the conservation of these resources it is need to control these pollutants and prohibited or ban on like these activities. The present comparative study of two different periods will be useful as new base line record for future floral studies on Nathia Gali coast.
Acknowledgement
Thanks and great regards to Prof. Dr. M. Shameel (Late) for help in identifying the species of seaweeds.
References
- Trono GCJr (1999) Diversity of the seaweeds flora of the Philippines and its utilization. Hydrobiologia 398: 1-6.
- Chapman VJ, Chapman DJ (1980) Seaweed and their uses. Chapman and Hall London. New York.
- Faveri C, Scherner F, Farias J, Oliveira EC, Paulo A, et al. (2010) Temporal changes in the seaweed flora in Southern Brazil and its potential causes, Pan-American. J of Aquatic Sci 5: 350-357.
- Hay CH, Villouta E (1993) Seasonality of the Adventive Asian Kelp Undariapinnatifidain New Zealand. Bot Mar 36: 461-476.
- Jagtap TG (1992) Marine flora of Nicobar group of islands in Andaman Sea. Indian J Mar Sci 21: 56-58.
- Ballesteros E (1991) Seasonality of growth and production of a deep-water population of Halimeda tuna (Chlorophyceae, Caulerpales) in the northwestern Mediterranean. Bot Mar 34: 291-301.
- Pedrini AG, Goncalves JEA, Fonseca MCS, Zau AS, Lacorte CC (1989) A survey of the marine algae of Trindade Island, Brazil. Bot Mar 32: 97-99.
- Basson PW, Mohamed SA, Arora DK (1989) A survey of the benthic marine algae of Bahrain. Bot Mar 32: 27-40.
- Chock JS, Mathieson AC (1983) Variations of New England estuarine seaweeds Biomass. Bot Mar 26: 87-97.
- Peckol P (1982) Seasonal occurrence and reproduction of some marine algae of the continental shelf, North Carolina USA. Bot Mar 25: 185-190.
- Gaur K, Ramakrishna T, Subbaraju DP, Murthy MS (1982) Ecological studies on UlvalactucaL. from Veraval, India. Hydrobiologia 89: 89-94.
- Whyte JNC, Englar JR, Saunders RG, landsay JC (1981) Seasonal variation in the biomass, quantity & quality of agar from the reproductive and vegetative stages of Gracilaria(Verrucosa type). Bot Mar 24: 493-501.
- Thom RM (1980) Seasonality in low intertidal benthic marine algal communities in Central Puget Sound Washington, USA Bot Mar 23: 7-11.
- Josselyn MN, Mathieson AC (1980) Seasonal influx and decomposition of autochthonous macrophytes litter in a north temperate estuary. Hydrobiologia 71: 197-208.
- Shameel M (1987) A preliminary survey of seaweed from the coast of Lasbella,Pakistan. Bot Mar 30: 511-515.
- Saleem KM (1965) The distribution of marine algae along Karachi. Bot Mar 8: 183-195.
- Saifullah SM (1973) A preliminary survey of the standing crop of seaweeds from Karachi coast. Bot Mar 16: 139-144.
- Qari R, Qasim R (1988) Seasonal change in the standing crop of intertidal seaweeds from the Karachi coast Pp 449-456 In: Proc Marine Science of the Arabian Sea. Thompson MF Tirmizi NM eds. American institutes of biological sciences, Washington DC.
- Qari R, Qasim R (1994) Seasonal change in the standing crop of intertidal seaweeds from Manora coast Karachi. In: Proc Nat Sem Fish Policy and Plan.Majid A, Khan MY, Moazzam M, Ahmed J (Editors). Marine Fisheries Dep Karachi p: 279-286.
- Qari R, Qureshi NA, Siddiqui SA (2014) Phytomass Studies on Natural bed of Seaweed at Paradise Point, Karachi Coast. Int J Econ Environ Geol 5(2): 11-17.
- Qari R, Siddiqui SA (2006) Nutrient dynamics in seawater of Paradise Point. Int J Biol Biotech 3: 329-337.
- Qari R, Siddiqui SA (2008) Heavy metal pollution in coastal seawater of NathiaGali, Karachi (Pakistan). J of Environ Res and Deve 3: 9-19.
- Chapman VJ (1964) Coastal vegetation. Pergaman Press Oxford.Anand PL (1940) Marine algae from Karachi 1. Chlorophyceae. Punjab University botanical publications p: 52.
- Anand PL (1943) Marine algae from Karachi 11. Rhodophyceae. Punjab University botanical publications p: 76.
- Round FE (1973) The biology of the algae. Edward Arnold Publishers London p: 278.
- Chapman VJ, Chapman DJ (1973) The Algae. Macmillan and Co New York p: 497.
- Dawes CJ (1974) Marine Algae of the west coast of Florida. University of Miami Press Coral Gables Florida p: 201.
- Morris I (1976) An introduction to the algae. Hutchinson and Co (Publishers) LTD London England p:189.
- Dring MJ (1982) The biology of marine plants. Edward Arnold Limited London p: 199.
- Boney AD (1969) A biology of marine algae. Hutchinson educational LTD London England.
- Aliya R, Shameel M (1996) Taxonomic study of coenocytic green algae commonly growing on the coast of Karachi. Pakistan J Mar Sci 5: 47-68.
- Johansen AM (2003) Chemical characterization of ambient aerosol collected during the northeast monsoon season over the Arabian Sea: Labile-Fe(II) and other trace metals. J of Geophysical Res 108 (D14, 4408, Ach 5-1-Ach 5-11.
- Chauhan VD (1968) An estimate of algin-bearing seaweeds in the Gulf of Kutch. Current Satheesh S, Wesley SG (2012) Diversity and distribution of seaweeds in the Kudankulam coastal waters, South-Eastern coast of India. Biodiversity J 3: 79-84.
- Renn D (1997) Biotechnology and the red seaweed polysaccharide industry: status, needs and prospects. Trends in Biotechnology 15: 9-14.
- Michalak I, Wilk R, Chojnacka K (2016) Bioconversion of Baltic seaweeds into organic compost. Waste Biomass Valor.
- Gavino C, TronoJr (1999) Diversity of the seaweed flora of the Philippines and its utilization. Hydrobiologia399: 1-6.
- Qari R and Siddiqui SA (1993) Biochemical composition and yield of agar from theGracilariacorticataof Karachi. Mar Res 2: 77-81.
- Zia Z, Zahid PB,Qari R (2012) Effect of seaweed manure on the growth of SpinaceaoleraceaL. (Spinach: Palak). Int J PhycolPhycochem 8: 1-6.
- Khan AR,Qari R (2012) Antibacterial activities of brown seaweed Sargassumboveanum(j. ag.) against diarrhea along the coast of Karachi, Pakistan. J ofEnviron Res and Dev6: 753-757.
- Khan F, Qari R (2009) Seasonal; variation in water soluble polysaccharides alginic acid extracted from Sargassumboveanum J. Agardh (Phaeophyta, Sargassaceae) along the different shore of Karachi coast, Pakistan. Int J Biol Biotech 6: 277-281.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 7606
- [From(publication date):
June-2017 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 6446
- PDF downloads : 1160