Case Report Open Access
An Abdominal Mass in a Child with IgA Deficiency: A Case Report
Maria Barbato1*, Ilaria Celletti1, Chiara Di Camillo1, Francesco Valitutti1, Stefania Leoni1, Vanessa Dionne2, Francesca Romana D’Attilia2, Alessandra De Grazia2 and Anna Clerico2
1Sapienza University of Rome, Department of Pediatric Gastroenterology and Liver Unit, Department of Pediatrics, Viale Regina Elena 324, 00161 Rome
2Sapienza University of Rome, Pediatric Oncology Unit, Department of Pediatrics, Viale Regina Elena 324, 00161 Rome
- *Corresponding Author:
- Maria Barbato
Paediatric Gastroenterology and Liver Unit
University Hospital Umberto I - Sapienza University of Rome
Viale Regina Elena 324 - 00161 Rome
Tel: +390649979257
Fax: +390649979325
E-mail: maria.barbato@uniroma1.it
Received date: February 27, 2014; Accepted date: March 19, 2014; Published date: March 26, 2014
Citation: Barbato M, Celletti I, Camillo C, Valitutti F, Leoni S, et al. (2014) An Abdominal Mass in a Child with IgA Deficiency: A Case Report. J Gastroint Dig Syst 4:180. doi:10.4172/2161-069X.1000180
Copyright: © 2014 Barbato M, et al. This is an open-access article distributed under the terms of the CreativeCommons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Introduction: We describe for the first time the case of a one-year old girl admitted to our hospital on the suspicion of an abdominal tumor who finally received the diagnosis of celiac disease and IgA deficiency.
Case presentation: A one-year old girl was admitted to the Pediatric Emergency Care Unit for severe bloating, diarrhea and vomiting for one month; she had been febrile for the last three days. Clinical examination revealed no guarding, a bloated and tender abdomen, and a palpable mass in the umbilical region. Abdominal ultrasonography was then performed, which identified a retroperitoneal mass resembling a tumor; therefore, she was transferred to the Paediatric Oncology Unit for further evaluations. Although deficit of serum IgA delayed the diagnosis, IgG serological markers (anti-deamidated gliadin peptide and anti-transglutaminase antibodies) and duodenal biospy confirmed celiac disease. She was discharged after 23 days on a gluten free diet. The patient was in good health and thriving normally at 12-month follow-up.
Conclusion: Celiac disease can mimic several conditions whose differential diagnoses could be wide. In this case, both IgA deficiency and malnutrition could have led to multiple mesenteric lymphadenopathies, completely regressed once the gluten-free diet was started. If unrecognized, IgA deficiency can jeopardize CD diagnosis since anti-tissue transglutaminase and anti-endomysial antibodies are commonly tested as IgA antibodies. Physicians should always be aware of this association and ascertain IgA serum levels when assessing CD serological markers: if a IgA deficiency is present, demanding for specific IgG serological tests is then mandatory.
Keywords
Celiac disease; IgA deficiency; Abdominal mass; Anti-deamidated gliadin peptide antibodies
Abbreviation
CD: Celiac Disease; Anti-tTG: Anti-Transglutaminase Antibodies; DGP: Anti-Deamidated Gliadin Peptide Antibodies; EMA: Anti-Endomysial Antibodies
Introduction
Celiac disease (CD) is a very frequent immune-mediated disorder triggered by gluten peptides from wheat and related cereals in genetic susceptible individuals (HLA-DQ2 and -DQ8). It is characterized by chronic inflammation of the small intestinal mucosa that gradually leads to the development of villous atrophy and malabsorption. Nowadays it is widely accepted that CD is not merely a gastrointestinal disease, but a systemic disorder. CD is associated with an increased prevalence of other diseases including: iron-deficiency anemia, osteoporosis, IgA deficiency, dermatitis herpetiformis, other autoimmune disorders and malignancies [1].
Selective IgA deficiency, defined as serum IgA levels <0.07 g/L with normal IgM and IgG levels, is the most common primary immunodeficiency worldwide [2]. The vast majority (≈80%) of individuals with selective IgA deficiency may be asymptomatic, while the rest can present clinical manifestations such as recurrent infections, chronic inflammatory disorder of autoimmune etiology, allergic disorders and also malignancies.
IgA antibodies neutralize toxins and microorganisms at mucosal interface by interfering with microbial receptors for epithelial adhesion sites [3]. Within the first six months of life, children rely on passive secretory IgA in breast milk to face infections and to adjust the developing gut microbiota [4].
Here we describe for the first time the case of a one-year old girl admitted to our hospital on the suspicion of an abdominal tumor who finally received the diagnosis of CD and IgA deficiency.
