Research Article Open Access
An Agenda for Naloxone Distribution Research and Practice: Meeting Report of the Surviving Opioid Overdose with Naloxone (SOON) International Working Group
Aaron M Orkin1,2,3*, Katherine Bingham1,4, Michelle Klaiman5, Pamela Leece1, Jason E Buick2 Fiona Kouyoumdjian6, Laurie J Morrison2,3,5 and Howard Hu11Dalla Lana School of Public Health, University of Toronto, Canada
2Rescu, Keenan Research Centre, Li Ka Shing Knowledge Institute, Toronto, Canada
3Institute of Health Policy, Management and Evaluation, University of Toronto, Canada
4Department of Family and Community Medicine, University of Toronto, Canada
5St. Michael’s Hospital, University of Toronto, Canada
6Centre for Research on Inner City Health, St. Michael’s Hospital, Toronto, Canada
- Corresponding Author:
- Aaron Orkin
Dalla Lana School of Public Health
University of Toronto
155 College St
Toronto ON, M5T 1P8, Canada
Tel: 6479237551
Email: aaron.orkin@mail.utoronto.ca
Received date: February 11, 2015; Accepted date: March 11, 2015; Published date: March 20, 2015
Citation: Orkin AM, Bingham K, Klaiman M, Leece P, Buick JE, et al. (2015) An Agenda for Naloxone Distribution Research and Practice: Meeting Report of the Surviving Opioid Overdose with Naloxone (SOON) International Working Group. J Addict Res Ther 6:212. doi:10.4172/2155-6105.1000212
Copyright: © 2015 Orkin AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Opioid-related mortality is a serious and growing issue in North America. Naloxone distribution and basic life support training for people at risk of overdose is a promising opportunity to improve access to potentially lifesaving bystander interventions and essential healthcare. We convened a unique international working group of experts in public health, resuscitation science, and health research methodology, along with clinical, community, policy, industry stakeholders and members of the lay public to explore and address key challenges and opportunities for rigorous research on this intervention.
The findings from the Surviving Opioid Overdose with Naloxone (SOON) International Working Group explored potential research opportunities and identified barriers in four priority areas: research methods, resuscitation guidelines, naloxone delivery device development, and knowledge translation. This novel collaborative effort:
• Identified key steps and challenges for developing an appropriate, feasible and rigorous pragmatic trial of naloxone distribution in various clinical settings;
• Identified emerging naloxone delivery devices and technologies, and described how these devices may alter standards of care for overdose prevention research and practice
• Engaged resuscitation experts in the development of bystander resuscitation protocols for opioid-associated resuscitative emergencies; and,
• Identified strategies to overcome knowledge translation barriers for patients and providers, as well as characteristics for effective educational tools and program implementation.
The SOON collaboration aims to advance the investigation, implementation, and practice of overdose education and naloxone distribution. Through diverse collaborations, we can use best science to improve practice for individuals at risk of opioid overdose.
Background
Opioid-related mortality is a serious and growing issue in North America [1,2]. In the United States, drug overdose accounted for over 16,000 deaths in 2010, and now exceeds motor vehicle collisions as the leading cause of death due to injury [1,3]. Premature opioid-related mortality in Ontario, Canada, rose from 12.2 deaths per million in 1991 (127 deaths annually) to 41.6 deaths per million in 2010 (560 deaths annually) [2]. The World Health Organization estimates that opioid overdose accounts for between 70,000 and 100,000 annual deaths worldwide [4]. This public health epidemic is driven, in part, by opioid prescriptions [5], and is pervasive across a wide diversity of socio-demographic strata [6,7].
Addressing this complex public health crisis requires comprehensive and multi-sectorial strategies [8]. Naloxone distribution and basic life support training for people at risk of overdose is a promising opportunity to improve access to potentially lifesaving bystander interventions and essential healthcare [9]. This approach has now received the attention of the World Health Organization, and national governments of Canada, the United States (U.S.), and the United Kingdom (U.K.) [4,8-11]. In the United States, nearly 200 local programs distributed naloxone to more than 53,000 individuals between 1996 and 2010 [9]. Additionally, over 25 states have adopted or are considering policies permitting first responders and public safety professionals such as police officers and fire-fighters, to carry and administer naloxone [12]. In Canada, several such program have been identified, with published reports and evaluations emerging from programs in British Columbia, Edmonton, and Toronto [13,14]. The Scottish Government has established and evaluated a national naloxone distribution program, while local pilot or experimental projects have been reported and celebrated in Russia, Ukraine, Georgia, Kazakhstan, Tajikistan, Afghanistan, China, Vietnam and Thailand [4,15-17]. The biomedical industry’s interest in naloxone has grown substantially, resulting in a new range of commercial naloxone formulations and purpose-engineered naloxone delivery devices.
