Mini Review Open Access
Alzheimer?s Disease, Cerebrovascular Disease and Dementia: APotentially Preventable and Modifiable Syndrome
Dennis A Davey*
Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory, Western Cape, South Africa
- Corresponding Author:
- Dennis A Davey
Faculty of Health Sciences
University of Cape Town
Anzio Road, Observatory
Western Cape, South Africa
Tel: +27217121314
E-mail: profdad@eject.co.za
Received date: February 12, 2015; Accepted date: March 10, 2015; Published date: March 17, 2015
Citation: Davey DA (2015) Alzheimer’s Disease, Cerebrovascular Disease and Dementia: A Potentially Preventable and Modifiable Syndrome. J Alzheimers Dis Parkinsonism 5:184. doi:10.4172/2161-0460.1000184
Copyright: © 2015 Davey DA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer’s disease (AD) and cerebrovascular disease (CVD) frequently co-exist and are part of a syndrome that may result in ageing-related cognitive impairment and dementia. Somemen and women with the neurodegenerative and cerebrovascular changes associated with dementia have normal cognition ascribed to a high cognitive reserve or resilience. Many measures claimed to decrease the risk of dementia are the same as those that prevent cardiovascular disease. In several countries the age-specific prevalence of dementia is decreasing and the decrease has been attributed to a decrease in cardiovascular risk factors andan increase in cognitive reserve associated with better education and healthier life-styles in recent generations.Modifiable risk factors are present in up to 50% of persons with dementia. By reducingrisk factors andpromotingprotective factors from early and mid-life onwards,cognitive impairment and dementia maybe prevented or delayedwell into old age in a significant proportion of men and women.
Keywords
Alzheimer’s disease; Cerebrovascular disease; Cognitive impairment; Dementia; Prevention
Background
Alois Alzheimer in 1906 reported the autopsy findings of the brain of a woman, Augusta D, who died of dementia at the age of 55. He described a “peculiar severe disease process of the cerebral cortex” with “miliary foci” (β-amyloid plaques)and “fibrils” (neurofibrillary tangles) [1]. The condition was given the name “Alzheimer’s Disease” in 1910 in Kraepelin’s textbook of psychiatry. Alzheimer’s findings were confirmed when the original histological slides were re-examined in 1998 using modern histochemical and genetic techniques [2]. Alzheimer’s report marked the beginning of research into ageing-related dementia and cognitive impairment.
Nomenclature
The term “Alzheimer’s Disease” has been used in different senses:
(a) The term “Alzheimer’s Disease” is used by neurologists and psychiatrists to mean the form of neurodegeneration characterized by β-amyloid plaques and neurofibrillary tangles in the brain as first described by Alois Alzheimer.
(b) The term “Alzheimer’s Disease” is also used loosely by some nonspecialists to include all forms of ageing-related cognitive impairment and dementia
(c) “Alzheimer’s Disease” is often used in non-medical circles instead of the word “dementia”.
The different uses of the term “Alzheimer’s Disease” has led to misunderstanding. It has been suggested that ageing-related cognitive impairment and dementia is best regarded as a syndrome - a complex of symptoms with multiple causes - that includes both AD and CVD making it similar to most late-life chronic diseases [3].
Dementia and mild cognitive impairment (MCI) are most commonly diagnosed clinically according to the 2011 criteria of the Alzheimer’s Association and the National Institute of Aging of the USA [4-6]. Three stages are recognized:
(a) Preclinical requiring changes in biomarkers or poor performance on challenging cognitive tests
(b) MCI with mild changes in memory and other cognitive abilities that do not interfere with day-to- day activities
(c) Dementia with changes in two or more aspects of cognition and behavior interfering with day-to-day function.
The use of biomarkers in the cerebrospinal fluid and plasma and structural and functional changes in the brain on MRI is usually reserved for research.
Cerebral pathology in ageing-related dementia and cognitive impairment and in normal cognition
Alzheimer’s disease (AD) as first described by Alzheimer is but one of several causes of ageing-related cognitive impairment and dementia (Table 1). The commonest are AD, Lewy Body Disease (LBD), and CVD. CVD changes include large infarcts, multiple micro-infarcts, lacunes, leukencephalopathy, cerebral atherosclerosis, arteriolar and capillary angiopathies, and microvascular changes resulting in bloodbrain barrier dysfunctions. The spectrum of neurodegenerative and cerebrovascular changes in the brains of persons dying with ageingrelated dementia and in those with normal cognition at the time of death has been investigated in several major studies.
| Brain Pathologies associated with Cognitive Impairment and Dementia |
|---|
| • Alzheimer’s Disease (β-amyloid plaques, neurofibrillary tangles) |
| • Cerebrovascular disease |
| • α-synucleinopathies (Lewy body disease) |
| • non-Alzheimer tauopathies (Supranuclear palsy, Pick’s disease) |
| • TDP-43 proteinopathies (Fronto-temporal lobe degeneration) |
| • Parkinson’s Disease |
| • HIV Immunodeficiency disease |
| • Prion Disease (Creutzfeldt-Jakob Disease) |
Table 1: Causes of ageing-related cognitive impairment and dementia.
