Short Communication Open Access
Amnesic Shellfish Poisoning: Emergency Medical Management
George Schroeder1*, Stephen S. Bates2 and John Spallino3
1American Academy of Urgent Care Medicine 2813 Hiawassee Road, Suite 206 Orlando, FL USA 32835
2Fisheries and Oceans Canada Gulf Fisheries Centre P.O. Box 5030 Moncton, NB E1C 9B6, Canada
3Laser Spine Institute 3001 N Rocky Point Dr. # 185 Tampa, FL 33607 USA
- *Corresponding Author:
- George Schroeder
American Academy of Urgent Care Medicine
2813 Hiawassee Road, Suite 206
Orlando, FL USA 32835
Tel: +13214740980
E-mail: george.schroeder@octapharmaplasma.com
Received date: October 12, 2015; Accepted date: December 21, 2015; Published date: December 29, 2015
Citation: Schroeder G, Bates SS, Spallino J (2015) Amnesic Shellfish Poisoning: Emergency Medical Management. J Marine Sci Res Dev 6:179. doi:10.4172/2155-9910.1000179
Copyright: © 2015 Schroeder G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Keywords
Amnesic shellfish poisoning; Diatom; Domoic acid; Excitotoxicity; Neurotoxin; Pseudo-nitzschia
Introduction
Human consumption of shellfish and certain finfish contaminated with the neurotoxin domoic acid causes Amnesic Shellfish Poisoning (ASP), a syndrome that results in preventable morbidity and mortality [1-5]. Although the incidence of ASP is rare around the world due to careful monitoring by government agencies since the original incident in 1987, patients can still present with clinical symptoms (Table 1) that may not be known to physicians. Hence, the goal of this report is to provide information to clinicians, along with relevant background material about the biological source of the neurotoxin.
History of the Event
ASP was first described in late 1987, in eastern Canada, when at least 153 human cases of intoxication and four deaths of elderly patients were reported, along with significant morbidity in other patients [6-8]. All were determined by Health Canada to have consumed aquacultured blue mussels (Mytilus edulis) from Prince Edward Island, eastern Canada.
Identification and Source of the Toxin
The toxin in the mussels was discovered to be domoic acid, a small (311 g/mol) polar amino acid [9]. Surprisingly, this toxin was identical to the one reported in Japan in 1959, from a marine red macroalga, Chondria armata. Seaweed extracts containing domoic acid had been used as an effective anthelminthic to treat intestinal worms in Japanese children. However, in the case of the Canadian outbreak, an order of magnitude higher dose (~290 mg) was consumed, compared to the “therapeutic” anthelminthic dose (~20 mg) used by the Japanese in treating for parasites [3,10]. Moreover, the elderly Canadian patients were more vulnerable and at a higher risk of mortality due to reduced renal function, as well as having a compromised blood-brain barrier [11]. The poisoning led to serious and potentially life-threatening neurological sequelae, including primarily serious and protracted generalized convulsions and seizures, as well as memory loss (hence the name of the syndrome) and even death [7,8]. The toxin also resulted in dermatologic, pulmonary, ophthalmic, as well as gastrointestinal and neurologic manifestations (Table 1) [12,13].
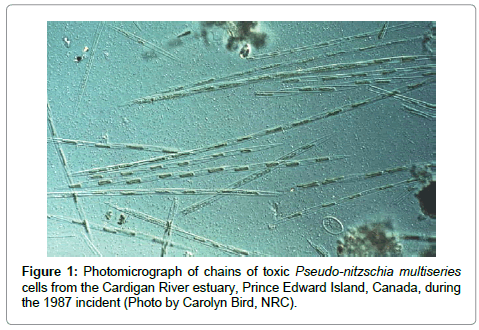
The biological source of domoic acid in the 1987 poisoning event was not seaweed, but rather species of phytoplankton, the marine pennate diatom Pseudo-nitzschia multiseries (Figure 1) [6]. At present, 45 species of the genus Pseudo-nitzschia have been discovered, of which 19 produce domoic acid [14]. These toxigenic species are found worldwide [3,4]; another diatom, Nitzschia navis-varingica, also produces domoic acid [15]. Mounting global research has discovered that repeated seasonal harmful algal blooms (HABs) of toxigenic Pseudo-nitzschia species exposed birds (e.g. pelicans, cormorants; [16]), marine mammals (e.g. sea lions, seals, dolphins, whales; [17]), and marine fish [18,19] to toxic and sublethal doses of domoic acid. Blooms of toxigenic Pseudo-nitzschia have become more prevalent along coastal waters worldwide. The 2015 toxic bloom along the entire west coast of North America resulted in numerous harvesting closures and human health concerns. It is not known why this diatom produces domoic acid, as this biotoxin does not appear to harm its immediate predators.
