Case Report Open Access
An Analysis of Prognostic Factors in Pancreatic Neuroendocrine Tumors
Moyana TN1*, Kendal WS2, Shabana W3, Walker A1, Balaa FK4, Nyiraneza C5, Dawkins Y6, Chatterjee A6, Goodwin R7, Asmis T7, and Chang N11Department of Pathology & Lab Medicine, the Ottawa Hospital and University of Ottawa, Ottawa, Ontario, Canada
2Division of Radiation Oncology, the Ottawa Hospital and University of Ottawa, Ottawa, Ontario, Canada
3Department of Radiological Sciences, the Ottawa Hospital and University of Ottawa, Ottawa, Ontario, Canada
4Division of Hepatobiliary Surgery, the Ottawa Hospital and University of Ottawa, Ottawa, Ontario, Canada
5Department of Epidemiology & Community Medicine, the Ottawa Hospital and University of Ottawa, Ottawa, Ontario, Canada
6Division of Gastroenterology, the Ottawa Hospital and University of Ottawa, Ottawa, Ontario, Canada
7Division of Medical Oncology, the Ottawa Hospital and University of Ottawa, Ottawa, Ontario, Canada
- *Corresponding Author:
- Terence N Moyana
Department of Pathology & Lab Medicine
The Ottawa Hospital–General Campus
501 Smyth Road, Ottawa, Ontario, Canada
Tel: 6137378290
Fax: 6137378853
E-mail: tmoyana@ottawahospital.on.ca
Received Date: June 21, 2016; Accepted Date: July 11, 2016; Published Date: Jul 13, 2016
Citation: Moyana TN, Kendal WS, Shabana W, Walker A, Balaa FK, et al. (2016) An Analysis of Prognostic Factors in Pancreatic Neuroendocrine Tumors. J Clin Exp Pathol 6:284. doi: 10.4172/2161-0681.1000284
Copyright: © 2016 Moyana TN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Background: Pancreatic neuroendocrine tumors (PNETs) generally behave indolently. However, some manifest an aggressive clinical course with poor prognosis. It is not clear whether this is due to intrinsic tumor aggressiveness, dedifferentiation, or simply silent progressive tumor accumulation that then triggers complications. This study analysed the features of PNETs to gain further insight into their biology.
Methods: A retrospective clinicopathologic review of 74 PNETs using Kaplan-Meier and Cox proportional hazard analyses.
Results: Median overall survival was 117 months; 5 year survivals were 92% where the PNETs were resected versus 35% for those not resected (p<0.001). Liver metastases developed in 33/74 (18/33 synchronous metastases; 15/33 metachronous). There was a significant difference in Ki67 grade between metastatic and non-metastatic tumors (p<0.001). However, there was considerable overlap of grades between synchronous and metachronous tumor groups with 5/10 (50%) of synchronous PNETs being well-differentiated. Most patients with metachronous metastases (10/15; 67%) showed non-progressive disease whereas the majority with synchronous metastases (15/18; 83%) manifested progressive disease (p=0.01). On Cox analysis, synchronous metastases and inoperability were significant predictors of survival whereas Ki67 grade and T/N stage were not.
Conclusion: Aggressive PNETs were associated with synchronous metastases and inoperability. By multivariate analysis, the strongest adverse prognostic factor was synchronous metastases and this was related to the poor outcome over and above what was indicated by the Ki67 grade on univariate analysis. While it is not entirely clear whether the presence of synchronous metastases reflects intrinsic tumor aggressiveness or simply disease in the later stages of its natural history, our findings suggest that they reflect intrinsic aggressiveness de novo. However larger studies with more statistical power are required to further substantiate this. While cell proliferation markers such as Ki67 have greatly improved our grading and understanding of PNETs, molecular studies of events involved in the metastatic cascade could also be meritorious in elucidating disease biology.
Keywords
Pancreatic neuroendocrine tumors; Tumor biology; Prognostic factors; Tumor grading; Synchronous metastases; Metachronous metastases
Introduction
Gastrointestinal and pancreatic neuroendocrine tumors (PNETs) are generally slow-growing tumors compared to their adenocarcinomatous or exocrine counterparts [1,2]. They were initially described as carcinoids or carcinoma-like, tumors in slow motion, given their indolent course [3,4]. Subsequent studies demonstrated a heterogenous group of neoplasms with unpredictable biologic behaviour [1,3,5,6]. More recently, it was observed that some of these tumors can manifest an aggressive clinical course with most deaths occurring within 2 years of diagnosis [7-9]. Whereas in the past a key cause of morbidity and mortality for these patients was the metabolic derangement secondary to hormonal effects, these effects have been curtailed by newer therapies [1,5-8,10]. Currently, most poor outcomes can be ascribed to tumor progression and mass effect [1,6,9-11].
