Review Article Open Access
Amniotic Membrane: A Novel Material for Regeneration and Repair
Aditi Chopra* and Betsy S Thomas
Department of Periodontology, Manipal College of Dental Sciences, Manipal University, Karnataka, India
- Corresponding Author:
- Aditi Chopra
Department of Periodontology
Manipal College of Dental Sciences
Manipal University, Karnataka, India
E-mail: draditichopra@gmail.com
Received date May 23, 2013; Accepted date July 07, 2013; Published date July 14, 2013
Citation: Chopra A, Thomas BS (2013) Amniotic Membrane: A Novel Material for Regeneration and Repair. J Biomim Biomater Tissue Eng 18:106. doi: 10.4172/1662-100X.1000106
Copyright: © 2013 Chopra A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
Amniotic membrane is the inner most lining of the human placenta that is normally discarded after parturition. The membrane has numerous growth factors, proteins and stem cell reserves that help in accelerating wound healing with regeneration of the lost tissues. The preserved human amniotic membrane is a novel tissue engineerined biomaterial that is recently tired in field of medicine and dentistry to regenerate the lost tissues and accelerate repair. This review paper unfolds the inherent structure, properties, mechanisms and the applications of this neglected tissue that makes it a potential material for regeneration especially in the field of oral and periodontal surgeries.
Keywords
Amnion membranes; Growth factors; Regeneration; Repair; Stem cells
Introduction
Wound healing is a complex phenomenon that requires the coordinated interplay of many extracellular matrix (ECM) proteins, growth factors, and cells. With manifold developments in our understanding on periodontal regeneration, biologic and materials sciences; complete regeneration still is an unrealistic situation in many clinical situations due to the complexity of the biological events, factors, and cells involved in regenerative process in the periodontium. Today, the concept of excluding the gingival epithelium with the mere use of barrier membranes has evolved to the incorporation of the correct signaling molecules like the growth factors and the presence of correct cell populations like the stem cells, fibroblast and cementoblast directly into the wound to promote regeneration. Mesenchymal stem cells (MSCs) are one of the major cells populations that play an important role in mediating each phase of the wound-healing process: inflammatory, proliferative, and remodeling. Stem cells therapy is emerging as a powerful tool to generate biological substitutes and regenerate for damaged tissue with high proliferability, differentiability and function. The incorporation of these cells in the periodontal wound may therefore accelerate the periodontal healing. Many efforts are under way to develop novel bioengineered wound-healing products, and include involvement of MSCs in the wound-healing process. Recently, the fetal derived mesenchymal stem cells (MSC) from the placenta or other gestational tissues like the amniotic fluid, umbilical cord are novel materials with rich stem cell reserves. The use of placental tissue for the treatment of wound started more than 100 years ago when by Davis in 1910 first used these fetal membranes as skin substitutes for the treatment of open wounds [1]. Later these membranes were also used for the treatment of burn and repair of conjunctiva defects and as a dressing of chronic ulcers [2]. With no ideal and practical means of preparation, sterilization and storage available at that time, the use of these membranes was very limited. In 1965, Dino et al. demonstrated for the first time that amniotic membrane could be separated, sterilized and safely used at a later date. Since then a lot of research has been initiated to understand the true regenerative potential of this membrane [3].
These cell populations in AM are easily accessible, non tumorigenic and capable of differentiating into a variety of cell types. These cells have the capabilities to stimulate the repair of injured tissues via paracrine actions, and acting as vectors for bio-delivery of exogenous factors. Amniotic epithelial cells (AECs) have several characteristics that make them a great source of stem cells [1]. These stem cells when cultured in vitro have the potential to form bone, soft tissue, muscle, nerve, fat and blood vessel [4-6]. Therefore amniotic membrane (AM) which is usually disposed after parturition can be preserved and used an alternative source of stem cells for basic research and clinical applications. Amnion-derived cells with multipotent differentiation ability have attracted lot of attention in tissue engineering, celltransplantation therapy and periodontal regeneration. This review paper unfolds the inherent structure, properties and mechanisms that support the physiological roles of the amnion membrane in the regeneration of oral and periodontal tissues.
