Research Article Open Access
Alzheimers Disease: The Novel Finding of Intracellular Biofilms
Herbert B Allen*, Rina Allawh, Andrew Touati, Christos Katsetos and Suresh G Joshi
Department of Dermatology, Drexel University College of Medicine, Philadelphia, USA
- *Corresponding Author:
- Herbert B Allen
Department of Dermatology, Drexel University College of Medicine
219 N. Broad St, 4th floor, Philadelphia, PA 19107, USA
Tel: 2157625550
Fax: 2157625570
E-mail: Herbert.Allen@drexelmed.edu
Received Date: March 28, 2017; Accepted Date: May 10, 2017; Published Date: May 12, 2017
Citation: Allen HB, Allawh R, Touati A, Katsetos C, Joshi SG (2017) Alzheimer’s Disease: The Novel Finding of Intracellular Biofilms. J Neuroinfect Dis 8:247. doi: 10.4172/2314-7326.1000247
Copyright: © 2017 Allen HB, et al. This is an open-access article distributed under the terms of the creative commons attribution license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
We previously have found biofilms in the hippocampi of Alzheimer’s disease (AD) post mortem brain specimens. We had seen them in an extracellular location and noted them to be present in the areas of pathological plaque formation. Other investigators have found the presence of spirochetes (Lyme and dental) in affected (AD) brains, and these have been correlated with Treponema pallidum. In a recent historical comparison of the pathology of syphilis, the histological findings of syphilis and AD were shown to be exactly the same. Further, spirochetes have been cultured from the affected brains and have been found to make biofilms and beta amyloid precursor protein. Utilizing the same pathological methods as in our prior study, we have found biofilms in an intracellular location. The similarity of this finding to other diseases has been presented; and, the impact of the “intra” versus the “extra” cellular location is discussed.
Keywords
Alzheimer’s disease; Immune system; Biofilms
Introduction
We, and others, have found biofilms in the brains of Alzheimer’s disease (AD) patients [1,2]. These biofilms have been located primarily in the pathological plaques of that disease. As such, they are in an extracellular location, and the amyloidogenic “curli” fibers of the biofilm activate Toll-like receptor 2 (TLR2) of the innate immune system [3]. The major pathway utilized by TLR2, in its role of inactivating invading pathogens, is the MyD88 pathway which eventuates in NFĸB and TNFα [4].
NFĸB, together with β amyloid converting enzyme (BACE), catalyzes β secretase which cleaves off the terminal portion of β amyloid precursor protein to form beta amyloid(Aβ) [4]. Aβ is antimicrobial [5] and it surrounds the plaques of AD; however, it cannot penetrate the biofilm [1,2]. The TNFα (produced by the innate immune system) cannot penetrate the biofilms either, so it has been postulated to kill the surrounding tissue instead [6]. Further, TNFα together with TNFα converting enzyme catalyzes α secretase which eventuates in α amyloid.
The biofilms have been shown to be made by spirochetes; the primary evidence for this is the growth in culture of Borrelia burgdorferi from the brains of AD patients. These cultured organisms, in turn, made biofilms when put under environmental stress. Also, produced by these organisms was β amyloid precursor protein and smaller amounts of Aβ [7]. Consequently, the paradigm thus elaborated would be:
Microbes (spirochetes)?biofilms?innate immune system--TNFα ? tissue destruction
Microbes?biofilms?innate immune system--NFĸB?βACE?β- secretase?Aβ?tissue destruction
Spirochetes are the primary organisms involved because of the evidence both from the cultures, [7,8] and from the PCR evidence implicating Borrelia and dental spirochetes in a 25%/75% ratio [9,10]. The dental organisms have been found in as many as 90% of patients and have also been strongly linked epidemiologically to AD [11].
Our current investigation involves examination of AD brains for intracellular (not extracellular) biofilms. Using the same staining patterns as in our previous pathological examinations, we have found “intracellular biofilms in all the AD brains, and none of the controls.
Methods
We re-examined hippocampal specimens with the techniques described previously [1]. Seven hippocampal specimens from patients who had previously been confirmed both clinically and pathologically (post mortem) to have Alzheimer’s disease were re-examined by five pathologists. Ten control hippocampal specimens, from age and sex matched patients who died of unrelated, non-cerebral diseases and/or causes were included for study. All specimens were stained with hematoxylin and eosin (H+E), periodic acid Schiff (PAS), Congo red routine stains; all specimens were also stained with Treponema pallidum (TPI), β amyloid, CD 282 (TLR2) and CD 284 (TLR4) immunostains. The technique for these stains was as previously published. PAS and β amyloid stains were applied sequentially to the specimens and were examined. Routine light microscopy was employed. The specimens were not “blinded” because by gross examination alone, the AD specimens could be distinguished from the controls.