Tumor staging systems have been used to assess mass effect and extent of disease within PNETs [6,12-14]. The classification system for gastrointestinal PNETs has evolved to where the term neuroendocrine neoplasm has almost completely replaced islet cell tumor, carcinoid and atypical carcinoid, and terms used to represent the spectrum of biologic aggressiveness have been replaced by new 3-tier World Health Organization (WHO) grading system [6,12-15]. This has resulted in improvements in tumor prognostication, treatment planning and comparison of data from different institutions. However, these classifications do not explain all the discrepancies in the behaviour of PNETs. For example, some patients with metastatic liver disease can live for many years whereas others follow an aggressive course [5-11,16-23]. It is not clear whether the poor prognosis can be attributed to intrinsic tumor aggressiveness, dedifferentiation, or simply a silent, progressive accumulation of tumor burden that at some point causes physiological processes to decompensate leading to system failure.
The aim of this study was to analyse the clinical, imaging and pathologic features of PNETs to gain further insight into the factors influencing morbidity and mortality.
Materials and Methods
Patient selection
Approval for the study was obtained from the Ottawa Hospital Research Ethics Board. Surgical, cytopathology and autopsy records were searched for all cases of neuroendocrine tumors between 1994 and 2014. More detailed information relating to clinical history, medical imaging, laboratory findings, patient management and followup were obtained from the health records database.
The tumors were categorized by site with the main groups being pulmonary, gastrointestinal, pancreas, and metastatic/unknown primary. Those cases that could be clearly defined as PNETs based on clinical, imaging and pathologic data were selected for this study. Cases with disseminated disease and involvement of the pulmonary and/or tubular gastrointestinal tract were excluded. Cases in which the lesions were <1 cm in maximum dimension were also excluded from the study.
Pathology
The diagnosis of PNET was based on conventional histology and immunohistochemistry (IHC) on the surgical biopsy, resection, or cytology specimens. Slides as well as the pathology reports were reviewed.
The tissue blocks were retrieved from storage. Immunohistochemical analysis was carried out on a representative tissue or cell block by staining for neuroendocrine markers as previously described [24]. Where necessary, additional stains were carried out to further confirm the diagnosis.
The Ki67 labelling index was based on tissue sections, and not on cytology material. Tissue was available from pancreas (n=51), both pancreas and liver (n=4) and liver metastases only (n=2). The Ki67 stain was carried out on both primary and metastases (Table 1). The counts were determined by 2 of the authors by counting at least 2000 cells from photographs of hot spots at 20x magnification as previously described [24].
| Discrete variates | No.(%) | |
|---|---|---|
| Females | 39 (52.7) | |
| Cancer-directed surgery | None | 19 (25.7) |
| Whipple | 14 (18.9) | |
| Distal pancreatectomy | 30 (40.5) | |
| Enucleation | 11 (14.9) | |
| T stage | 1 | 18 (24.3) |
| 2 | 18 (24.3) | |
| 3 | 34 (45.9) | |
| 4 | 4 (5.4) | |
| N stage | 0 | 44 (59.5) |
| 1 | 29 (39.2) | |
| Liver metastases | none | 42 (55.4) |
| synchronous | 18 (24.3) | |
| metachronous | 15 (20.3) | |
| Deaths from all causes | 15 (20.3) | |
| Cancer specific deaths | 13 (17.6) | |
| Pancreatic tumor site | head | 29 (39.2) |
| uncinate process | 4 (5.4) | |
| neck | 11 (14.9) | |
| body | 10 (13.5) | |
| tail | 20 (27.0) | |
| RECIST criteria at last staging exam | Complete response | 5 (6.8) |
| (33 evaluable cases) | Stable disease | 8 (10.8) |
| Progressive disease | 20 (27.0) | |
| Continuous variates | Mean | Range |
| Age at diagnosis (years) | 58 | 32-84 |
| ki67 Grade primary (55 evaluable cases) | 1.6 | 1-3 |
| ki67 Grade liver (6 evaluable cases) | 2.8 | 2-3 |
Table 1: Clinicopathologic features of individuals with pancreatic neuroendocrine tumours.
Medical imaging
All patients underwent CT of the abdomen (Figure 1), pelvis and chest. A few selected cases had MRI or ultrasound depending on their clinical status. All the cases were performed with IV contrast. Some cases were scanned in both arterial and portal venous phases while others had just the portal venous phase.
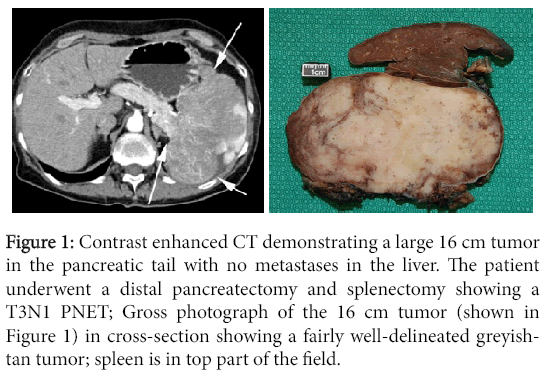
Figure 1: Contrast enhanced CT demonstrating a large 16 cm tumor in the pancreatic tail with no metastases in the liver. The patient underwent a distal pancreatectomy and splenectomy showing a T3N1 PNET; Gross photograph of the 16 cm tumor (shown in Figure 1) in cross-section showing a fairly well-delineated greyishtan tumor; spleen is in top part of the field.