Basic Structure of Amnion Membrane
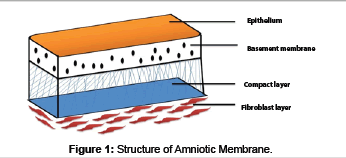
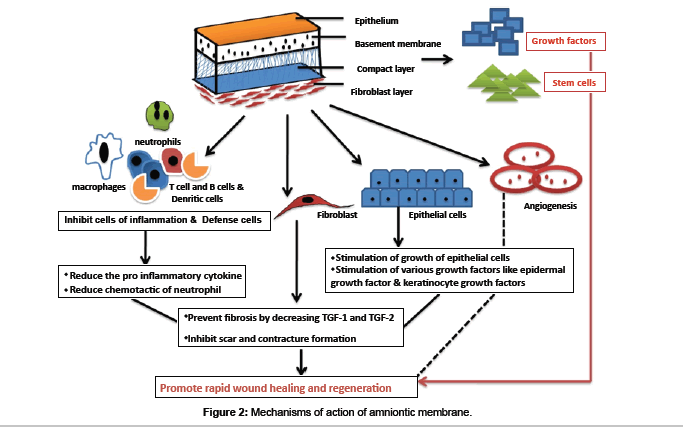
The human placenta is a complex organ starts developing within a few days after fertilization and is very important for the development and survival of the fetus throughout the gestation. It is about 10-15 micrometer thick which consists of two fetal membranes; the inner amniotic membrane and the outer chorion [7]. The AM encases the amniotic fluid and fetus, and is highly flexible because of which it is easily be separated from the chorion. AM has two types of cells with different embryological origins: amnion epithelial cells derived from embryonic ectoderm and amnion mesenchymal cells from embryonic mesoderm. At ultra structural level AM is a thin, tough, transparent, avascular composite membrane composed of three major layers: a single epithelial layer, a thick basement membrane, and an avascular mesenchyme consisting mainly of collagen [8-10]. The amniotic epithelial cell layer is a single layer of flat, cuboidal and columnar cells that are in direct contact with the amniotic fluid. It is from this layer that amniotic MSC (AMSC) are isolated and stored to be used for regenerating tissues. There are no nerves, muscles, or lymphatics in the amniotic membrane [7,11]. These HAE cells and human amniotic mesenchymal cells (HAM cells) express pluripotency and are potent stem cells reservoirs. The amniotic mesoderm layer consists of macrophages and fibroblast-like mesenchymal cells [7]. The basement membrane of the amnion is very similar to the basement membrane found in the other parts of the body like the conjunctiva or gingiva (Figure 1). This makes AM an ideal carrier for ex vivo culture and transplantation of embryonic stem cells (ESCs). The amniotic cells are connected to each other by numerous desmosomes and tight junctions occluding the lateral intercellular spaces with limited paracellur transport [12]. The basal lamina contains a large amount of proteoglycans like heparan sulfate that is one of the major proteoglycan in the gingiva. The amniotic epithelium has a specialized arrangement of intracellular cytoskeletal filaments such as actin, α-actinin, spectrin, ezrin, cytokeratins, vimentin, and desmoplakin indicating their role in the structural integrity and modulation of cell shape of the healing tissue [13]. The spongy layer on the stromal portion of the amnion has an abundance of hydrated proteoglycans and glycoproteins that form a non fibrillar network along with collagen [14]. AECs secrete collagen type III and IV and non collagenous glycoproteins like laminins, nidogen, fibronectin and vitronectin within the basement membrane that serve as adhesion ligands transmitting signals and interacting at cell surface receptors [15]. Laminin and its isoforms mostly 2, 4, 5, 6, 7, 10, and 11 contributes significantly to cell differentiation, cell shape and movement, maintenance of tissue phenotypes and promotion of tissue survival via cell surface receptors such as integrins and dystroglycans [16]. Laminin-5 being the most prevalent in the amnion membrane helps in cellular adhesion of gingival cells, invasive growth of fibroblasts and angiogenesis in the early phases of wound healing. The stroma also contains mitogenic factors, antiangiogenic and anti-inflammatory proteins and natural inhibitors to proteases and anti scarring properties that allow the wound to heal in a much faster and efficient way [17-19]. In addition, the matrix of human AM contains abundant growth factors like keratinocyte growth factor (KGF), basic-fibroblast growth factor (b-FGF), transforming growth factor-beta (TGF-β), nidogen growth factor (NGF), and epidermal derived growth factor (EDGF) which promote periodontal regeneration [12]. These growth factors provide a natural healing environment, accelerate healing and mimic the stem cell niche for ex vivo growth [16]. In addition, the presence of integrin a6/b4 as the main ligands in the basement membrane which participates in the construction of the hemidesmosome like structure favours the adhesion and anchoring of ESCs to the healing wound. This facilitates migration of epithelial cells, reinforces adhesion of basal epithelial cells, promotes epithelial differentiation, and prevents apoptosis [20,21]. Various stem cell markers such as octamer-binding transcription factor (OCT) - 4, hepatocytes nuclear factor-3β (HNF-3β), nestin and nanog which are specific stem cells markers are also found in the amnion membrane [22,23]. The stem cell markers like epidermal marker CA125 [24] and general epithelial markers such as cytokeratins and vimentin are present in large amount in the amniotic epithelial cells [25]. Moreover, human amniotic mesenchymal cells are positive for CD44 and desmin, CK19/ vimentin, indicating co expression of both epithelial and mesenchymal cell markers. In addition, TNF alpha, NGF, BDNF, noggin and activin has been detected in AECs [26,27]. Human MSC have a characteristic cell surface phenotype of CD90+, CD105+, CD73+, CD44+, HLA I+, CD45, CD34, CD11b, HLA II [28]. These markers suggest that the amnion derived cells are completely differentiated into epithelial or mesenchymal cells but remain as undifferentiated stem cells [13]. These MSCs have the ability to accelerate the inflammatory phase towards the proliferative phase which is critical for treating chronic wounds like periodontitis. The mechanisms involved in accelerated wound healing by amnion membrane can be divided as follows (Figure 2):
• Immunomodulative and Immune privilege
• Anti-microbial (broad spectrum effect against bacteria, fungi, protozoa and viruses)
• Reduction of pain
• Anti-scarring and anti-Inflammatory
• Tissue reparative activities with enhanced bone remodeling, osteogenesis and chondrogenesis
• Speed fibrogenesis and angiogenesis
• Increased extracellular matrix deposition
• Potent source of mesenchymal stem cells
Immunomodulative and immune privilege
AM has a unique molecular surface architecture and biochemical properties that is derived from the layer of trophoblast cells which renders it insusceptible to maternal immune attack. The native AECs express the non-polymorphic, non-classical human leukocyte antigen (HLA-G) [29] but lack the polymorphic antigens HLA-A, B (Class IA) and HLA-DR (Class II) on their surfaces [30]. The class I antigen is seen in almost all cells of the amniotic membrane unlike the class II antigen which is only present in some fibroblasts. These mesenchymal stem cells are different from other nucleated mammalian cells as stimulate they show little allogeneic reactivity when administered to MHCunmatched adult immune competent recipients. This immune barrier is due to lack of expression of co-stimulatory cell surface molecules such as CD80 and CD8 mostly in the human. Furthermore, they are actively suppressive of T cell, dendritic cell and B cell function that down-modulate exuberant inflammation. These finding suggest that AECs may be immunologically inert with reduced risk of rejection or immune reaction upon transplantation [31,32].