The Medical research council cognitive function and ageing (MRC-CFAS) study: The epidemiology and neuropathology of 456 brain donations from an elderly population were studied comprehensively [7]. Both AD and CVD type changes were common and there was a high prevalence of mixed pathology. A significant proportion of the donors had more limited pathology than traditionally associated with dementia and a proportion of the donors with significant pathology remained cognitively intact until death. The dissociation between clinical dementia and the pathology of AD and CVD increased with increasing age of the subjects at the time of death.
Vienna trans-danube aging study: A community-based autopsy study of the brains of 233 elderly individuals, with and without dementia, found a wide spectrum of lesions and proteinopathies [8]. Virtually all the cases showed some degree of neurofibrillary degeneration but amyloid-β deposits were only present in 68.7%. Other pathologies included vascular lesions (48.9%), α-synucleinopathies (24.9%), nonalzheimertauopathies (23.2%), TDP-43 proteinopathies (13.3%) and others including inflammation and tumours (15.1%). The authors concluded that (a) pure AD pathology with dementia was infrequent (b) a number of non-AD neurodegenerative pathologies may result in dementia and that their prevalence has been underestimated. The commonest of the other underestimated pathologies were vascular lesions which were most probably due to CVD.
Religious orders study and rush memory and aging project: In 483 consecutive autopsies of elderly participants followed for several years with a battery of neuropsychological tests, the clinical diagnosis according to NINDCDS/AARDA 1984 criteria was probable AD in 179, mild cognitive impairment (MCI) in 134, and no cognitive impairment in 170 [9]. Of those diagnosed as probable AD, 87.7% had pathologically-confirmed AD, 45.8% had mixed pathologies most commonly AD combined with macroscopic infarcts. Of those diagnosed as MCI, 54.4% had pathologically confirmed AD, and 19.4% had mixed pathologies. Of those with no cognitive impairment 45.3% had AD pathology, 9.4% had mixed pathology. Major neuropathological lesions only accounted for 25% - 30% of the variance in cognition measured using a global measure of cognitive function assessed proximate to death [10]. Many individuals suffered varying degrees of cognitive and neurological deterioration whilst others maintained normal cognitive functioning in spite of significant brain pathology and the authors advanced the concept of cognitive resilience and resilient aging. The macroscopic infarcts were most likely the result of CVD and the author’s state “Infacts are the most common unrecognized contributing pathology in clinical AD, prevention of infarcts is likely to be most important in delaying onset and slowing cognitive decline in persons with AD pathology. From a public health perspective, measures that can improve vascular health, such as life-style changes and prevention and treatment of hypertension and diabetes, could decrease both the incidence and severity of clinical AD in the population.”
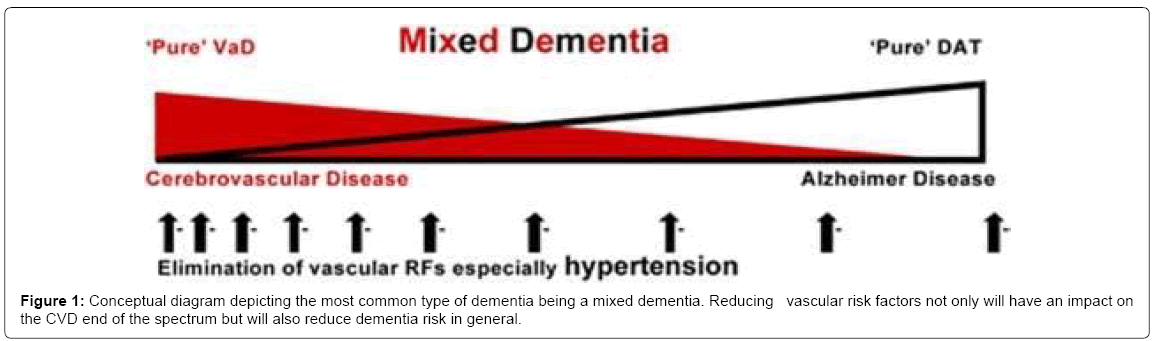
The National Alzheimer’s coordinating centre USA study: In an analysis of 4,629 cases in with autopsy-confirmed neurodegenerative diseases, 79.9% were classified as also having CVD [11]. Concurrent CVD was a common finding in aged subjects with dementia and was more common with AD than in other neurodegenerative diseases. The occurrence of CVD changed the threshold for dementia due to AD and α-synucleinopathies. CVD accelerated β amyloid production, aggregation and deposition. CVD changes act both additively and synergistically with AD to cause dementia and cognitive impairment. The prevalence of cardiovascular risk factors was hypertension 56.2%, hypercholesterolemia 47.4%, coronary heart disease 18.0%, atrial fibrillation 13.7%, and diabetes 12.2%. AD and CVD pathologies appeared to have additive and synergistic effects on ageing-related cognitive decline and dementia. In persons with ageing-related cognitive impairment and dementia AD, LBD, and CVD changes frequently co-exist and the neurodegenerative and cerebrovascular changes form a spectrum from the extremes of “pure AD” to “pure CVD”. A combination of changes is the most common and results in “mixed dementia” (Figure 1).