Case Presentation
A one-year old girl was admitted to the Pediatric Emergency Care Unit for severe bloating, diarrhea and vomiting for one month; she had been febrile for the last three days. At admission the child appeared fatigued and her temperature was 38.5°C. Her weight was at 5th percentile (8 kilograms) and her height was at 50th percentile (74 centimetres). Clinical examination revealed no guarding, a bloated and tender abdomen, and a palpable mass in the umbilical region. Abdominal ultrasonography was then performed, which identified a retroperitoneal mass resembling a tumor; therefore, she was transferred to the Paediatric Oncology Unit for further evaluation.
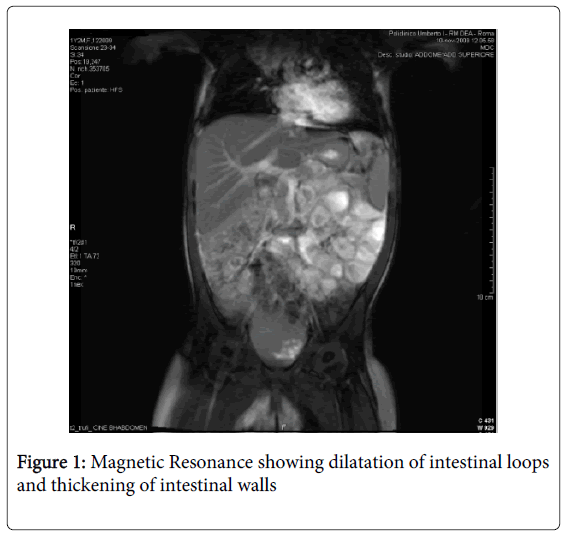
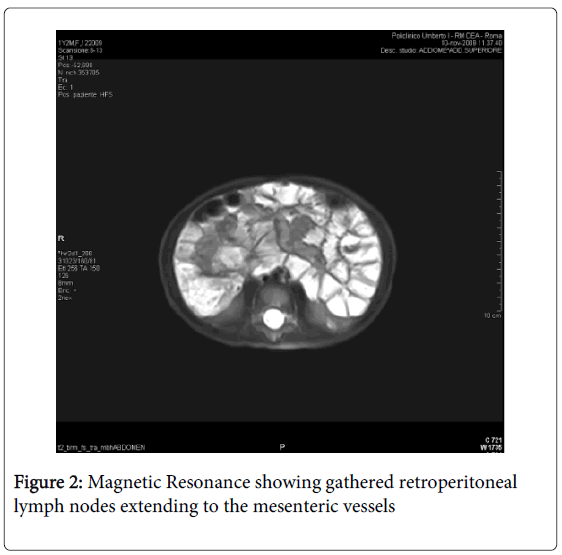
Magnetic resonance imaging (MRI) was performed: a massive abdominal lymphadenopathy (diameter 1-1.5 centimetres) involving gathered retroperitoneal lymph nodes and extending to the mesenteric vessels was identified; dilatation of intestinal loops and thickening of intestinal walls were also present (Figures 1 and 2). First laboratory findings revealed: white blood count 10.1/μl, haemoglobin 11.2 g/dl, haematocrit 67%, platelets 314,000, neutrophils 61.9%, monocytes 7.5%, lymphocytes 26%. The liver and renal function tests were normal, lactic dehydrogenase (LDH) was 300 IU/l (50-450) and the prothrombin time was prolonged. An increased level of chromogranin A (CgA), 159 ng/mL (normal<90), and thymidine kinase (TK), 20 U/L (normal<6), were present. Herpes virus testing, stool cultures for Yersinia, Campylobacter, Giardia, and Clostridium were negative. The child was empirically placed on metronidazole for 7 days (15 mg/kg/day) and fever and diarrhea disappeared within 2 days. Three days after commencing metronidazole therapy, assessment of Serum Immunoglobulins, showed an IgA deficiency with a value of 0.009 g/L (normal values: 0.15-1-50 g/L). IgG serologic markers for celiac disease were then assessed: IgG anti-Deamidated Gliadin Peptide (DGP) antibodies and IgG anti-tissue transglutaminase antibodies (anti tTG-IgG) were positive (both DGP IgG and anti tTG-IgG>100 IU/L). Therefore, the child underwent upper GI endoscopy with multiple duodenal biopsy: during the procedure no macroscopic alteration was present, whereas the biopsy specimens showed total villous atrophy, crypt hyperplasia and intraepithelial lymphocytes. These histological findings correspond to a Stage III C of the Marsh classification for CD. Furthermore, HLA typing was performed: a DQ2 haplotype was present, thus strengthening the diagnosis of CD.