Most patients also underwent octreotide scan as part of their workup. Staging was based on this imaging. Synchronous metastases were defined as those present at the time of diagnosis of the primary tumor or within 1 year of curative surgery; metachronous metastases occurred thereafter [11].
The extent of hepatic parenchymal involvement was calculated as a percentage of liver, and incorporated into modified Response Evaluation Criteria in Solid Tumors (RECIST) scores [25]. The dominant liver metastases had their greatest diameter measured; along with the tumor configuration these measurements were used to estimate the total area occupied by the metastases (Figure 2). Findings were classified as: i) complete response (CR) if the tumor was completely eradicated by metastasectomy or radio-frequency ablation (RFA) ii) stable disease (SD) if the tumor had not changed in size by 20% or iii) progressive disease (PD) if the tumor increased in size by >20% or new lesions appeared.
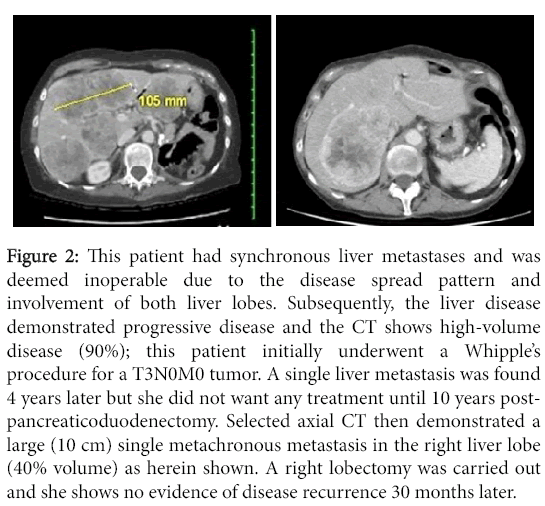
Figure 2: This patient had synchronous liver metastases and was deemed inoperable due to the disease spread pattern and involvement of both liver lobes. Subsequently, the liver disease demonstrated progressive disease and the CT shows high-volume disease (90%); this patient initially underwent a Whipple’s procedure for a T3N0M0 tumor. A single liver metastasis was found 4 years later but she did not want any treatment until 10 years postpancreaticoduodenectomy. Selected axial CT then demonstrated a large (10 cm) single metachronous metastasis in the right liver lobe (40% volume) as herein shown. A right lobectomy was carried out and she shows no evidence of disease recurrence 30 months later.
Statistics
Statistical comparisons of tabular data were based on chi-square analysis, Student’s t-test and analysis of variance (ANOVA). Box and whisker plots provided visual comparisons of different population variables; the mean and its 95% confidence interval (CI) were estimated for each population. Survival analysis was based on the method of Kaplan and Meier, using the log rank test for comparisons. Cox proportional hazards analysis was performed based on overall survival. The 95% CI were provided for each hazard ratio and the strengths of the resulting relationships evaluated with the Wald chisquare test [26].
Results
Characteristics of the study population
Data were retrieved from 74 cases (Table 1). Fifty-five individuals underwent potentially curative resection. Fifteen cases were deemed unresectable by medical imaging or at laparotomy. Another 4 patients were technically operable but because of comorbidity or advanced age were treated non-operatively. After a median follow-up of 33 months, 34 patients were alive and well, 23 alive with disease, 13 dead of disease, 2 dead of other causes and 2 were lost to follow-up.
Tumor grading
The results of the Ki67 grading are provided in Table 1 (Figures 3 and 4). In some cases, the liver metastases were extirpated using RFA and therefore no tissue was available for pathologic assessment. In other instances, the diagnosis of PNET was based on cytology (from the primary or liver metastases) and we elected not to use this material for grading purposes.
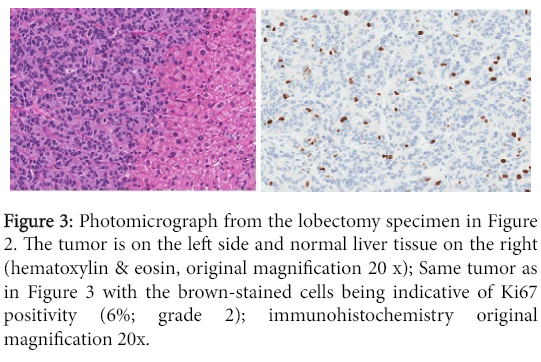
Figure 3: Photomicrograph from the lobectomy specimen in Figure 2. The tumor is on the left side and normal liver tissue on the right (hematoxylin & eosin, original magnification 20 x); Same tumor as in Figure 3 with the brown-stained cells being indicative of Ki67 positivity (6%; grade 2); immunohistochemistry original magnification 20x.