Valenzuela M, Esler M, Ritchie K, Brodaty. [12] Adapted with permission from the authors and publishers Translational Psychiatry .
The separation of AD and “vascular dementia” has been claimed to be a false dichotomy [12]. The changes of AD and CVD overlap and both pathologies have both additive and synergistic effects on cognitive decline [13]. The interaction between the cerebrovascular and neurodegenerative changes is important in the pathogenesis of ageing-related cognitive impairment and dementia [14-16]. Both cerebrovascular and neurodegenerative brain changes are frequently found in persons with normal cognition at the time of death and protective factors are important in the prevention of ageing-related cognitive impairment and dementia.
Cognitive reserve, cognitive resilience and cognitive health
The maintenance of normal cognition in a significant proportion of persons with the pathological changes of AD, LBD or CVD is prima facie evidence of the importance of cognitive reserve and cognitive resilience [17]. Cognitive reserve refers to high cognitive ability from an early age and the maintenance of high cognitive ability in mid-life and a consequent prevention or delay of ageing-related impaired cognition or dementia [18]. Cognitive resilience or resilient aging refers to retention of normal cognition and delay of the onset of impaired cognition or dementia in spite of the development of neuropathological changes of AD, LBD or CVD10. Cognitive health means the achievement and maintenance of high cognitive reserve, the promotion of protective factors throughout life and the avoidance and preventive treatment of modifiable risk factors that affect the development of impaired cognition and dementia.
Risk factors for cognitive impairment and dementia
Age is the most important factor determining the incidence and prevalence of cognitive impairment and dementia. In a prospective cohort study in the USA the age specific rates of all-cause dementia increase from 4.85 per 1,000 person-years in the 65-69 age groups to 84.19 per 1,000 person-years in the 90+ age group [19]. These figures are consistent with other US and European studies. Both neurodegenerative and cardiovascular changes have a long preclinical phase, commonly starting in mid-life. With increasing age converging genetic, environmental, and lifestyle factors trigger a pathophysiological cascade that over decades results in the neurodegenerative and cerebrovascular changes that may manifest in cognitive impairment and dementia. It is estimated that seven potentially modifiable factors are present in about 50% of persons with AD [20] (Table 2). These estimates do not take into account the non-independence of risk factors and the combined population-attributable risks factors have been estimated to be about 30% for Europe, UK and USA [21]. A meta-analysis of 31 studies of incident AD found that low education, high homocysteinemia and smoking were significant risk factors and that higher physical activity and n-3 fatty acids were significant protective factors [22]. Because current therapies are of limited value and do not appear to significantly prevent the progression of ageing-related cognitive impairment and dementia, increasing attention is being focused on modifiable risk factors, protective factors and measures that may prevent, delay the onset or modify ageing-related cognitive impairment and dementia [23].