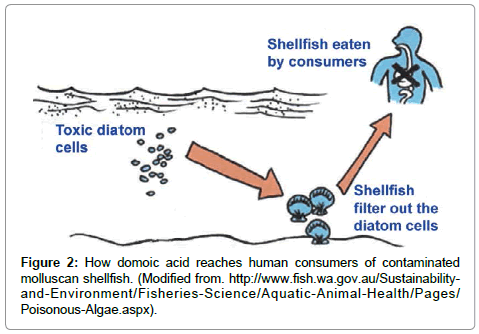
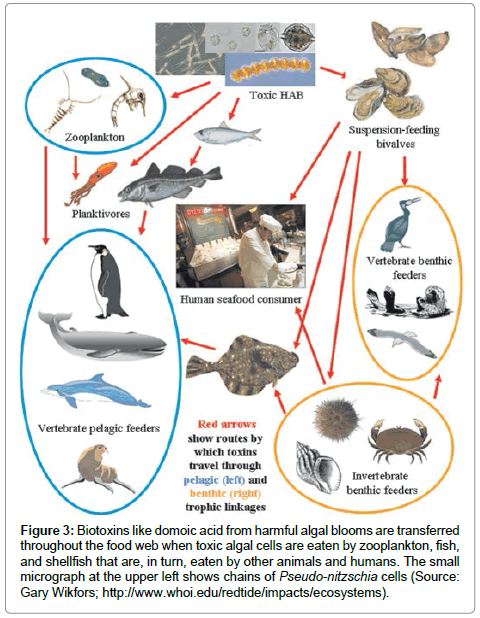
Humans become poisoned after consuming molluscan shellfish (e.g., mussels, clams, oysters, scallops, cockles) that have filtered the toxic diatom cells out of the water, therefore concentrating the toxin in their digestive system (Figure 2). The toxin can be present at very high in bivalve shellfish, particularly within the viscera and intestinal tracts [6]. Humans can also become poisoned by eating planktivorous fish (e.g., anchovies, sardines) that have fed on the toxic diatoms, recreational fish (e.g., white croaker, staghorn sculpin, coho salmon) that feed on other contaminated pelagic fish, or benthic organisms (e.g., Dungeness crabs) (Figure 3). The serious impact of chronic low doses of domoic acid on human health are not understood, compared to what is known about exposure to high doses [10,19,20]. This is especially problematic for coastal indigenous populations that consume molluscan shellfish on a regular basis.
Figure 3: Biotoxins like domoic acid from harmful algal blooms are transferred throughout the food web when toxic algal cells are eaten by zooplankton, fish, and shellfish that are, in turn, eaten by other animals and humans. The small micrograph at the upper left shows chains of Pseudo-nitzschia cells (Source: Gary Wikfors; http://www.whoi.edu/redtide/impacts/ecosystems).
ASP is one of several marine biotoxin poisonings, along with Paralytic Shellfish Poisoning (PSP; saxitoxin and derivatives), Diarrhetic Shellfish Poisoning (DSP; okadaic acid and derivatives), Azaspiracid Shellfish Poisoning (AZP; azaspiracid and derivatives), Neurotoxic Shellfish Poisoning (NSP; brevetoxins) and Ciguatera Fish Poisoning (CFP; ciguatoxin), caused by another group of marine phytoplankton, dinoflagellates [21-23]. Other biotoxins include pectenotoxins, spirolide toxins and yessotoxins, produced by other species of dinoflagellates [23].