Naloxone distribution programs center around teaching the lay public to activate emergency medical services and deliver potentially lifesaving opioid antagonists and resuscitative maneuvers to critically ill overdose patients. Building and optimizing these programs therefore requires the integration of addictions medicine, drug policy and harm reduction, with bystander resuscitation, prehospital medicine and emergency toxicological expertise and systems. In March 2014, we convened the Surviving Opioid Overdose with Naloxone (SOON) International Working Group, a meeting of experts and key stakeholders to consider challenges and opportunities to advance naloxone distribution implementation and research in Canada. The meeting was held in Toronto, Ontario, and co-hosted by the University of Toronto Dalla Lana School Of Public Health and St. Michael’s Hospital Li Ka Shing Knowledge Institute.
This report summarizes our proceedings and proposes future directions for research and practice related to the distribution of naloxone to people at risk of overdose. Our goal was to convene the international and inter-professional expertise necessary to advance the practice, investigation, and implementation of bystander naloxone administration for opioid-associated resuscitative emergencies. Working Group members included clinicians and researchers in resuscitation science, emergency medicine, primary care, addictions, rehospital care, public health, and harm reduction, as well as decision-makers, educators, and community members. Working group members also included representatives from local and regional naloxone distribution programs in Canada and the United States, leadership in mental health and addictions from the World Health Organization, and representatives from biomedical technology firms engaged in the development of naloxone delivery devices. Prior to the meeting, the planning committee identified four priority topics related to naloxone distribution programs: [1] advancing research strategies and methods, [2] identifying and developing appropriate technologies for the user-friendly and safe administration of bystander naloxone, [3] identifying and synthesizing consistent bystander basic life support and resuscitation practices, and [4] developing widely available and broadly applicable knowledge translation strategies for this intervention. Participants were divided into four meeting streams, each corresponding with each of the four priority topics, based on their expertise and perspectives.
Research Strategies and Methods
Although naloxone distribution is expanding rapidly, there are no completed experimental studies on this intervention that examine mortality as an outcome. Most of the existing literature describes self-reporting of naloxone administration from participants returning for naloxone refills, with limited active follow-up of participants [18]. For nearly 15 years, practitioners and scientists have identified that the evidence to support a decrease in mortality from naloxone distribution remains thin, and called for higher quality research [18,19]. Clark, at al. 2014 systematic review on community naloxone distribution programs identified 19 published studies, including 14 uncontrolled longitudinal studies, three descriptive papers with limited summary statistics, and two qualitative studies. All relied on participant self-reports and most suffered from elevated attrition rates. No randomized studies or controlled cohort studies were identified, and the authors were unable to draw a summary conclusion regarding the overall effectiveness of naloxone distribution to prevent opioid-related deaths [18]. Walley, et al. 2013 time-series analysis found that areas in Massachusetts with greater enrollment in naloxone distribution programs had lower rates of opioid-related overdose death than comparators lower enrollment [20]. We are aware of two prospective randomized trials, both in the recruitment phase that is designed to test the effects of this intervention on opioid-related morbidity and mortality. The Seattle-based “Project OOPEN” is designed to test the effects of emergency department-based naloxone distribution and overdose prevention education on rates of fatal and non-fatal overdose rates and drug use behaviours [21]. The U.K.-based “NALIVE” trial focuses on mortality following release from prison in people with a history of opioid use [22].