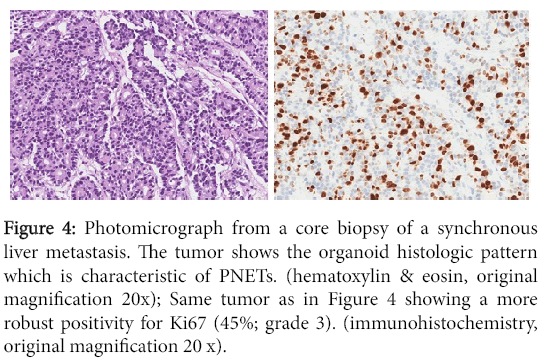
Figure 4: Photomicrograph from a core biopsy of a synchronous liver metastasis. The tumor shows the organoid histologic pattern which is characteristic of PNETs. (hematoxylin & eosin, original magnification 20x); Same tumor as in Figure 4 showing a more robust positivity for Ki67 (45%; grade 3). (immunohistochemistry, original magnification 20 x).
Metastatic disease
The liver was the dominant site of metastasis (synchronous 18/33 and metachronous 15/33). In 5/33 of the liver metastases cases, there were also extrahepatic metastases: to bone (5/5), brain (1/5) and uterine serosa (1/5).
Of the 18 cases of synchronous metastases, 16 were present at diagnosis and 2 occurred within one year. Extirpation of liver metastases was attempted in 8 cases by RFA, metastasectomy, or both; for 5 of these cases, there was no further recurrence.
Surgery versus no surgery
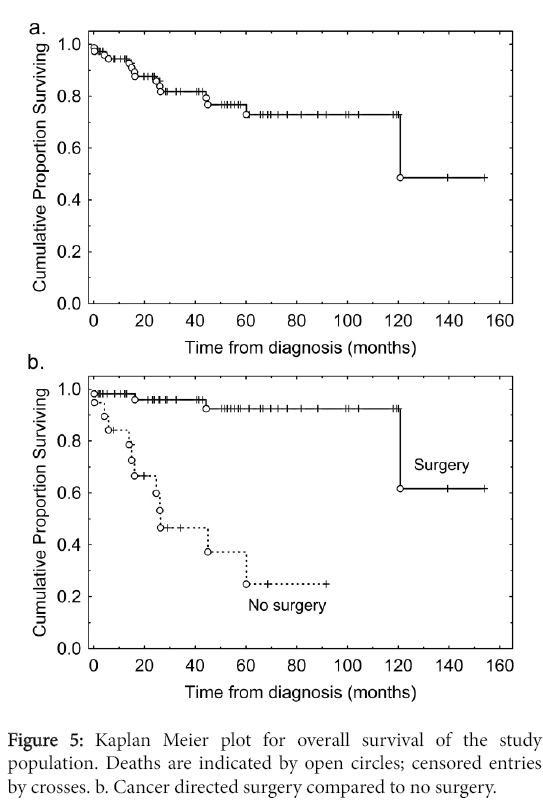
The median overall survival was 117 months (Figure 5). Patients who underwent cancer-directed surgery had better overall survival than those who did not (Figure 5) (p<0.001).
These surgical patients represented a highly selected subpopulation, with better Tumor/Nodes/Metastases (TNM) staging and younger ages (Table 2).
| Discrete variates | No surgery, No. cases (%) |
Cancer-directed surgery No. cases (%) | Chi-squared (d.f.) | p |
|---|---|---|---|---|
| Female gender | 8 (42.1) | 31 (56.4) | 1.2 (1) | N.S. |
| T stage | ||||
| T1 | 3 (15.8) | 15 (27.3) | 12.5 (3) | 0.006 |
| T2 | 4 (21.1) | 14 (25.5) | ||
| T3 | 8 (42.1) | 26 (47.3) | ||
| T4 | 4 (21.1) | 0 (0) | ||
| N stage | ||||
| N0 | 7 (38.9) | 37 (67.3) | 4.6 (1) | 0.033 |
| N1 | 11 (61.1) | 18 (32.7) | ||
| Ki67 grade primary | ||||
| 1 | 2 (28.6) | 26 (54.2) | 8.5 (2) | 0.01 |
| 2 | 2 (28.6) | 19 (39.6) | ||
| 3 | 3 (42.9) | 3 (6.25) | ||
| Liver metastases at diagnosis | ||||
| No | 8 (42.1) | 48 (87.3) | 15.7 (1) | <0.001 |
| Yes | 11 (57.9) | 7 (12.7) | ||
| Death from all causes | 11 (57.9) | 4 (7.3) | 22.4 (1) | <0.001 |
| Continuous variates | No surgery, Mean |
Cancer directed surgery, Mean | t (d.f.) | p |
| Age at diagnosis | 66.1 yr | 55.7 yr | -3.1 (72) | 0.002 |
Table 2: Comparison of surgical and non-surgical cases.