Antimicrobial effect
The anti-microbial activity of MSCs in the amnion helps to protect the wound from infection. It forms an early physiologic “seal” with the host tissue precluding bacterial contamination. It forms a firm adherence barrier with the wound via fibrin and elastin linkages that seals the wound and prevent contamination [33]. This tight adherence helps in restoring lymphatic integrity, protects circulating phagocytes from exposure and allows faster removal of surface debris and bacteria from the wound [34]. This antimicrobial activity of MSC is mediated by two mechanisms: direct; via secretion of antimicrobial factors such as LL-37 [35] and indirectly, via secretion of immunomodulative factors which will upregulates bacterial killing and phagocytosis by immune cells [36]. These mechanisms will reduce the bacterial counts in the wound and promotes healing. Many bactericidal products of purine metabolism and lysozyme are also found in the amnion membrane. Defensins, mostly beta 3 defensins that helps the epithelial surfaces to resist microbial colonization form a major group of anti-microbial peptides found in the amniotic membranes [37,38]. Apart from defensins, secretary leukocyte proteinase inhibitor (SLPI) and elafin form an important anti-inflammatory and antimicrobial molecule in the amniotic cells. They act as components of the innate immune system to provide protection from infection. Cystatin E, an analogue of cysteine proteinase inhibitors and a powerful antiviral is secreted in large amount by the amniotic cells [39].
Reduction of pain
Amnion membrane (AM) has the unique ability to reduce the pain during the surgical procedure as it diminishes inflammation and provides a better state of hydration that soothes the wound bed to promote faster healing [40]. The soft mucoid lining of amniotic membrane also protects the exposed nerve endings from external irritant that help to decrease pain sensation by preventing nerve stimuli. CAM also supported the growth of the epithelium and facilitates migration and reinforced adhesion.
Anti scarring and anti inflammatory properties
Wound healing stimulates recruitment of various cells like neutrophils, macrophages and giant cells to the surgical site to combat the invading microorganisms and to control the ongoing inflammatory process. Macrophages and giant cells at the surgical site produce cytokines that attract fibroblasts leading to development of fibrosis at the surgical site. Fibroblasts are activated by the transforming growth factor (TGF) beta that is secreted by macrophages and fibroblast in the wound area. AM secretes vascular endothelial growth factor (VEGF), hepatocytes growth factor (HGF) that maintain a proper balance between TGF-1 and TGF-3 that prevents scarring [41]. AM downregulates TGF-beta and its receptor expression by fibroblast that causes a reduced fibrosis at the site. This property helps to modulate the healing of a wound by promoting tissue reconstruction rather than promoting scar tissue formation by excessive fibrosis [19,42]. The direct apoptosis of macrophages by the TGF-beta down regulates inflammation facilitates rapid healing The MSCs in the AM decrease the secretion of the proinflammatory cytokines like tumor necrotic factor-alpha ( TNF-alpha) and interferon (IFN) while simultaneously increasing the production of anti-inflammatory cytokines interleukin (IL) 10, IL -4, IL-1alpha and IL-1beta. Amniotic membrane stromal matrix markedly suppresses lipopolysaccharides induced up regulation of both IL-1alpha and beta [43]. Various immune cells like T cell, dendritic cell and B cell are actively suppressed that prevents pathological remodeling and excessive fibrosis [31,32]. These anti-inflammatory properties of MSCs are beneficial for the treatment of chronic wounds like oral ulcers, herpetic lesions, and healing after any surgical procedures. Both HAE and HAM cells express various anti angiogenic and anti inflammatory proteins such as interleukin 1 receptor antagonist; tissue inhibitors of metalloproteinase (TIMPs) 1, 2, 3, 4 and IL-10 [44]. HAE cells expressed mRNA of tumor necrosis factor TNF alpha, Fas ligand, TNFrelated apoptosis-inducing ligand (TRAIL), TGF beta, and macrophage migration-inhibitory factor (MIF) that prevent fibrosis and scar tissue formation [45]. The supernatants from HAE cell cultures have shown to inhibit the chemotactic activity of neutrophils and macrophages toward macrophage inflammatory protein 2, reduced the proliferation of T- and B-cells after mitogenic stimulation and induced apoptosis of T and B cells. AM also reduces recruitment of various inflammatory cells including polymorphonuclear cells, CD3 cells, CD4 T cells, and CD11b cells to the injured site thereby reducing the inflammation (Figure 2). The facilitation of lipid peroxidation and apoptosis of keratinocytes (programmed cell death) is also reported [46].
Increase vascularization or revascularization
There is an enhanced induction of Vascular Endothelial Growth Factor (VEGF) both for VEGF receptors 1 and 2 by the cells of the AM. Extensive neovascularization observed immediately after its application is attributed to the release of angiogenic factor like insulin derived growth factor (IGF) that promote granulation tissue formation and epithelialization [47]. This angiogenic potential helps in the development of tissue engineered vascular grafts which are useful in revascularization of ischemic tissues, chronic ulcers, repair of bone and cartilage. The increase formation of blood capillary blood flow to the lyophilized amniotic membranes when used as graft material in vestibuloplasty have also shown rapid revascularization and promotes faster healing [48].