Pathophysiology of Amnesic Shellfish Poisoning
Domoic acid and its 11 natural isomers (some with less toxicity) act as potent excitatory neurotransmitters. The molecule is heat-stable and binds strongly to the same receptor sites as its biochemical analogues, glutamic acid and kainic acid. However, domoic acid is three times more potent than kainic acid and 30-100 times more so than glutamic acid [24].
An acute neuronal hyperexcitation syndrome is caused by toxic levels of domoic acid in humans. As well, peripheral neurons are affected, followed by a chronic loss of function in neurological systems [11,12,25,26]. These are most susceptible to excitotoxic degeneration, especially in the hippocampus amygdala and anterior horn cells of the human spinal cord.
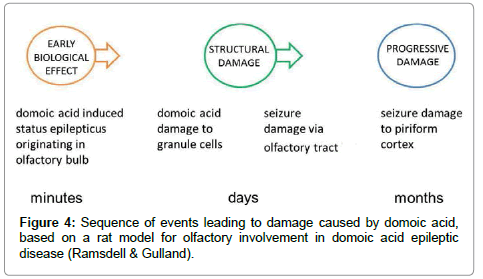
Neurotoxic synergism is postulated to occur by voltage-dependent magnesium block at the NMDA receptor channel, followed by domoic-acid-mediated activation of non-NMDA receptors. This leads eventually to chronic loss of function in short-term memory, as well as certain cognitive functions, and has even been associated with dementia [27,28]. A rat model has shown the sequence of events leading to damage caused by domoic acid (Figure 4) [29]. Mice exposed to domoic acid in the laboratory get limbic seizures, gait abnormalities and demonstrable degeneration of their hippocampus [26]. The effects of domoic acid on the nervous system and memory have triggered interest in those studying Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease, and other dementias.
Clinical Manifestations, Symptoms and Signs
Extensive research on patients of the 1987 intoxication revealed a wide range in the onset time of symptoms: 15 min to 38 hrs (mean, 5.5 hrs) after ingestion, depending on the amount and cumulative absorption of ingested toxins [7,8]. The maximal neurologic deficits were observed, four hours post-ingestion, in patients exhibiting the least effect, versus 72 hours in patients most affected by domoic acid. A summary of the symptoms is given in Table 1.
| Symptom | Percent |
|---|---|
| Acute Symptoms and Signs | |
| Dizziness | 80 |
| Nausea and vomiting | 76 |
| Abdominal cramps | 51 |
| Severe headache | 43 |
| Diarrhea | 42 |
| Palpitations | 35 |
| Agitation | <25 |
| Chronic | |
| Amnesia (short-term memory loss) | 25 |
| Diplopia | 15 |
| Confusion, disorientation and coma | <5 |
Table 1: Acute symptoms of Amnesic Shellfish Poisoning in 99 patients, after consuming mussels contaminated with domoic acid [Perl et al. 1990].
Patients presented with permanent neurologic sequelae, amnesia and cognitive abnormalities, including coma, mutism, seizures, and purposeless chewing and grimacing. The cognitive dysfunction occurred in patients manifesting neurologic signs and symptoms within 48 to 72 hrs. Some patients were described with hiccups and emotional lability, with uncontrolled crying or aggressiveness [7]. In addition, patients exhibited seizures, myoclonus, hemiparesis, hyporeflexia, profuse respiratory secretions, and hemodynamic instability with labile blood pressure. Patients had a predominantly anterograde memory disorder. However, some severely affected patients also had retrograde amnesia, extending several years prior to the mussel-induced intoxication [8]. Nearly a year after the ASP event, an 84-year-old male survivor reexperienced severe seizures and was diagnosed with temporal lobe epilepsy caused by domoic acid intoxication [29,30].