Results
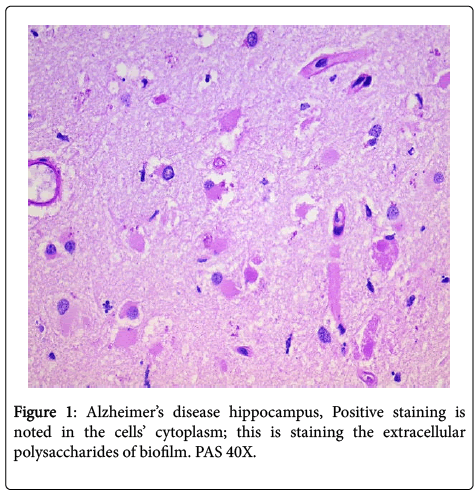
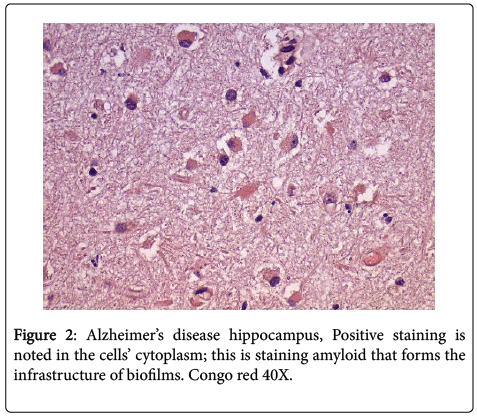
In all the hippocampal specimens from AD patients, we found positive intracellular staining with both PAS and Congo red. The positive PAS indicates the presence of polysaccharides, and the positive Congo red indicates the presence of amyloid (Figures 1 and 2). The other results that had been previously presented were re-affirmed [1].
Discussion
Intracellular biofilms have not been noted previously in AD; however, they have been found in urinary tract disease and in pulmonary disease [12,13]. The intracellular biofilms in cystitis have been referred to as “pods”, and these “pods” when extruded into the surrounding tissue are fully capable of creating new, and larger, biofilms.
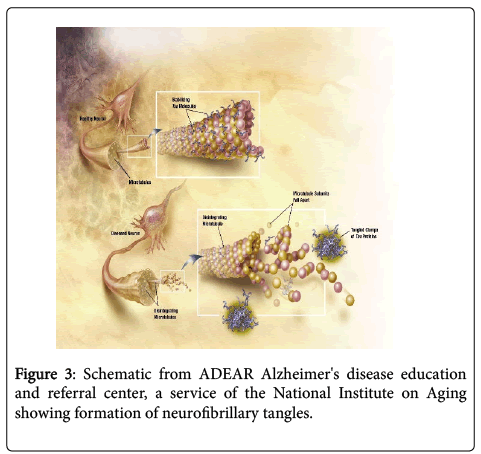
One possible impact of intracellular biofilms in AD is shown in the diagram (Figure 3). The intracellular biofilms could be extruded from the neurons as portrayed and become neurofibrillary tangles. These tangles have been shown to be present in tertiary syphilis as in AD [14]. They have also been shown, in the same historical comparison of the two diseases, to contain spirochetes (T. pallidum in syphilis and Lyme/dental in AD).
Biofilms are present inside the epithelial cells lining the bladder. Because of the intracellular location in the bladder, lungs and brain, the organisms have another level of protection in addition to the extracellular polysaccharide, “slime” coating. Situated inside the cell walls, they do not appear to be recognized by the immune system (as they are not in the urinary intracellular location). Thus, they remain a “nidus” of infection that contributes to the chronicity of the diseases.
As “pods”, the biofilms retain all the capabilities of extracellular biofilms: immune system avoidance, gene transfer, and reduced antibiotic diffusion [15]. Further, although it is not employed, they retain the ability to down regulate the immune response [16]. In the future, we will show this phenomenon of intracellular biofilms is not unique to the organs mentioned, but is also present in such diverse diseases as leprosy and psoriasis. Consequently, it is another example of nature behaving similarly as regards the pathogenesis of many diseases.