Increased extracellular matrix deposition
The ability of MSCs to accelerate the transition from the inflammatory to the proliferative phase is critical for treating chronic wounds with high levels of inflammation is an additional advantage of using AM. MSCs differentiation helps in regenerating the damaged tissue, whereas MSC paracrine signaling regulates the local cellular responses to injury. The paracrine signaling by the MSC help in cell survival, proliferation, migration and gene expression of epithelial cells, endothelial cells, keratinocytes, and fibroblasts [49]. MSC-conditioned medium acts as a chemoattractant for macrophages, endothelial cells, epidermal keratinocytes, and fibroblasts which accelerate wound closure [50]. All the above mentioned properties and inherent stem cell reservoirs makes amniotic membrane a novel material for accelerating wound healing. The stem cells obtained from AM is often compared to other stem cell reservoir most commonly being the bone marrow.
Amniotic Stem Cells as Compared to Bone Marrow Stem Cells
The recovery of MSCs and their in vitro expansion is much greater from the amniotic membrane than from any other source like as the bone marrow stroma. The ex vivo expansion of MSCs from the amniotic membrane is a non-invasive procedure that yields much higher amount of stem cells than many other sources like bone marrow [51]. The ability to ensure complete regeneration from a stem cell depends on the age of the MSC from the donor tissue [52]. Bone marrow mesenchymal stem cell and progenitor cells unlike AM mesenchymal cells show decreasing proliferative potential with increasing age [53-55]. The flow cytometry analysis has even shown an immunophenotypical profile of AM-MSCs consistent with that for bone marrow derived MSCs with AM- hMSCs positive for CD105, CD73, CD29, CD44 and CD166 while it is negative for CD14, CD34 and CD45 [56]. In addition, AM-isolated cells undergo in vitro osteogenic, adipogenic, chondrogenic and myogenin expression along with CD34 and von willebrand factor positive cells which permits unrestricted use, distribution and reproduction of these MSC in culture medium [56]. These cells also express the surface markers associated with embryonic stem cell, e.g. SSEA-3, SSEA-4, TRA-1-60, TRA-1-81, pluripotent stem cell-specific transcription factors such as Oct-4 and Nanog [57]. The AECs may be a superior source of MSC than the adult bone marrow is their method of culturing. These cells can proliferate without any need of a second cell type serving as a feeder layer as they create their own feeder layer with some cells spreading out at the bottom of the culture dish. The basal layer of AECs that attaches to the culture dish may play the role of an autologous feeder layer, serving as a substrate for attachment or possibly providing secreted factors that help induce or maintain undifferentiated AECs. An average yield is more than 100 million AECs per amnion that is much higher than those collected from the other stem cell reserves [57].
Preparation of Amniotic Membrane Isolation and Cultivation of Amniotic Cells
All donated tissue should follow a strict guideline for procurement, processing and distribution before they can be used clinically. The AM can be used either alone with only amniotic epithelium (intact AM) or without it (denuded AM). In denuded AM only the cellular components are removed leaving the structural components like the basement membrane intact. The native and intact AM contains higher levels of EGF, KGF, HGF and FGF beta compared to epithelial denuded AM as removal of epithelium eliminates nearly all important growth factors [58]. To isolate fetal-derived MSC-like cells from the amniotic membrane the technique of enzymatic digestion is used. A detergentbased protocol with sodium dodecyl sulphate (SDS) is used to remove the amniotic epithelium from AM while maintaining the histoarchitecture of the matrix. The amniotic cells were isolated by sequential trypsin and collagenase digestion that remove the epithelial layer of cells and digested the stromal cells respectively. The amnion is then washed in phosphate-buffered saline (PBS) and then cut into pieces in PBS containing 0.03% hyaluronidase and 0.025% deoxyribonuclease I. The resulting minced amnion is digested with 0.2% trypsin and incubated at 37°C with constant stirring at 100 rpm for 30 min. The mixture is then poured to separate the dispersed amnion epithelial cells from the tissue pieces. The dispersed epithelial cells are collected by centrifugation and suspended in DMEM supplemented with heat-inactivated 10% Fetal Calf Saline and 1% antibiotic-antifungal solution. It is then seeded into culture dishes at a concentration of 3.0×104 cells /cm2 and incubated at 37°C under 5% carbon dioxide in air. Trypsinization of the amnion is repeated several times until no more HAE cells were obtained. The dispersed mesenchymal cells of amnion are collected by filtration through gauze and centrifugation [59].
Special processing and sterilization is recommended to ensure consistent quality and preservation of the properties of AM. Various methods have been tried to preserve the AM include: hypothermic storage at 4°C, freeze drying through liquid nitrogen at -196°F, γ-sterilization, glycerol preservation and cryopreservation. The media and storage temperature used for the preservation process affects the viability of cells and growth factors in the AM. Sterilization with γ-rays has no significant effect on growth factor content in the human AM. While storage of AM in glycerol at 4°C will result in immediate cell death, cooling will preserves the membrane for an indefinite time and make it bacteriologically pure and immunologically inert [60]. Cryopreservation with dimethylsulphoxide (DMSO) at -80°C is an important modality for preservation of these tissues as it keeps the viable for a longer period of time but causes loss of some angiogenic factors and cell rupture [22,60]. To overcome these problems with cryopreservation, freeze dried - irradiated (Lyophilized) is the one the most commonly used preservation technique that preserves the original size and shape with minimum cell rupture [61]. The membrane is first freeze dried at -60°C under vacuum (atmospheric pressure 102 mm of Hg) for 48 hours and then irradiated with 2.5 mega Rads (25 K Gray) in a batch type cobalt-60 irradiator [62,63].