Those most affected were males, older patients (>60 years of age), as well as immunocompromised younger patients. Those <65 years of age had pre-existing illnesses, such as insulin-dependent diabetes, chronic renal disease, autoimmune conditions requiring chronic steroid therapy, hypertension with a history of TIA’s, chronic hepatic dysfunction and alcoholic liver disease. Animal studies have demonstrated that the placental barrier is unable to prevent fetal exposure to domoic acid and that the toxin can transfer to neonatal rats during lactation [31]. Thus, pregnant women, infants and children must remain particularly vigilant to avoid consuming high-risk shellfish.
Diagnosis
Patients can be diagnosed by: CBC; EKG (cardiac dysrhythmias); EEG (if seizures); CMP (Comprehensive Metabolic Panel), focusing on Mg, Ca and K; and stool sample testing (C & S, Gram stain, ova and parasites). Motor testing can identify unsteadiness, generalized weakness, symmetric transient hyperreflexia, and Babinski signs. As well, patients can be observed for fasciculations and distal atrophy, hyporeflexia, loss of distal sensitivity to pain and temperature changes [8]. Neuropsychological testing identifies confusion, disorientation, and memory loss. In extreme cases involved amnesia lasting five years. Long-term testing can be carried out using enhanced CT, MRI and PET scans of the brain to assess structural changes to hippocampus and amygdala.
Testing for the presence of domoic acid in seafood consumed by humans is carried out by government agencies. However, the toxin can also be detected in blood by competitive ELISA [32].
Treatment
Currently, the only approved therapies for humans intoxicated with domoic acid are anticonvulsant drugs and maintenance therapy; although a number of glutamatergic antagonists are in preclinical development [26]. Treatment for domoic acid toxicity therefore consists of supportive symptomatic therapies, including:
1. I.V. hydration with Lactated Ringer’s solution/normal saline.
2. Antiemetics for nausea, vomiting; properistaltic/prokinetic to increase transit time through GI tract such as metoclopramide.
3. Analgesics for headache, abdominal pain (parenteral toradol, dicyclomine).
4. Administration of intravenous anticonvulsants, e.g., Diazepam and/or Phenobarbital, to treat seizures (but seizures in these cases are resistant to treatment with Phenytoin).
5. Clinical management of cardiac dysrhythmias; these are typically exacerbated by massive electrolyte disturbances as we as shifts in Mg and Ca.
6. Continuous ECG monitoring, along with correction of underlying electrolyte abnormalities, infusion of I.V. magnesium sulfate if necessary; also therapeutic for seizures and/or cardioversion if patient is hemodynamically unstable and exhibits organ hypoperfusion.
Some neurons undergo excitotoxic cell death very quickly after exposure to domoic acid. However, toxicity may also develop over time, during which domoic acid can initiate a “neurotoxic cascade” [33], long after the toxin is gone. Treatments could therefore catch patients during the early stages of toxicity, preventing them from experiencing some of the later effects, notably seizures that intensify over time and are responsible for memory loss. Promising research indicates that administration of troxerutin (a flavanol) [34], as well as ursolic acid (a natural triterpenoid) [35], to domoic-acid-treated mice results in reversal of memory impairment. Both are potential agents for prevention and treatment of cognitive deficits resulting from domoic acid toxicity. Furthermore, guidance for developing other treatments can be gained from experimental animal studies [26].
Contacts in the Event of Amnesic Shellfish Poisoning
In the USA, contact Poison Control (1-800-222-1222; http:// www.poison.org/). In Canada, contact Health Canada (1-866-225- 0709; http://www.hc-sc.gc.ca/fn-an/securit/chem-chim/toxin-natur/ index-eng.php) or the Canadian Food Inspection Agency (1-800-442- 2342; http://www.inspection.gc.ca/food/information-for-consumers/ fact-sheets/specific-products-and-risks/fish-and-seafood/toxins-inshellfish/ eng/1332275144981/1332275222849).