People at risk of dying from opioid overdose deserve high quality, evidence-based care. We proposed a working definition of people at risk of opioid overdose based on the available literature (Table 1). As naloxone distribution to opioid users is rolled out in harm reduction settings, the window of opportunity closes for prospective trials or observational studies comparing overdose prevention interventions with and without naloxone. Participants identified methodological and design challenges to researching the effectiveness of bystander administered naloxone for opioid-associated resuscitative emergencies, and outlined opportunities for future intervention research.
| People at Risk of Opioid Overdose may be defined as people who use opioids and also meet one or more of the following criteria: |
| • Known or suspected prescription opioid dependence or heroin use [30] |
| • History of emergency care for opioid overdose [31] |
| • Opioid use with known or suspected use of alcohol or benzodiazepines, or other drugs known to increase overdose risk [32,33] |
| • Release from prison with a history of opioid dependence [34] |
| • Discharge from a treatment program for opioid dependence [35] |
| •Enrollment in opioid dependence treatment with methadone during specific times such as induction or discharge [36,37] |
| • High doses of prescribed opioids [38] |
Table 1: A Working Definition of People at Risk of Opioid Overdose.
Most existing overdose prevention and naloxone distribution programs enroll individuals who use opioids and access harm reduction services [18]. Harm reduction programs may have limited interaction with the full diversity of people at risk of opioid overdose, and especially limited interaction with those who use primarily oral opioid formulations or prescribed medications. There remains significant uncertainty about whether harm reduction literature can be generalized to other populations at risk of overdose, such as chronic pain patients, prison populations, or non-injection opioid users. There is a lack of data on fatal or non-fatal overdose event rates in many of the potential populations that could be used in planning future research.
Participants in the research methods stream did not generally think that there is clinical equipoise to support an effectiveness trial in all settings, and advocated for future research to focus on implementation trials [23]. There was support for high quality ecological studies and for research designs involving staggered introduction of overdose prevention training and naloxone distribution, such as a stepped wedge design [24]. Other SOON participants thought that the effectiveness of naloxone distribution to people at risk of overdose had not yet been established in all relevant clinical settings, such as emergency departments, and may present new opportunities for a pragmatic randomized controlled trial.
Naloxone Administration Devices
Existing naloxone kits are cumbersome and inconsistent, involving a collection of glass ampoules, syringes, needles or atomizers, printed instructions, and personal protective equipment, all designed and packaged by individual programs. Rescue medications for other conditions have received comparatively more investment from both clinical and biomedical engineering communities, for example epinephrine auto-injectors (e.g.: Epi-Pen©) for the treatment of anaphylaxis. Technological innovations in naloxone distribution and delivery may have profound effects on both the scientific and evidentiary landscape to support this intervention, and on the socio-political will to support research, implementation, and investment in this area [25].
We aimed to build the collaborations necessary to advance user-friendly and purpose-made naloxone delivery for research and practice, and to integrate them with appropriate knowledge translation and educational tools. We reached out to biomedical firms with active naloxone related projects, to gather information about emerging products, and understand how technological innovations in this field may alter naloxone distribution practices and administration by bystanders.
Naloxone delivery devices and kits entered a period of fast-paced transformation in 2013 and 2014, driven largely by three key biopharmaceutical players:
1. Lightlake Therapeutics, a U.K.-based biopharmaceutical company, participated in the SOON Working Group and provided information about their intranasal naloxone delivery device. In December 2013, Lightlake announced the initial findings of its clinical trial with the U.S. National Institute on Drug Abuse, showing that its nasal spray may deliver circulating naloxone doses equivalent to parenteral formulations [26]. Lightlake has since filed an investigational new drug application in the U.S. (July 2014).
2. Kaléo Pharma is a U.S.-based pharmaceutical firm with experience in epinephrine autoinjector devices. In April 2014, Kaléo announced U.S. Food and Drug Administration approval for its naloxone autoinjector, called Evzio, as well as financing plans for its commercialization.
3. Anti-OP, a U.S.-based pharmaceutical company with expertise in intranasal delivery systems, announced its plans to develop a naloxone nasal spray in partnership with Reckitt Benckiser pharmaceutical group in May 2014.
These and other new products have the potential to transform the future of naloxone distribution, delivery, research and practice by providing mainstream commercial alternatives to ad hoc naloxone kits. Uncertainty remains about how these new devices will alter educational practices, basic life support standards, and associated program and end-user costs. The introduction of these devices may also challenge the external validity of existing studies on the effectiveness of naloxone distribution programs using soon-to-be obsolete kits.