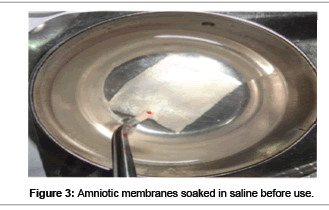
The far-infrared rays and microwaves are also used for sterilization of amniotic membrane which is known as the Hyper-dry-amnion. During the drying process, the temperature inside the hyperdrying device should not exceed 35°C as high temperatures on the surface that can reach 60°C can decrease the degradation of tissue-protein. Compared to cryopreserved amnion, which can be preserved for less than 3 months at 80°C, “Hyper-dry amnion” can be preserved at room temperature indefinitely until the packet is cut open. It is easily cut to the desired size and shape just before application. The freeze dried membrane can be readied for use by soaking in normal saline for 1 minute (Figure 3). It returns to a layered structure similar to that of fresh amnion when it absorbs water unlike the hyperdry AM. However upon hydration, the freeze dried amnions did not recover their thickness, and their histologic appearance was different from a fresh AM [64]. Gluteraldehyde fixation is recently introduced method to fix the AM that provide better stability and properties. This requires neither antibiotics nor the use of special storage techniques and renders the amnion sterile and non-immunogenic. Gluteraldehyde treated amnion (SAM) is employed successfully as a microvascular interpositional graft in many experimental animals and is the area of further research [65].
Even after proper sterilization by any technique, a proper screening to test for infectious diseases such as human immunodeficiency virus (HIV) type 1 and 2 antibodies, human T-lymphotropic virus (HTLV) type 1 and 2 antibodies, Hepatitis C antibody, Hepatitis B surface antigen, Hepatitis B core total antibody, serological test for Syphilis, HIV type 1 nucleic acid test, and Hepatitis C virus nucleic acid test form an mandatory step. Though amnion membrane is considered totally non immunogenic, low-grade inflammatory responses occurs with viable amniotic epithelial cells. The immunogenicity of cryopreserved tissue is less than that of fresh tissue as more than 50% of AECs remain viable for two months [66]. Although cryopreservation makes the tissue non immunogenic, it also introduces many problems like incomplete sterilization that which may transmit diseases. Biological and ultrastructural properties of a freeze-dried irradiated human amniotic membrane are different from the decellularized and dehydrated human amniotic membrane (DDHAM) product with cryopreserved human amniotic membrane (CHAM). Immunohistochemical examination and transmission electron microscopy revealed the disruption of the trilaminar structure of the basement membrane in CHAM and loss of collagen IV and VII, laminin and fibronectin in DDHAM. Lower levels of growth factors were seen in DDHAM compared to CHAM. Though significant differences in composition and ultrastructure exist between DDHAM and CHAM, no difference in cell survival has been noted in vivo [67].
Before the membrane is applied, the wound should be prepared after thorough removal of granulation tissue. Membrane is applied with rough (chorionic) surface next to the wound. Care is taken to ensure no that there is no air bubbles trapped between the membrane and wound. Freeze dried irradiated membrane is also used as described above, but before application it is soaked in sterile saline for 1-2 minutes. CAM is manufactured with the stromal side of the graft attached to a white nitrocellulose filter paper that is sticky unlike the epithelial side which is non-sticky and shiny. Fibrin glue sticks to the stromal side and permits adherence of the membrane to the wound even without suturing. If the tissue damage is deep, multiple layers of CAM can be placed to fill the defect. CAM does not fuse with the host epithelium or prevent epithelialization.
Uses of the Amniotic Membrane
Though the amniotic membrane has been used in general surgery for a long period of time, it use in dentistry especially in oral and periodontal surgeries is increasing with promising results. Its biological properties of rapid re epithelialization, vascularization and formation of granulation tissue makes AM a good biological dressing. These fetal membranes are being used as a graft or dressing in the management of burns; in the reconstruction of the oral cavity, bladder, and vagina; tympanoplasty; arthroplasty and so forth. Its adhesive and tight contact with the injured surface promotes hemostasis and good pain relief due to exposition of nerve fibres. The ECM of the AM is an effective conduit for peripheral nerve regeneration and is used as a biodegradable scaffold with unique biochemical because of this it is used for neuronal regeneration and differentiation [68]. AM has shown to reduce the acute inflammatory response in scalpel and laser surgery, chemical and thermal burns and as covering for epithelial defects after flap necrosis following surgery in the head and neck region [69]. Dried irradiated human amniotic membrane as a biological dressing for facial burns and wounds with promising results [70]. Good biocompatibility and mechanical properties like permeability, stability, elasticity, flexibility, plasticity, and resorbability also makes it a promising scaffolding material in tissue engineering as in cell adhesion and the potential for delivery of biomodulatory agents such as growth factors and genetic materials [71,72]. This amnion membrane is an excellent micro carrier for culture technique as it involves a development of a three dimensional space that is suitable for the growth and proliferation of embryonic stem cells (ESCs). Most micro carriers available at present are made of artificially synthesized biomaterials that lack the basement membrane structure and are unable to mimic the stem cell niche or microenvironment for adhesion and proliferation of ESCs. AM has structure similar to the basement membrane of gingiva because of which it can be utilized as a feeder layer for stem cells culture and amplification for tissue engineering. The graft of amniotic membrane is a viable and reliable method to cover the exposed periosteum as they serve as a good alternative to mucosal and skin grafts [73].