Metastasis versus no metastasis
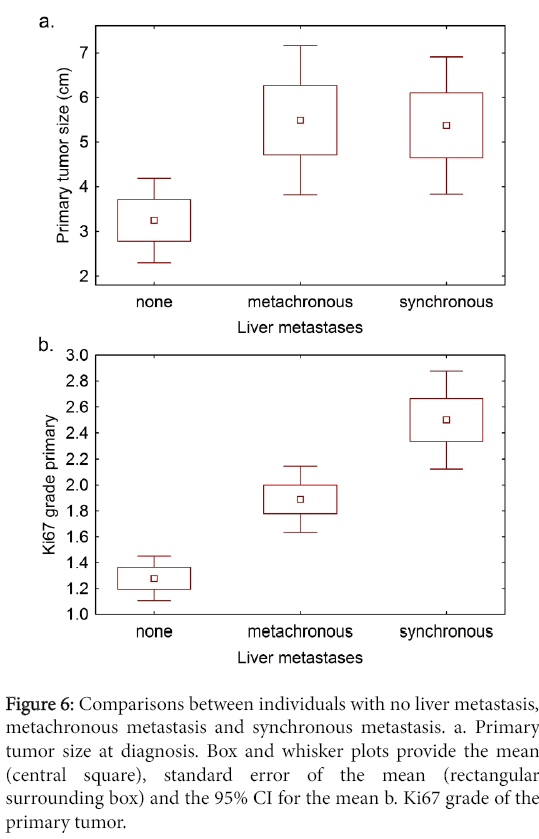
To determine whether primary tumor size was related to metastatic behavior, we compared non-metastatic and metastatic tumor groups, and there was a significant difference between them (Figure 6). However, there was no significant difference in primary tumor size between the individuals with metachronous and synchronous metastases.
Comparison of non-metastatic and metastatic tumor groups with regard to Ki67 grade of the primary tumor showed a significant difference (Figure 6) and differences were seen between the metachronous and synchronous metastases groups.
Figure 6: Comparisons between individuals with no liver metastasis, metachronous metastasis and synchronous metastasis. a. Primary tumor size at diagnosis. Box and whisker plots provide the mean (central square), standard error of the mean (rectangular surrounding box) and the 95% CI for the mean b. Ki67 grade of the primary tumor.
However, there was considerable overlap in the tumor grades with 5 (50%) of the synchronous tumors being well-differentiated (Table 3).
| Ki67 grade primary | No metastases | Metachronous metastases | Synchronous metastases |
|---|---|---|---|
| 1 | 27(75.0%) | 1(11.1%) | 0(0%) |
| 2 | 8(22.2%) | 8(88.9%) | 5(50%) |
| 3 | 1(2.8%) | 00% | 5(50%) |
Table 3: Ki67 grade categorized by type of metastases.
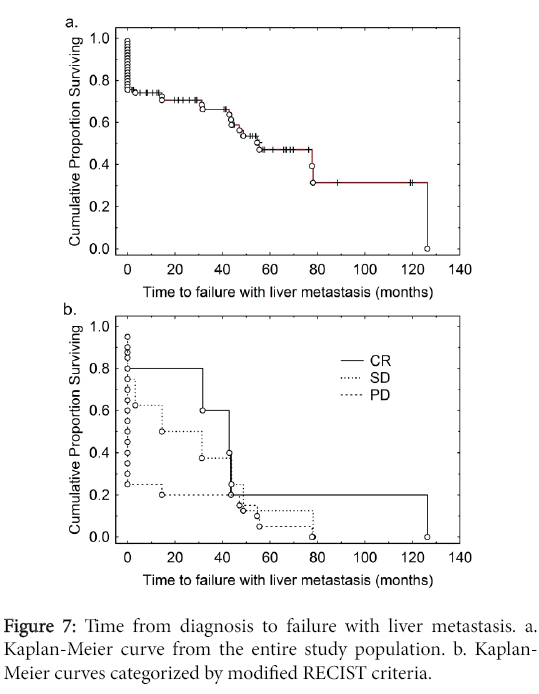
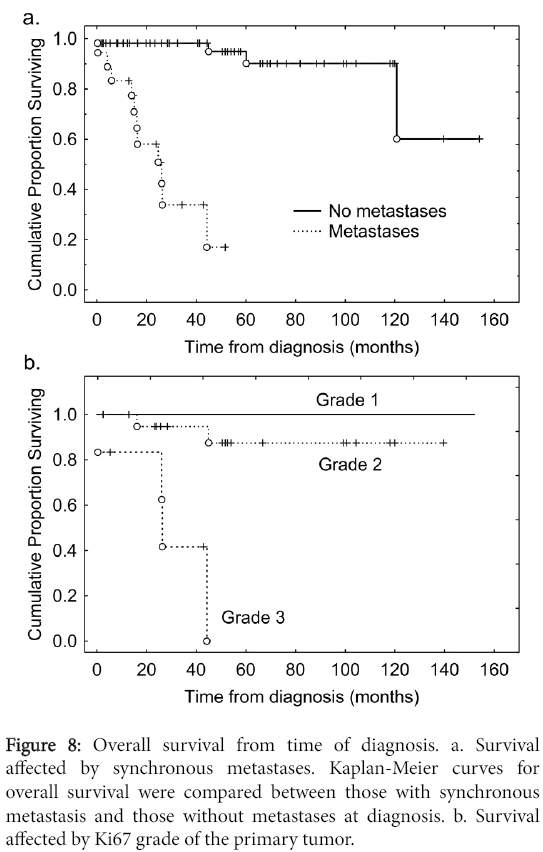
We carried out an analysis of the time to failure with liver metastases from the time of diagnosis (Figure 7). The median time to failure with liver metastases was 54 months. Of individuals with synchronous metastases 50% were dead by 2 years of diagnosis whereas of those without metastases at diagnosis more than 95% remained alive over the follow-up period (Figure 8). We then carried out an analysis of time to development of liver metastasis stratified for the modified RECIST criteria (Figure 7).