Basic Life Support and Bystander Resuscitation Guidelines
Limited evidence and inadequate collaboration with resuscitation experts has hampered the development of consistent and effective bystander overdose resuscitation techniques. In 2010, the American Heart Association (AHA) had insufficient data to support the use of specific antidotes in a cardiac arrest due to opioid overdose [27]. The AHA recommended that “Naloxone has no role in the management of cardiac arrest” [28]. Existing basic life support guidelines provide little specific guidance regarding bystander or layperson best practices in the management of suspected opioid-related emergencies. In the absence of expert or evidence-based guidelines, individual programs have developed or adopted a variety of resuscitation protocols (Table 2). Since our meeting, the World Health Organization released guidelines on community management of opioid overdose, with some instructions on resuscitation practices. These are not aligned or coordinated with international resuscitation science and basic life support groups like the American Heart Association and European Resuscitation Council, leaving room for further cooperation in this area [29]. This also creates challenges for programs and groups that are seeking to deliver recommendations or basic life support training to people at risk of opioid overdose. We aim to conduct a systematic review of the literature on best basic life support practice for opioid-associated emergencies when naloxone is used in the community.
| Program | Call EMS | Rescue Breathing | Chest Compressions | Naloxone dose & route |
|---|---|---|---|---|
| Preventing Overdose in Toronto (POINT) Program | ✓ | ✘ | ✓ | 0.4mg IM prn x 2 |
| Toronto Public Health | ||||
| Take Home Naloxone Program | ✓ | ✓ | ✓ | 0.4mg IM prn x 2 |
| Scottish Drug Forum, Scottish Government | ||||
| Community Based Naloxone Overdose Prevention Program | ✓ | ✓ | ✓ or ✘ | 0.4mg IM prn x 2 |
| Streetworks, Edmonton | ||||
| Towards the Heart | ✓ | ✓ | ✘ | 0.4mg IM prn x 2 |
| British Columbia Centre for Disease Control | ||||
| Chicago Recovery Alliance | ✓ | ✓ | ✘ | 0.4mg IM prn x 2 |
| Chicago, Illinois | ||||
| Overdose Education and Naloxone Distribution (OEND) | ✓ | ✓ | ✘ | 2mg IN prn x 2 |
| State of Massachusetts Department of Public Health | ||||
| New York State Overdose Prevention Program, | ✓ | ✓ | ✘ | 0.4mg IM prn x 2 |
| NY State Department of Health, AIDS Institute |
Table 2: Program Resuscitation Training Protocols ( ✓= included; ✘= not included).
EMS: Emergency Medical Services; IM: Intramuscular; IN: Intranasal; prn: as needed
We aimed to clarify the role of bystander naloxone administration, rescue breathing, and chest compressions in the management of unresponsive opioid overdose patients, and to develop a strategy for the incorporation of a protocol into future basic life support guidelines. We conducted an expert-facilitated discussion process to arrive at a consensus on appropriate bystander resuscitation practices for opioid-associated emergencies.
The group recommended a standard basic life support algorithm with the integration of naloxone prior to chest compressions. Lay rescuers should presume that cardiac arrest has occurred whenever an unresponsive person is not breathing normally or showing obvious signs of life, even if the etiology is presumed to be opioid toxicity. Bystanders should not perform pulse checks. In all cases of presumed cardiac arrest, bystanders should activate emergency medical services (EMS) promptly, should perform chest compressions, and should use an automated external defibrillator if available. The group was unable to agree on the role of rescue breathing in opioid-associated emergencies. The group concluded that a rigorous guideline based on expert opinion from an internationally recognized organization, such as the American Heart Association, is essential to standardize resuscitation in opioid-associated emergencies.
Knowledge Translation
Naloxone distribution programs find their origins in harm reduction programs, but physicians in a variety of clinical setting, first responders, and other community services have contact with people at risk of opioid overdose and may be uniquely situated to translate research on overdose prevention into practice. If, for example, evidence syntheses and ongoing inquiry suggest that naloxone distribution is helpful, it is important to identify and address a range of challenges and barriers to wider implementation of effective therapies. Participants engaged in a facilitated discussion on barriers and opportunities for the development of widely available and broadly applicable clinical and educational tools for a variety of settings beyond addictions services and harm reduction programs.