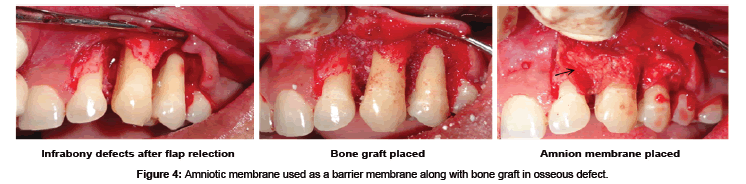
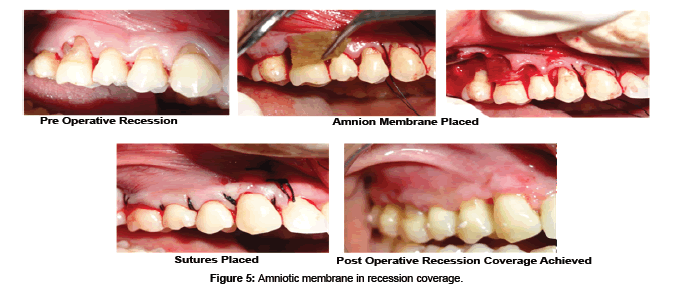
AM may be used as alternative treatment to manage wounds in the oral cavity like the tongue, buccal mucosa, vestibule, palatal mucosa, and floor of the mouth [74]. Antiinflammatory and antiscarring property of AM have shown decreased necrosis and rapid healing of ulcers with herpes simplex virus (HSV), varicella zoster virus-infected tissues, erythema multiforme major (Stevens-Johnson syndrome) and cervical necrotizing fasciitis [75]. The inflammatory response after reactivation of HSV from its latent stage is mediated by a cluster of differentiation positive (CD4+) cells with the contribution of neutrophils and macrophages. AM are known to decrease the neutrophils migration to the site in the ulcer and thus provide a good anti-inflammatory property with reduction in necrosis. Murine fetal submandibular gland (SMG) explants when cultured on different preparations of AM scaffolds have resulted in a well- developed branching morphogenesis in the gland. SMG epithelial cell converted to a spindle-shape with loss of intercellular connections, changed cytoskeleton organization, and exhibited scattering behaviors. Hepatocytes growth factor (HGF), a bioactive epithelial scattering factor found abundantly in the cultivated AM epithelia influenced the cell behaviors and structure formation of SMG [75]. HAM has been tried in the reconstruction of TMJ ankylosis as it prevents fibrosis and reankylosis when used as an interpositional material [76]. AM is even used as a carrier for local delivery of the various drugs like antibiotic netilmycin (NTM) and antiviral drugs like acyclovir (ACV) and trifluridine (TFU) [77]. Amnion has been tried as a graft material after vestibuloplasty where it prevents secondary contraction after the surgery and maintains the postoperative vestibular depth [78]. Hyperdry amnion or cryopreserved amniotic membrane tissue is used as a barrier membrane in the treatment of periodontal osseous defect with or without bone graft (Figure 4) [79] and even tried in the management of gingival recession with guided tissue regeneration (Figure 5) [80].
These membranes are extremely thin around 300 nm in cross sectional thickness unlike the other collagen membranes used for guided tissue regeneration which are around 700 - 800 nm [81]. Because of its thin diameter it intimately molds according to the defect anatomy and root surfaces easily. However further long term clinical trial and research on the stability of the hyperdry AM in the oral environment remains to be elucidates. Though it is known to keeps its strength and morphology at least one month in vitro when soaked in sterilized physiologic saline solution at room temperature, further investigation is needed to evaluate its ability to resist the masticatory forces, biodegradable rate for subsequent repair and maturation of the mucosal tissues when used as a barrier membrane.
Conclusion
The use of this novel biological membrane is rising in various fields of in tissue engineering, medicine, regeneration biology and stem cell research. However, further research and long term clinical trials investigating the full potential of this potential stem reservoir are still warranted to strengthen the fact amniotic membrane is indeed a reservoir for regeneration and repair.
References
- Naughton GK (2002) From lab bench to market: critical issues in tissue engineering. Ann N Y Acad Sci 961: 372-385.
- De Rotth A (1940) Plastic repair of conjuctival defects with fetal membranes. Arch opthalmol 23: 522-525.
- Dino BR, Eufemio GG, DeVilla M, Reysio-Cruz M, Jurado RA (1965) The use of fetal membrane as homografts in local management of burns. J Philipp Med Assoc 41: 890-898.
- Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, et al. (2004) Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation 78: 1439–1448.
- In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH et al. (2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22: 1338–1345.
- Yen BL, Chien CC, Chen YC, Chen JT, Huang JS, et al. (2008) Placenta-derived multipotent cells differentiate into neuronal and glial cells in vitro. Tissue Eng Part A 14: 9–17.
- Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, et al. (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 26: 300-311.
- Burgos H (1986) Angiogenic factor from human term placenta. Puri?cation and partial characterization. Eur J Clin Invest 16: 486–93.
- Onerci M (1991) The effects of lyophilized homograft amniotic membrane on wound healing on rabbits. Acta Otorhinolaryngol Ital 11: 491–6.
- Benirschke K, Kaufman P (2000) Pathology of the human placenta. New York: Springer-Verlag; 273-281.
- Blackburn S (2003) Maternal, Fetal and Neonatal Physiology: a clinical prospective: Saunders: an imprint of Elsevier Science 345.
- King BF (1982) Cell surface specializations and intercellular junctions in human amniotic epithelium: an electron microscopic and freeze-fracture study. Anat Rec 203: 73-82.