Most individuals with metachronous metastases (10/15; 67%) showed non-progressive disease while the majority of those with synchronous metastases (15/18; 83%) manifested progressive disease (p=0.01) (Table 4).
| Metachronous metastases | Synchronous Metastases | |
|---|---|---|
| Complete response | 4 (27%) | 1 (5.6%) |
| Stable disease | 6 (40%) | 2 (11%) |
| Progressive disease | 5 (33%) | 15 (83%) |
Table 4: Modified RECIST criteria for metachronous and synchronous metastases.
Survival and response analysis
The median time to failure with liver metastases was 54 months from time of diagnosis (Figure 7). By RECIST criteria, individuals with CR had the longest survival, those with SD had an intermediate survival, whereas those with PD had poor survivals (Figure 7). Of individuals with synchronous metastases 50% were dead by 2 years of diagnosis whereas of those without metastases at diagnosis more than 95% remained alive by that time (Figure 8). Most individuals with metachronous metastases had non-progressive disease whereas for most individuals with synchronous metastases manifested progressive disease (Table 4).
Prognostic factors for overall survival
We had observed a poor prognosis in three situations: where cancer-directed surgery was not performed (Figure 5), for individuals with synchronous metastasis (Figure 8), and where the Ki67 grade of the primary tumor was 3.
In order to clarify the relationship of these and other potentially prognostic explanatory variables, a Cox proportional hazards analysis was performed based on overall survival (Table 5).
| Explanatory variables | Hazard ratio | 95% CI | Wald statistic | p | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Date of diagnosis | 0.999 | 0.999 | 1.000 | 3.471 | 0.062 |
| Male gender | 0.922 | 0.262 | 3.244 | 0.016 | 0.899 |
| Age at diagnosis | 0.989 | 0.923 | 1.060 | 0.096 | 0.757 |
| Primary tumor size | 1.069 | 0.760 | 1.504 | 0.146 | 0.702 |
| T stage | 3.719 | 0.573 | 24.126 | 1.895 | 0.169 |
| N stage | 1.897 | 0.164 | 21.907 | 0.263 | 0.608 |
| Ki67 grade primary | 1.434 | 0.337 | 6.101 | 0.238 | 0.625 |
| Cancer-specific surgery done | 0.060 | 0.005 | 0.793 | 4.56 | 0.033 |
| Liver metastasis at diagnosis | 33.231 | 2.530 | 436.557 | 7.11 | 0.008 |
Table 5: Cox proportional hazards analysis. Cox proportional hazards analysis.
Chi-squared=52.38, d.f.=9, p<0.001.
The date of diagnosis was included as an explanatory variable in order to adjust for secular effects over the study period such as improvements in surgical or diagnostic techniques. Other explanatory variables included gender, age at diagnosis, T and N stage, as well as primary tumor size. Synchronous metastasis was the strongest prognostic factor with a hazard ratio of 33; if cancer-directed surgery was performed the hazard ratio was reduced to 0.06. The remaining factors revealed no significant associations on this analysis, in particular in the context of synchronous metastases and cancerdirected surgery Ki67 grade no longer associated with survival.
Discussion
The findings of this study lend support to the WHO classification in that most patients with early stage tumors did well while those with advanced tumors had significantly worse outcomes (Table 2). Our results also show that individuals who had their tumors resected had much better outcomes than those who did not. This could mean that surgery had a beneficial effect on survival. Indeed, it is known that currently, the only curative modality for PNETs is surgery [1,6,11,16,22,23,27]. However, these surgical patients were highly selected, they were younger, had less extensive disease, and better performance status.