Participants in the knowledge translation stream referred to a summary of potential barriers to provider and patient participation in naloxone distribution and related research, in the categories of philosophical, logistical, liability and medicolegal, and educational barriers. Philosophical barriers included concerns that the public will “abuse” naloxone by treating it like safety net to allow riskier use of opioids. Participants identified that these barriers were likely related to stigma about addiction and overdose, and suggested educational and de-stigmatizing approaches for both providers and patients. Logistical barriers relate to accessing training, appropriate naloxone kits, and addressing costs. Barriers related to public liability included fears of being arrested while intervening in an overdose event. Medicolegal concerns included considerations that naloxone may be prescribed to and administered by a third party. The group suggested advocacy for laws to protect bystanders and prescribers. Education with stakeholder groups, along with wider support from professional organizations and government, could also reduce this barrier.
Having considered these barriers and solutions, the group developed a strategy to engage providers and patients in further naloxone-related research and practice. Educational approaches for providers and patients should [1] be tailored to the needs of specific groups (health professional, public, etc.), [2] provide simple instructions for how to use the naloxone device and other basic life support interventions, [3] consider timing and delivery of educational tools (i.e., where and when they are available), and [4] involve educators or “champions” to be a resource within provider or patient subgroups. Participants also identified additional future partners, including government and professional regulatory bodies, correctional facilities, pain medicine practitioners, withdrawal management programs, and First Nations communities.
Conclusion
Advancing research and practice
While naloxone has a history of pharmacological safety and effectiveness for opioid overdose from a perspective, uncertainties remain regarding its use in the context of bystander intervention in opioid-associated prehospital emergencies.
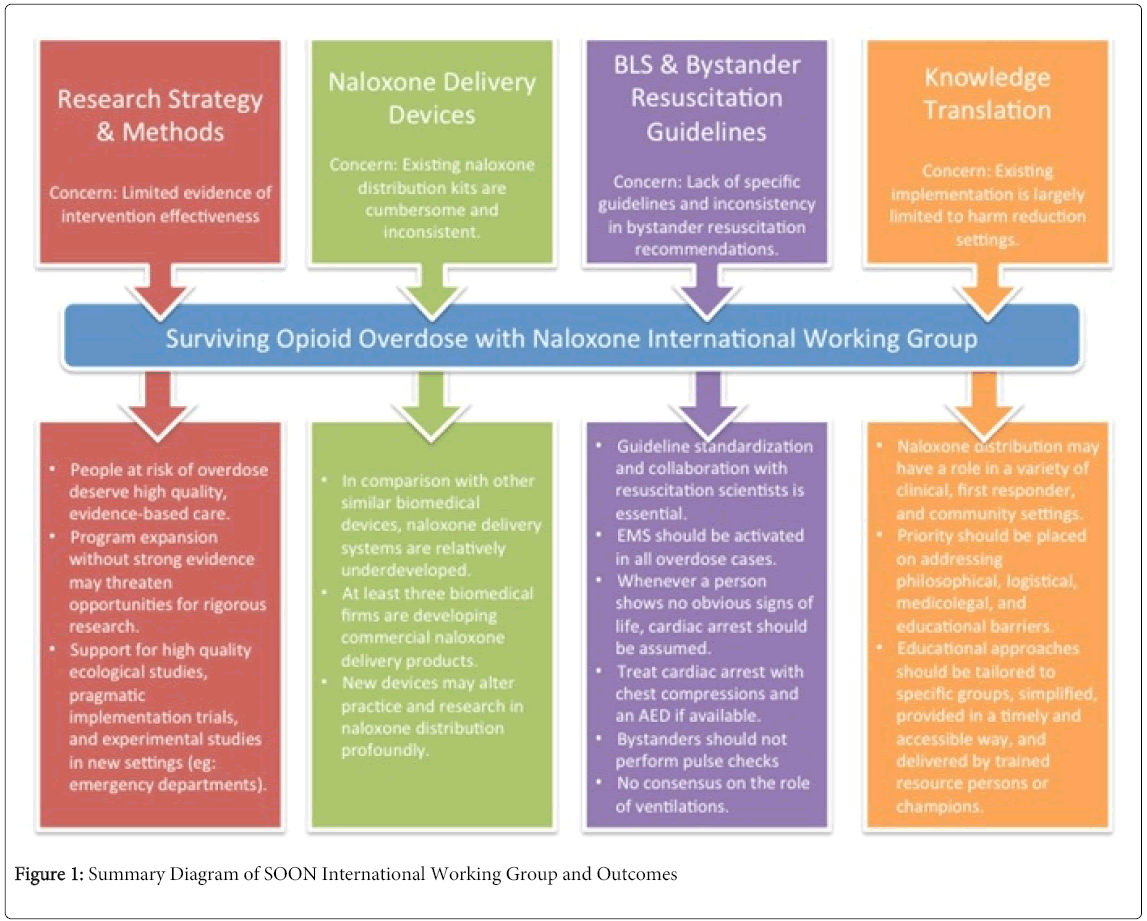
The findings from the SOON Working Group advance a research and evaluation agenda for naloxone distribution. Advancing naloxone distribution science and practice likely involves both high quality program evaluations in settings where naloxone distribution is underway, and prospective designs and trials in new settings. The findings of our International Working Group are summarized in Figure 1.