- Bani-Yaghoub M, Wilson P, Hengstschlager M, Nikaido T (2012) Amniotic Stem Cells: Potential in RegenerativeMedicine. Stem Cells International.
- Parry S, Strauss JF 3rd (1998) Premature rupture of the fetal membranes. N Engl J Med 338: 663-670.
- Sniadecki NJ, Desai RA, Ruiz SA, Chen CS (2006) Nanotechnology for cell-substrate interactions. Ann Biomed Eng 34: 59-74.
- Akashi T, Miyagi T, Ando N, Suzuki Y, Nemoto T, et al. (1999) Synthesis of basement membrane by gastrointestinal cancer cell lines. J Pathol 187: 223-228.
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ (1995) Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267: 891-893.
- Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwoood NJ, Quantock AJ, et al. (2000) Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 20: 173-180.
- Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC (2000) Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res 20: 325-334.
- Diwan SB, Stevens LC (1976) Development of teratomas from the ectoderm of mouse egg cylinders. J Natl Cancer Inst 57: 937-942.
- Enders AC, King BF (1988) Formation and differentiation of extra embryonic mesoderm in the rhesus monkey. Am J Anat 181: 327-340.
- Kubo M, Sonoda Y, Muramatsu R, Usui M (2001) Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 42: 1539-1546.
- Witkowska-Zimny M, Walenko K (2011) Stem cells from adipose tissue. Cell Mol Biol Lett 16: 236-257.
- Nanbu Y, Fujii S, Konishi I, Nonogaki H, Mori T (1989) CA 125 in the epithelium closely related to the embryonic ectoderm: the periderm and the amnion. Am J Obstet Gynecol 161: 462-467.
- Beham A, Denk H, Desoye G (1988) The distribution of intermediate filament proteins, actin and desmoplakins in human placental tissues as revealed by polyclonal and monoclonal antibodies. Placenta 9: 479-492.
- Koyano S, Fukui A, Uchida S, Yamada K, Asashima M, et al. (2002) Synthesis and release of activin and noggin by cultured human amniotic epithelial cells. Dev Growth Differ 44: 103-112.
- Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, et al. (2000) Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res 62: 585-590.
- Roubelakis MG,Trohatou O, Anagnou NP (2012) Amniotic Fluid and Amniotic Membrane Stem Cells: Marker Discovery. Stem Cells International.
- Lefebvre S, Adrian F, Moreau P, Gourand L, Dausset J, et al. (2000) Modulation of HLA-G expression in human thymic and amniotic epithelial cells. Hum Immunol 61: 1095-1101.
- Sakuragawa N, Tohyama J, Yamamoto H (1995) Immunostaining of human amniotic epithelial cells: possible use as a transgene carrier in gene therapy for inborn errors of metabolism. Cell Transplant 4: 343-346.
- Wei JP, Zhang TS, Kawa S, Aizawa T, Ota M, et al. (2003) The Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transplant 12: 545–552.
- Weiss ML, Anderson C, Medicetty S, Seshha Reddy KB, Wiess RJ, et al. (2008) Immune response of human umbilical wharton jelly-derived cell. Stem Cells 26: 2865-74.
- Walker AB, Cooney DR, Allen JE (1977) Use of fresh amnion as burn dressing. J Pediatr Surg 12: 391.
- Rao TV, Chandrasekhram V (1981) Use of dry human and bovine amnion as a biological dressing. Arch Surg 116: 891-896.
- Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, et al. (2010) Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem cells 28: 2229-38.
- Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y (1991) Antibacterial properties of human amniotic membranes. Placenta 12: 285-288.
- Krisanaprakornkit S, Weinberg A, Perez CN, Dale BA (1998) Expression of the peptide antibiotic human beta defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun 66: 4222-4228.
- Harder J, Bartels J, Christophers E, Schroder JM (2001) Isolation and characterization of human beta defensin-3: a novel human inducible peptide antibiotic. J Biol Chem 276: 5707-5713.
- King AE, Paltoo A, Kelly RW, Sallenave JM, Bocking AD, et al. (2007) Expression of natural antimicrobials by human placenta and fetal membranes. Placenta 28: 161-169.
- Roa TV, Chandrashekharam V (1981) Use of dry human bovine amnion as a biological dressing. Arch Surg 116: 891-896.
- Ono I, Yamashita T, Hida T, Jin HY, Ito Y, et al. (2004) Combined administration of basic fibroblast growth factor protein and the hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. Wound Repair Regen 12: 67-79.
- Tseng SC (2001) Amniotic membrane transplantation for ocular surface reconstruction. Bioscience Reports 21: 481-9.
- Solomon A, Rrosenblatt M, Monroy D, Ji Z, Pflugfelder SC, et al. (2001) Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol 85: 444-449.
- Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F (2000) Identification of antiangiogenic and anti-inflammatory proteins in human amniotic membrane. Cornea 19: 348-352.
- Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, et al. (2005) Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci 46: 900-907.
- Park WC, Tseng SCG (2000) Modulation of acute inflammation and keratocyte death in blood and amniotic membrane in PRK. Invest Ophthalmol Vis Sci 41: 2906-2914.
- Kim JS, Kim JC, Na BK, Jeong JM, Song CY (2000) Amniotic membrane patching promotes healing and Inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res 70: 329-337.
- Samandari MH, Yaghmaei M, Ejlali M, Moshref M, Saffar AS (2004) Use of amnion as a graft material in vestibuloplasty: a preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97: 574-8.
- Hocking AM, Gibran NS (2010) Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 316: 2213-2219.
- Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA (2012) Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem Cells Trans Med 1: 142-149.