Although it is recognized that the liver is the organ mostly commonly involved by PNET metastasis, the degree to which this occurred in our study was quite striking: all 33 cases with distant metastasis (100%) involved the liver, and in 28/33 (85%) of cases, the liver was the only organ so affected. It is possible that our selection criteria may have excluded bona fide PNETs that had metastasis to the lung since it can be difficult to distinguish poorly differentiated PNETs from their pulmonary counterparts even with immunohistochemistry [28]. However, other studies also showed that the liver was the dominant organ for PNET metastasis [1,6,16,18,23,29,30]. This being the case, it is therefore not surprising that most of the morbidity and mortality of these patients were due to liver metastasis. This is also in line with the findings of other studies [1,6,9,10,17-23,29,30].
Since metastatic liver disease plays such a critical role in the morbidity and mortality of these patients, it deserves more scrutiny in the way it gets evaluated. In this study, we assessed not just the presence or absence of liver metastases but also i) whether the metastases were present at the time of diagnosis or appeared subsequently ii) the extent of liver involvement and iii) disease progression. This is important because some patients with liver metastasis can live for a long time despite the disease [1,5,10,17]. Furthermore, we quantitated the presence of metastasis in a manner that can provide a measure of the metastatic burden. Indeed this not something new. A number of reports have previously incorporated this approach by, for example, stating whether disease was uni- or bilobar, the number of metastases and volume of liver occupied by metastasis [8-11,17,18,30].
There was correlation between primary tumor size and metastatic potential, i.e. larger tumors were more likely to metastasize compared to smaller ones. However, when we compared the synchronous metastases group to the metachronous one, there was no difference in primary tumor size between the 2 groups. This suggests that the poor prognosis seen with the synchronous metastases is not merely due to tumor progression as measured by the size of the primary tumor but rather an intrinsic property of the tumor de novo.
With respect to tumor grading, our findings are consistent with the WHO classification in that there was a significant difference between the metastatic and non-metastatic groups (p<0.001). However, as shown in Table 3, 50% of the synchronous tumors were grade 2. Since most of these tumors demonstrated an aggressive clinical course, this suggests that there could well be other factors to account for the biologic aggressiveness of the synchronous metastasis group besides tumor grade. Indeed some tumors, for example basal cell carcinomas, tend to have a high proliferation rate yet hardly metastasize. Other factors that suggest an intrinsic biologic aggressiveness for synchronous tumor group are their tendency towards multicentric or diffuse metastases [11]. On the other hand, it has been observed that large and/or isolated metastases can be associated with long survival (Figure 4) [5,9-11,20,30].
The inherent biologic aggressiveness of the synchronous metastasis group can also be seen from the analysis of the modified RECIST data. Most of these tumors (83%) (Table 4) showed tumor progression that could be measured in months (Figure 7). In contrast, a significant proportion of patients (67%) with metachronous metastases had nonprogressive disease over a relatively long period of time with some (27%) even showing complete response following metastasectomy and/or radiofrequency ablation. This is in line with other studies [10,11].
The 4 cases for which grading could be done on paired samples from both the primary and metastatic tissue are too few for statistical analysis. However, other studies showed that there was no difference in the Ki67 grade between the primary and the corresponding liver metastases. This suggests that the biologic behaviour of these tumors might be determined de novo as opposed to the selection of aggressive tumor clones for metastasis (de-differentiation).
Recognition of PNETs with an aggressive course is important because, while it is known that the presence of liver metastases is the rate-limiting step on survival, the real problem is predicting when death would occur since some of these patients can live for many years [15,17,18,29]. In this regard, the new WHO classification has been useful in that most patients with grade 3 tumors tend to do poorly as we also show (Figure 8) [31]. What is less clear to prognosticate are patients with well-differentiated PNETs metastatic to the liver. Our study suggests that the finding of synchronous liver metastases, even in well-differentiated PNETs, indicates a proclivity towards biologic aggressiveness.
Surgery is the only potentially curative form of treatment for these patients [28,30]. However, the best treatment for patients with liver PNET metastasis remains controversial [11,17]. This is due to the fact that these tumors are uncommon, often described retrospectively in small series and prospective clinical trials comparing alternative therapies are limited [15,17,18,23,28,30]. As a result, patients may end up being managed on a case-by-case basis. Therefore this kind of information relating to synchronous liver PNET metastasis, Ki67 grade and outcome could be helpful in selecting the most appropriate type of patient management in the pre- or post-operative setting. Likewise, for patients with metastatic liver PNETs who are being considered for other forms of treatment such as chemotherapy, hepatic artery embolization/chemo-embolization and liver transplant, knowledge about the biologic aggressiveness of the disease is helpful [19,29-33].
In conclusion, this study shows that a poor prognosis for PNETs appears to be associated with synchronous metastases and patients ineligible for cancer-directed surgery. After the potentially prognostic factors had been adjusted for by multivariate analysis, the strongest adverse prognostic factor was the presence of synchronous metastases at diagnosis and this thus appears to be related to poor outcome over and above what might be indicated by metachronous metastases or the Ki67 grade of the primary on univariate analysis. Overall, this suggests that the presence of synchronous metastases may reflect intrinsic tumor aggressiveness though the idea that this may reflect individuals later in the natural history of their disease cannot be completely discounted. While cell proliferation markers such as Ki67 have greatly improved our grading and understanding of PNETs, molecular studies of events involved in the metastatic cascade could also be meritorious in elucidating disease biology.