Report the following information:
1. Age, weight and condition of patient.
2. Type of shellfish or finfish ingested.
3. Amount of shellfish or finfish ingested.
4. Where the shellfish or finfish was eaten.
5. Where the shellfish or finfish was harvested.
6. Time of ingestion.
7. Patient’s medication list (which may impact G.I. transit time and absorption).
Prevention
Patients with multiple co-morbidities, hepatic and renal dysfunction, and compromised immune systems should avoid raw seafood (especially in endemic coastal regions in late summer and early fall) and heed posted public service announcements posted during HABs.
Do not eat shellfish sold as bait. Bait products do not meet the same food safety regulations as seafood for human consumption. Be careful of anchovies and sardines harvested in late summer and early fall (especially in endemic coastal regions) and most importantly during reported HABs. Check with local health officials before collecting shellfish (especially mussels, oysters, clams, scallops and certain verities of crab, particularly Dungeness crabs). Separate and discard the more toxic viscera of shucked scallops from to adductor muscle to reduce the toxic load ingested. Avoid consuming large amounts of any potentially toxic marine species over a short period of time, to minimize the likelihood of more severe sequelae. A searchable list of references on domoic acid and ASP is found in [36].
References
- Jeffery B, Barlow T, Moizer K, Paul S, Boyle C (2004) Amnesic shellfish poison. Food ChemToxicol 42: 545-557.
- Bates SS, Trainer VL (2006) In: Granéli E, Turner J (eds.) The ecology of harmful diatoms. Ecological Studies, Springer-Verlag, Heidelberg 189: 81-93.
- Lelong A, Hégaret H, Soudant P, Bates SS (2012) Pseudo-nitzschia(Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: revisiting previous paradigms. Phycologia 51: 168-216.
- Trainer VL, Bates SS, Lundholm N, Thessen AE, Cochlan WP, et al. (2012) Pseudo-nitzschiaphysiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 14: 271-300.
- Zabaglo K, Chrapusta E, Bober B, Kaminski A, Adamski M, et al. (2016) Environmental roles and biological activity of domoic acid: a review. Algal Res 13: 94-101.
- Bates SS, Bird CJ, de Freitas ASW, Foxall R, Gilgan M, et al. (1989) Pennate diatom Nitzschiapungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can J Fish AquatSci 46: 1203-1215.
- Perl TM, Bédard L, Kosatsky T, Hockin JC, Todd ECD, et al. (1990) An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. New Engl J Med 322: 1775-1780.
- Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, et al. (1990) Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. New Engl J Med 322: 1781-1787.
- Wright JLC, Boyd RK, de Freitas ASW, Falk M, Foxall RA, et al. (1989) Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from Eastern Prince Edward Island. Can J Chem 67: 481-490.
- Lefebvre KA, Robertson A (2010) Domoic acid and human exposure risks: a review. Toxicon 56: 218-230.
- Ramsdell JS (2007) In: Botana L (ed.) The molecular and integrative basis to domoic acid toxicity. Blackwell Publishing Professional, Cambridge, MA 223-250.
- Pulido O (2008) Domoic acid toxicologic pathology: a review. Mar Drugs 66: 180-210.
- Lefebvre KA, Robertson A (2010) Domoic acid and human exposure risks: a review. Toxicon 56: 218-230.
- Pseudo-nitzschia. Wikipedia.
- Kotaki Y, Lundholm N, Onodera H, Kobayashi K, Bajarias FFA, et al. (2004)Wide distribution of Nitzschianavis-varingica, a new domoic acid-producing benthic diatom found in Vietnam. Fish Sci 70: 28-32.
- Work TM, Barr B, Beale AM, Fritz L, Quilliam MA, et al. (1993) Epidemiology of domoic acid poisoning in brown pelicans (Pelecanusoccidentalis) and Brandt’s cormorants (Phalacrocoraxpenicillatus) in California. J Zoo Wildlife Med 24: 54-62.
- Scholin CA, Gulland, F, Doucette GJ, Benson S, Busman M, et al. (2000) Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 403: 80-84.