- Brooke G, Tong H, Levesque JP, Atkinson K (2008) Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev 17: 929-940.
- Ringe J, Kaps C, Burmester GR, Sittinger M (2002) Stem cells for regenerative medicine: advances in the engineering of tissues and organs. Naturwissenschaften 89: 338-51.
- Caplan AI (1994) The mesengenic process. Clin Plast Surg 21: 429-35.
- Bergman RJ, Gazit D, Kahn AJ, Gruber H, Mcdougall S, et al. (1996) Age-related changes in osteogenic stem cells in mice. J Bone Miner Res 11: 568-77.
- Stenderup K, Justesen J, Clausen C, Kassem M (2003) Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33: 919-26.
- Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, et al. (2007) Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol 7: 11-15.
- Miki T, Lehmann T, Cai H, Stolz DB, Strom SC (2005) Stem cell characteristics of amniotic epithelial cells. Stem Cells 23: 1549-1559.
- Branski LK, Kulp G, Jeschke MG, Norbury WB, Herndon DN (2007) Amniotic membrane as wound coverage: The effects of irradiation and different processing methods on growth factor content. J Surg Res 137: 339-346.
- Wilshaw SP, Kearney JN, Fisher J, Ingham E (2006) Production of an acellular amniotic membrane matrix for use in tissue engineering. Tissue Eng 12: 2117-2129.
- Hennerbichler S, Reichl B, Wolbank S, Eibl J, Gabriel C, et al. (2007) Cryopreserved amniotic membrane releases angiogenic factors. Wound Rep Reg 15: A51.
- Nazri MM (1990) Freeze-drying: the latest in food technology. Friday Dawn Magazine, Karachi, February II: 16.
- Notea E, Hirshowitz B, Karev A, Levi J, Mahler D (1975) [Lyophilized amnion in burns and skin loss]. Harefuah 88: 265-267.
- Siddiqui MA (1981) Freeze-dried, radiated sterilized human amniotic membrane as a biological dressing for burns and chronic ulcers. Liaquat Medical College, Jamshoro. (unpublished report)
- Kitagawa K, Yanagisawa S, Watanabe K, Yunoki T, Hayashi A, et al. (2009) A hyperdry amniotic membrane patch using a tissue adhesive for corneal perforations and bleb leaks. Am J Ophthalmol 148: 383-389.
- Thomson PD, Parks DH (1984) Amnion as a wound dressing. In: Wise DL (Ed.), Burn wound coverings, Boca Raton: CRC Press 48-49.
- Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I (1981) Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 2: 1003-1005.
- Lim LS, Poh RW, Riau AK, Beuerman RW, Tan D, et al. (2010) Biological and ultrastructural properties of acelagraft, a freeze-dried γ-irradiated human amniotic membrane. Arch Ophthalmol 128: 1303-1310.
- Meng XT, Chen D, Dong ZY, Liu JM (2007) Enhanced neural differentiation of neural stem cells and neurite growth by amniotic epithelial cell co-culture. Cell Biol Int 31: 691-698.
- Talmi Y, Finkelstein Y, Zohar Y (1990) Use of human amniotic membrane as a biologic dressing. Eur J Plast Surg 13: 160-2.
- Bujang-Safawi E, Halim AS, Khoo TL, Dorai AA (2010) Dried irradiated human amniotic membrane as a biological dressing for facial burns--a 7-year case series. Burns 36: 876-882.
- Baguneid MS, Seifalian AM, Salacinski HJ, Murray D, Hamilton G, et al. (2006) Tissue engineering of blood vessels. Br J Surg 93: 282-290.
- Walgenbach KJ, Voigt M, Riabikhin AW, Andree C, Schaefer DJ, et al. (2001) Tissue engineering in plastic reconstructive surgery. Anat Rec 263: 372-378.
- Yang S, Leong KF, Du Z, Chua CK (2001) The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng 7: 679-689.
- Arai N, Tsuno H, Okabe M, Yoshida T, Koike C, et al. (2012) Clinical application of a hyperdry amniotic membrane on surgical defects of the oral mucosa. J Oral Maxillofac Surg 70: 2221-2228.
- Hsiao YC, Lee HW, Chen YT, Young TH, Yang TL (2011) The impact of compositional topography of amniotic membrane scaffold on tissue morphogenesis of salivary gland. Biomaterials 32: 4424-4432.
- Florian B, Lukas MH, Klaus-Dietrich W, Marco R (2013) Temporomandibular joint arthroplasty with human amniotic membrane. A case report. E plasty journal 13: 129-130.
- Mencucci R, Menchini U, Dei R (2006) Antimicrobial activity of antibiotic-treated amniotic membrane: An in vitro study. Cornea 25: 428-431.
- Kothari CR, Goudar G, Hallur N, Sikkerimath B, Gudi S, et al. (2012) Use of amnion as a graft material in vestibuloplasty: a clinical study. Br J Oral Maxillofac Surg 50: 545-549.
- Holtzclaw DJ, Toscano NJ (2012) Amnion-chorion allograft barrier used for guided tissue regeneration treatment of periodontal intrabony defects: A retrospective observational report. Clinical Advances in periodontics 3: 131-137.
- Gurinsky B (2009) A novel dehydrated amnion allograft for use in the treatment of gingival recession: An observational case series. The Journal of Implant & Advanced Clinical Dentistry 1: 124-30.
- Rothamel D, Schwarz F, Sager M, Herten M, Sculean A, et al. (2005) Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res 16: 369-378.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 27612
- [From(publication date):
July-2013 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 22127
- PDF downloads : 5485