The following essential elements should form the basis of efforts to continue building
1. High quality research and evaluation methods;
2. Ongoing appraisal of emerging technologies and their impacts on scientific knowledge, practice, and markets;
3. Appropriate resuscitation guidelines and related science; and
4. Knowledge translation approaches that consider barriers to implementation and educational needs.
Through diverse collaborations, we can address critical scientific uncertainties, synthesize and translate existing knowledge, and use best science to improve practice in many settings that serve individuals at risk of opioid overdose.
Acknowledgements
We thank all of the participants at the SOON Working Group: Dr. Marc Berg, Dr. Jane Buxton, Dr. Douglas Campbell, Angela Carol, Dr. Nicholas Clark, Dr. Roger Crystal, Dr. Katie Dainty, Doug (community member), Dr. Rajesh Ghirdari, Catherine Ford, Tara Gomes, Dr. Curtis Handford, Dr. Claire Heslop, Shaun Hosein Dr. Stephen Hwang, Julie (community member), Dr. Stephen Jones, Dr. David Klein, Natascha Koslowski, Dr. Steve Lin, Dr. Kieran Moore, Dr. David Ooi Poon, Paul Raftis, Dr. Rita Shahin, Dr. Sharon Stancliff, Dr. John Strang, Dr. Margaret Thompson, Dr. Lisa Thurgur, Dr. Richard Verbeek, Garrie Wright, Dr. Matthew Young.
We especially wish to acknowledge Daven Seebarran, Karen Born, and Liisa Sorsa for their essential contributions to the success of the SOON Working Group.
The project was funded through a Partnership for Health Systems Improvement Planning Grant from the Canadian Institutes of Health Research.
References
- Jones CM, Mack KA, Paulozzi LJ (2013) Pharmaceutical overdose deaths, United States, 2010. JAMA 309: 657-659.
- Gomes T, Mamdani MM, Dhalla IA, Cornish S, Paterson JM, et al. (2014) The burden of premature opioid-related mortality. Addiction 109: 1482-1488.
- Centers for Disease Control and Prevention. (2012) Wide-ranging OnLine Data for Epidemiologic Research (WONDER).
- United Nations Office on Drugs and Crime, World Health Organization (2013) Discussion paper UNODC/WHO 2013: Opioid overdose: preventing and reducing opioid overdose mortality.
- Wright ER, Kooreman HE, Greene MS, Chambers RA, Banerjee A, et al. (2014) The iatrogenic epidemic of prescription drug abuse: county-level determinants of opioid availability and abuse. Drug Alcohol Depend 138: 209-215.
- Centers for Disease Control and Prevention (CDC) (2011) Vital signs: overdoses of prescription opioid pain relievers-United States, 1999--2008. MMWR Morb Mortal Wkly Rep 60: 1487-1492.
- Paulozzi LJ (2012) Prescription drug overdoses: a review. J Safety Res 43: 283-289.
- National Advisory Committee on Prescription Drug Misuse. (2013) First do no harm: Responding to Canada prescription drug crisis.
- Centers for Disease Control and Prevention (CDC) (2012) Community-based opioid overdose prevention programs providing naloxone - United States, 2010. MMWR Morb Mortal Wkly Rep 61: 101-105.
- Advisory Council on the Misuse of Drugs. (2012) Consideration of naloxone.
- Department of Justice. (2014) Attorney General Holder, Calling Rise in Heroin Overdoses Urgent Public Health Crisis, Vows Mix of Enforcement, Treatment.