As regards treatment of biofilm-related diseases especially AD, it is extremely important to treat before the microbes make biofilms [1]. The example for this is syphilis which in its tertiary stage is a biofilmrelated disease. If treatment with penicillin is administered at any stage of the disease prior tertiary, syphilis is “cured”. Consequently, if Lyme disease is treated similarly, one would expect similar results. Also, if antimicrobials are given prior to dental work, similar results predictably would be forthcoming [1].
Treating after the biofilms have formed is much more challenging. A biofilm dispersing agent must be taken along with the antibiotics to allow the antibiotics to work. This has been shown to be effective in leprosy and in chronic arthritis [17,18]. Difficulty in AD, compared to the other diseases, arises because the debris needs to be cleared from the brain after the biofilm is dispersed and the organisms are killed. The sheer amount of debris would be overwhelming for the microglia [19]. Intracellular biofilms add immeasurably to the challenge.
References
- Allen HB (2016) Alzheimer’s disease: Assessing the role of spirochetes, biofilms, the immune system, and beta amyloid with regard to potential treatment and prevention. J Alzheimers Dis 53:4.
- MacDonald AB (2015) Blood-borne borrelia biofilms coated with beta-amyloid. https://spirodementia.wordpress.com/featured-new-discovery-blood-borne-borrelia-biofilms-coated-with-beta-amyloid-7-oct-21053
- Tukel C, Wilson RP, Nishimori M, Pezeshki M, Chromy BA, et al. (2009) Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell Host Microbe 6: 45-53.
- Allen HB, Morales D, Jones K, Joshi SG (2016) Alzheimer’s disease: A novel hypothesis for the development and the subsequent role of beta amyloid. J Neuroinfect Dis 7:2.
- Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson Met al. (2010) The Alzheimer’s disease-associated β-protein is an anti-microbial peptide. PLoS ONE 5: e9505.
- Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sørensen SR, Moser C, et al. (2013) The in vivo biofilm. Trends Microbiol 21: 466-474.
- Miklossy J (2016) Bacterial amyloid and DNA are important constituents of senile plaques: Further evidence of the spirochetal and biofilm nature of senile plaques. J Alzheimers Dis 53:4.
- MacDonald AB (1988) Concurrent neocortical borreliosis and Alzheimer’s disease: Demonstration of a spirochetal cyst form. Ann NY Acad Sci 539: 468-470.
- Miklossy J (2011) Alzheimer’s disease: Aneurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria. J Neuroinflammation 8:90.
- Riviere GR, Riviere KH, Smith KS (2002) Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer's disease. Oral Microbiol Immunol 17: 113-118.
- Kamer AR, Dasanayake AP, Craig RG(2008) Alzheimer's disease and peripheral infections: The possible contribution from periodontal infections, model and hypothesis. J Alzheimers Dis 13: 437-449.
- Scott VC, Haake DA, Churchill BM, Justice SS, Kim JH(2015)Intracellular bacterial communities: A potential etiology for chronic lower urinary tract symptoms. Urology 86: 425-431.
- Garcia-Medina R, Dunne WM, Singh PK, Brody SL (2005) Pseudomonas aeruginosa acquires biofilm-like properties within airway epithelial cells. Infect Immun 73: 8298-8305.
- Miklossy J (2015) Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease. Front Aging Neurosci 7:46.
- Gupta P, Sarkar S, Das B, Bhattacharjee S,Tribedi P (2016) Biofilm, pathogenesis and prevention:A journey to break the wall: A review. Arch Microbiol 198: 1-15.
- Scherr TD, Roux CM, Hanke ML, Angle A, Dunman PD,et al.(2013) Global transcriptome analysis of Staphylococcus aureus biofilms in response to innate immune cells. Infect Immun 81: 4363-4376.
- Allen HB, Moschella SL (2017) The role of rifampin in leproleprosy through a new lens. JAMAdermatol 153: 261-262.
- Allen HB, Hossain C, Abidi N, Larijani M, Joshi SG (2017) Penicillin: The old/new wonder drug.Adv Tech Biol Med 5:1.
- Allen HB, Joshi SG (2016) Nicotine and Alzheimer’s disease: Mechanism how the fog of smoke increases the fog of dementia. J Neuroinfect Dis 7:4.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 5791
- [From(publication date):
June-2017 - Apr 27, 2025] - Breakdown by view type
- HTML page views : 4832
- PDF downloads : 959