- Fire SE, Silver MW (2005) Domoic acid in the Santa Cruz wharf fishery. Calif Fish Game 91: 179-192.
- Lefebvre KA, Frame ER, Gulland F, Hansen JD, Kendrick PS, et al. (2012) A novel antibody-based biomarker for chronic algal toxin exposure and sub-acute neurotoxicity. PLoS ONE 7: e36213.
- Hiolski EM, Kendrick PS, Frame ER, Myers MS, Bammler TK, et al. (2014) Chronic low-level domoic acid exposure alters gene transcription and impairs mitochondrial function in the CNS. AquatToxicol 155: 151-159.
- Baden D, Fleming LE, Bean JA (1995) In: de Wolff FA (ed.) Marine toxins. Elsevier Press, Amsterdam. 141-175.
- Erdner DL, Dyble J, Parsons ML, Stevens RC, Hubbard KA, et al. (2008) Centers for Oceans and Human Health: a unified approach to the challenge of harmful algal blooms. Environ Health 7 (Suppl 2): S2.
- Berdalet E, Fleming LE, Gowen R, Davidson K, Hess P, et al. (2016) Marine harmful algal blooms, human health and wellbeing: challenges and opportunities in the 21st century. J BiolAssoc UK (in press).
- La Barre S, Bates SS, Quilliam MA. In: La Barre S, Kornprobst JM (eds.) Domoic acid. Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, Germany. 189-216.
- Ramsdell JS (2010) Neurological disease rises from ocean to bring model for human epilepsy to life. Toxins 2: 1646-1675.
- Tasker RA (2015) In: Gopalakrishnakone P, Haddad V Jr., Kem WR, Tubaro A, Kim E (eds.) Domoic acid and other amnesic toxins: toxicological profile. Springer Science+Business Media Dordrecht, Dordrecht Netherlands. (in press).
- Debonnel G, Beaaushene L, Demonigny C (1989) Domoic acid, the alleged mussel toxin, might produce its neurotoxic effect through kainite receptor activation, an electrophysiologic study in the rat dorsal hippocampus. Can J PhysiolPharmacol 67: 29-33.
- Novelli A, Kispert J, Fernández-Sánchez T, Torreblanca A, Zitko V (1992) Domoic acid-containing toxic mussels produce neurotoxicity in neuronal cultures through a synergism between excitatory amino acids. Brain Res 577: 41-48.
- Ramsdell JS, Gulland FM (2014) Domoic acid epileptic disease. Mar Drugs 12: 1185-1207.
- Cendes F, Andermann F, Carpenter S, Zatorre RJ, Cashman NR (1995) Temporal lobe epilepsy caused by domoic acid intoxication: evidence for glutamate receptor-mediated excitotoxicity in humans. Ann Neurol 37: 123-126.
- Maucher JM, Ramsdell JS (2005) Domoic acid transfer to milk: evaluation of a potential route of neonatal exposure. Environ Health Persp 113: 461-464.
- Maucher JM, Ramsdell JS (2005) Ultrasensitiv e detection of domoic acid in mouse blood by competitive ELISA using blood collection cards. Toxicon 45: 607-613.
- Dawson R, Beal MF, Bondy SC, Dimonte DA, Isom EG (1995) Excitotoxins, aging, and environmental neurotoxins: implications for understanding human neurodegenerative diseases. ToxicolApplPharmacol 134:1-17.
- Lu J, Wu DM, Zheng Y-L, Hu B, Cheng W, et al. (2013) Troxerutin counteracts domoic acid-induced memory deficits in mice by inhibiting CAAT/enhancer binding protein β-mediated inflammatory response and oxidative stress. J Immunol 190: 3466-3479.
- Wu DM, Lu J, Zhang Y-Q, Zheng Y-L, Hu B, et al. (2013) Ursolic acid improves domoic acid-induced cognitive deficits in mice. J ToxicolApplPharmacol 271: 127-136.
- Bates SS (2016) Domoic acid and Pseudo-nitzschia references.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 15052
- [From(publication date):
February-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 14041
- PDF downloads : 1011