- Goodloe JM (2014) Not so fast on naloxone? There's growing support for non-paramedic use, but keep these cautions in mind. EMS World 43: 51-52.
- Oluwajenyo Banjo MPHc, Tzemis D, Al-Qutub D, Amlani A, Kesselring S, et al. (2014) A quantitative and qualitative evaluation of the British Columbia Take Home Naloxone program. CMAJ Open 2: E153-161.
- Dong KA, Taylor M, Wild C, Villa-Roel C, Salvalaggio G, et al. (2012) Community-based Naloxone: A Canadian pilot program. Can J Addiction Medicine 3: 4-9.
- Leece PN, Hopkins S, Marshall C, Orkin A, Gassanov MA, et al. (2013) Development and implementation of an opioid overdose prevention and response program in Toronto, Ontario. can J public Health 104: e200-e204.
- McCauley A, Best D, Taylor A, Hunter C, Robertson R (2012) From evidence to policy: The Scottish national naloxone programme. Drugs: Education, Prevention, Policy 19: 309-319.
- Simini B (1998) Naloxone supplied to Italian heroin addicts. Lancet 352: 967.
- Clark AK, Wilder CM, Winstanley EL (2014) A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med 8: 153-163.
- Lenton SR, Hargreaves KM (2000) Should we conduct a trial of distributing naloxone to heroin users for peer administration to prevent fatal overdose? Med J Aust 173: 260-263.
- Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, et al. (2013) Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ 346: f174.
- Banta-Green C (2014) Project OOPEN: Opioid Overdose Prevention, Education and Intervention. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). NCT01788306.
- Strang J, Bird SM, Parmar MK (2013) Take-home emergency naloxone to prevent heroin overdose deaths after prison release: rationale and practicalities for the N-ALIVE randomized trial. J Urban Health 90: 983-996.
- Freedman B (1987) Equipoise and the ethics of clinical research. N Engl J Med 317: 141-145.
- Brown CA, Lilford RJ (2006) The stepped wedge trial design: a systematic review. BMC Med Res Methodol 6: 54.
- Timmermans S, Berg M (2003) The practice of medical technology. Sociol Health Illn 25: 97-114.
- Lightlake Sinclair Ltd (2012) Clinical Trial on Binge Eating Disorder, Treatment with Naloxone Spray (BED) In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
- Morrison LJ, Deakin CD, Morley PT, Callaway CW, Kerber RE, et al. (2010) Part 8: Advanced Life Support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 122: S345-S421.
- Vanden Hoek TL, Morrison L, Shuster M, Donnino M, Sinz E (2010) Part 12: Cardiac Arrest in Special Situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122: S829-S861.
- World Health Organization. Community Management of Opioid Overdose.
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, et al. (2011) Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 106: 32-51.
- Stoové MA, Dietze PM, Jolley D (2009) Overdose deaths following previous non-fatal heroin overdose: record linkage of ambulance attendance and death registry data. Drug Alcohol Rev 28: 347-352.
- Chan GM, Stajic M, Marker EK, Hoffman RS, Nelson LS (2006) Testing positive for methadone and either a tricyclic antidepressant or a benzodiazepine is associated with an accidental overdose death: analysis of medical examiner data. Acad Emerg Med 13: 543-547.
- Substance Abuse and Mental Health Services Administration (2012) Drug Abuse Warning Network, 2010: National Estimates of Drug-Related Emergency Department Visits. 12-4733, DAWN SeriesD-38.
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, et al. (2007) Release from prison--a high risk of death for former inmates. N Engl J Med 356: 157-165.
- Davoli M, Bargagli AM, Perucci CA, Schifano P, Belleudi V, et al. (2007) Risk of fatal overdose during and after specialist drug treatment: the VEdeTTE study, a national multi-site prospective cohort study. Addiction 102: 1954-1959.
- Caplehorn JR1 (1998) Deaths in the first two weeks of maintenance treatment in NSW in 1994: identifying cases of iatrogenic methadone toxicity. Drug Alcohol Rev 17: 9-17.
- Woody GE, Kane V, Lewis K, Thompson R (2007) Premature deaths after discharge from methadone maintenance: a replication. J Addict Med 1: 180-185.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN (2011) Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 171: 686-691.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 16130
- [From(publication date):
March-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 11629
- PDF downloads : 4501