After the diagnosis, the patient went on a gluten-free diet with tremendous clinical improvement: vomiting was not presented anymore during her stay at the hospital. Abdominal ultrasound at day 20 did not show any residual lymphoadenopathy. She was discharged after 23 days with a diagnosis of IgA immunodeficiency and celiac disease.
12-month follow up showed clinical improvement: the patient was healthy, her weight was between 75th- 90th percentile (13,400 Kilograms) and her height was at 75th percentile (88 centimetres).
Discussion
In this patient, investigating the nature of the mesenteric lymphadenopathy was the clinical priority. The child presented with a palpable abdominal mass, abdominal distension, fever, diarrhea, and vomiting. Blood, urine and stool cultures were negative for bacteria, viruses, ova and parasites. The laboratory finding of IgA deficiency (0.009 g/L; normal range 0.15- 1.50 g/L) raised the index of suspicion of CD in the differential diagnosis. The diagnosis of CD was established by serological marker assessment including DGP IgG and tTG IgG in addition to duodenal biopsy. Albeit selective IgA deficiency is the most common primary immunodeficiency with a prevalence of 1/600 among Caucasians, patients with primary IgA immunodeficiency may be very often asymptomatic or, conversely, can present clinical manifestations such as recurrent infections, chronic inflammatory disorder of autoimmune etiology, allergic disorders and also malignancies [2].
CD is the most common non-infectious gastrointestinal disease associated with selective IgA deficiency: in fact, 7.7% of IgA deficiency patients also suffer from CD [5]. Compared to the general population, this prevalence is much higher due to the strong association of IgA deficiency with particular human leukocyte antigens (HLA), among which DQ2 haplotype [6]. This association complicates serological testing for CD and often results in false negative findings at diagnosis since CD serological markers are commonly IgA antibodies [7].
We are tempted to speculate that IgA deficiency and malnutrition led to multiple mesenteric lymphadenopathies with the complicity of a bacterial gastrointestinal infection: the improvement of clinical conditions after an empiric therapy with metronidazole could be speculated accordingly.
Serological positivity for DGP-IgG and tTG-IgG and HLA typing (DQ2), in addition to the histologic findings including total villous atrophy, crypt hyperplasia and intraepithelial lymphocyte infiltration, confirmed the diagnosis of CD. The clinical condition of the child kept improving at three-month follow up visit: abdominal pain and distension were markedly reduced; her weight significantly increased by 1.6 kilograms after three months on a strict gluten-free diet.
Afterward, the whole family was screened for CD by measuring serum IgA, anti-endomysial antibodies (EMA) and tTG IgA. Patient’s older male sibling was positive for EMA and tTG-IgA: he underwent upper endoscopy which confirmed as well the suspicion of CD.
Conclusion
CD may mimic several conditions whose differential diagnoses can be wide. In this case, both IgA deficiency and malnutrition might have led to multiple mesenteric lymphadenopathies resembling a retroperitoneal tumor, completely regressed once the gluten-free diet was started.
If unrecognized, IgA deficiency can jeopardize CD diagnosis since anti-tissue transglutaminase and anti-endomysial antibodies are commonly tested as IgA antibodies. Physicians should always be aware of this association and ascertain IgA serum levels when assessing CD serological markers: if a IgA deficiency is present, demanding for specific IgG serological tests is then mandatory.
References
- Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, et al. (2012) ESPGHAN Working Group on Coeliac Disease Diagnosis: European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J Pediatr Gastroenterol Nutr 54:136-160.
- Yel L (2010) Selective IgA deficiency. J Clin Immunol 30: 10-16.
- Mazanec MB, Nedrud JG, Kaetzel CS, Lamm ME (1993) A three-tiered view of the role of IgA in mucosal defense. Immunol Today 14: 430-435.
- Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, et al. (2014) Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A 111: 3074-3079.
- Meini A, Pillan NM, Villanacci V, Monafo V, Ugazio AG, et al. (1996) Prevalence and diagnosis of celiac disease in IgA-deficient children. Ann Allergy Asthma Immunol 77: 333-336.
- Olerup O, Smith CI, Hammarström L (1990) Different amino acids at position 57 of the HLA-DQ beta chain associated with susceptibility and resistance to IgA deficiency. Nature 347: 289-290.
- Korponay-Szabó IR, Dahlbom I, Laurila K, Koskinen S, Woolley N, et al. (2003) Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency. Gut 52: 1567-1571.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15503
- [From(publication date):
April-2014 - Jul 03, 2025] - Breakdown by view type
- HTML page views : 10853
- PDF downloads : 4650