References
- Metz DC, Jensen RT (2008) Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 135: 1469-1492.
- Reid MD, Bagci P, Adsay NV (2013) Histopathologic assessment of pancreatic cancer: does one size fit all? J SurgOncol 107: 67-77.
- Modlin IM, Shapiro MD, Kidd M (2004) Siegfried Oberndorfer: origins and perspectives of carcinoid tumors. Hum Pathol 35: 1440-1451.
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, et al. (2008) One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J ClinOncol 26: 3063-3072.
- Moertel CG, Johnson CM, McKusick MA, Martin JK Jr, Nagorney DM, et al. (1994) The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Intern Med 120: 302-309.
- Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, et al. (2008) Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9: 61-72.
- di Bartolomeo M, Bajetta E, Buzzoni R, Mariani L, Carnaghi C, et al. (1996) Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology Group. Cancer 77: 402-408.
- Delaunoit T, Rubin J, Neczyporenko F, Erlichman C, Hobday TJ (2005) Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumors. Mayo ClinProc 80: 502-506.
- Sutliff VE, Doppman JL, Gibril F, Venzon DJ, Yu F, et al. (1997) Growth of newly diagnosed, untreated metastatic gastrinomas and predictors of growth patterns. J ClinOncol 15: 2420-2431.
- Yu F, Venzon DJ, Serrano J, Goebel SU, Doppman JL, et al. (1999) Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J ClinOncol 17: 615-630.
- Chu QD, Hill HC, Douglass HO Jr, Driscoll D, Smith JL, et al. (2002) Predictive factors associated with long-term survival in patients with neuroendocrine tumors of the pancreas. Ann SurgOncol 9: 855-862.
- Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, et al. (2007) TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 451: 757-762.
- Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S(2010) The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 39:707-712.
- Couvelard A(2012) Neuroendocrine tumours of the pancreas: recent developments in staging and grading. Diagnostic Histopathology 18: 1-7.
- Adsay V (2012) Ki67 labeling index in neuroendocrine tumors of the gastrointestinal and pancreatobiliary tract: to count or not to count is not the question, but rather how to count. Am J SurgPathol36:1743-1746.
- Norton JA, Warren RS, Kelly MG, Zuraek MB, Jensen RT (2003) Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery 134: 1057-1063.
- Weber HC, Venzon DJ, Lin JT, Fishbein VA, Orbuch M, et al. (1995) Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology 108: 1637-1649.
- Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, et al. (2000) Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am CollSurg 190: 432-445.
- Lo CY, van Heerden JA, Thompson GB, Grant CS, Söreide JA, et al. (1996) Islet cell carcinoma of the pancreas. World J Surg 20: 878-883.
- McEntee GP, Nagorney DM, Kvols LK, Moertel CG, Grant CS (1990) Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery 108: 1091-1096.
- Hurtuk MG, Godambe AS, Shoup M, Yong S, Aranha GV (2011) Support for a postresection prognostic score for pancreatic endocrine tumors. Am J Surg 201: 406-410.
- Norton JA, Kivlen M, Li M, Schneider D, Chuter T, et al. (2003) Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg 138: 859-866.
- Saxena A, Chua TC, Sarkar A, Chu F, Liauw W, et al. (2011) Progression and survival results after radical hepatic metastasectomy of indolent advanced neuroendocrine neoplasms (NENs) supports an aggressive surgical approach. Surgery 149: 209-220.
- Moyana TN, Kendal WS, Chatterjee A, Jonker DJ, Maroun JA, et al. (2014) Role of fine-needle aspiration in the surgical management of pancreatic neuroendocrine tumors: utility and limitations in light of the new World Health Organization classification. Arch Pathol Lab Med138:896-902.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205-216.
- Woodward M (1999) Epidemiology: Study design and data analysis. Chapman & Hall, London.
- Norton JA, Sugarbaker PH, Doppman JL, Wesley RA, Maton PN, et al. (1986) Aggressive resection of metastatic disease in selected patients with malignant gastrinoma. Ann Surg 203: 352-359.
- Rekhtman N (2010) Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 134: 1628-1638.
- Sarmiento JM, Que FG, Grant CS, Thompson GB, Farnell MB, et al. (2002) Concurrent resections of pancreatic islet cell cancers with synchronous hepatic metastases: outcomes of an aggressive approach. Surgery 132:976-983.
- Chen H, Hardacre JM, Uzar A, Cameron JL, Choti MA (1998) Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am CollSurg 187: 88-92.
- Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, et al. (2014) Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J SurgPathol 38:437-447.
- Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, et al. (2011) Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet378:2005-2012.
- Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, et al. (2010) NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 39: 735-752.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 12859
- [From(publication date):
August-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 11918
- PDF downloads : 941