| RISK FACTOR | POPULATION PREVALENCE | RELATIVE RISK (95% CI) | PAR% (confidence range) | NO. OF CASES ATTRIBUTABLE (thousands; confidence range) |
|---|---|---|---|---|
| Worldwide | ||||
| Diabetes mellitus | 6·4% | 1·39 (1·17–1·66) | 2·4% (1·1–4·1) | 826 (365–1374) |
| Midlife hypertension | 8·9% | 1·61 (1·16–2·24) | 5·1% (1·4–9·9) | 1746 (476–3369) |
| Midlife obesity | 3·4% | 1·60 (1·34–1·92) | 2·0% (1·1–3·0) | 678 (387–1028) |
| Depression | 13·2% | 1·90 (1·55–2·33) | 10·6% (6·8–14·9) | 3600 (2295–5063) |
| Physical inactivity | 17·7% | 1·82 (1·19–2·78) | 12·7% (3·3–24·0) | 4297 (1103–8122) |
| Smoking | 27·4% | 1·59 (1·15–2·20) | 13·9% (3·9–24·7) | 4718 (1338–8388) |
| Low education | 40·0% | 1·59 (1·35–1·86) | 19·1% (12·3–25·6) | 6473 (4163–8677) |
| Combined (maximum) | .. | .. | 50·7% | 17187�??28* |
| USA | ||||
| Diabetes mellitus | 8·7% | 1·39 (1·17–1·66) | 3·3% (1·5–5·4) | 174 (77–288) |
| Midlife hypertension | 14·3% | 1·61 (1·16–2·24) | 8·0% (2·2–15·1) | 425 (119–798) |
| Midlife obesity | 13·1% | 1·60 (1·34–1·92) | 7·3% (4·3–10·8) | 386 (226–570) |
| Depression | 19·2% | 1·90 (1·55–2·33) | 14·7% (9·6–20·3) | 781 (506–1078) |
| Physical inactivity | 32·5% | 1·82 (1·19–2·78) | 21·0% (5·8–36·6) | 1115 (308–1942) |
| Smoking | 20·6% | 1·59 (1·15–2·20) | 10·8% (3·0–19·8) | 574 (159–1050) |
| Low education | 13·3% | 1·59 (1·35–1·86) | 7·3% (4·4–10·3) | 386 (236–544) |
| Combined (maximum) | .. | .. | 54·1% | 2�??866�??951* |
Table 2: Alzheimer's disease cases attributable to potentially modifiable risk factors worldwide and in the USA.
Measures to prevent delay or modify ageing –Related cognitive impairment and dementia
There have been few long-term, randomized, control investigations but a large number of observational studies suggest that various measures may prevent, delay the onset or minimize the severity of ageing related cognitive impairment and dementia [24] (Table 3). Many of these measures are similar, if not identical, to those shown to prevent or reduce the incidence of cardiovascular disease. The measures can be divided into (a) those aimed at preventing and reducing CVD and (b) those aimed at improving and maintaining functional cognition irrespective of whether or not there are associated neuropathologies.
| Measures that may Prevent, Delay or Minimize Ageing-related cognitive impairment or dementia | |
|---|---|
| Related to cardiovascular and cerebrovascular health | Mediterranean diet and specific nutrients |
| Exercise, maintenance of cardiovascular fitness and healthy life-style | |
| Prevention of obesity and diabetes | |
| Early and active treatment of hypertension | |
| Early and active treatment of hyperlipidaemia | |
| Aspirin and non-steroidal anti-inflammatory drugs | |
| Related to cognition reserve and cognitive health | Improved level of education |
| Maintenance of cognitive activity | |
| Social interventions | |
| Depression management | |
| Correction of sleep disorders | |
| Specific cognitive interventions | |
Table 3: Severity of ageing related cognitive impairment and dementia.
Measures aimed at prevention CVD: The measures aimed to prevent or protect against CVD have been well documented and include exercise, Mediterranean diet and omega-3-fatty acids and the aggressive treatment of hypertension, hyperlipidaemia and diabetes from early and mid-life onwards [24].
Measures aimed at the improvement and maintenance of cognition: These measures include education, maintenance of cognitive and social activity throughout life and the treatment of depression and sleep disorders. These measures can modify the relation between the degree of neuropathology and the level of cognition and the occurrence of dementia [17].
Education: The level of education is an important factor in relation to the later development of impaired cognition and dementia irrespective of the presence, amount or type of brain pathology. Education appears to delay the onset of cognitive decline but is associated with a more rapid progression once decline commences [25].
Cognitive activity: A systematic review of 22 cohort studies including 29,000 individuals concluded that complex patterns of mental activity in early, mid- life was associated with a significant reduction in dementia incidence in late life (RR 0.54 95% CI 0.49-0.59) [18]. In the Rush Studies cognitive stimulating activities over the life span, including games and puzzles, was associated with slower late-life cognitive decline and was independent of the development of common neuropathological conditions [26]. More frequent participation in cognitive activity was associated with reduced incidence of AD (HR 0.58 95%CI 0.44-0.77). Frequent cognitive activity was associated with reduced incidence of mild cognitive impairment and less rapid decline in cognitive function.
Social factors: Social isolation and loneliness appear to increase cognitive decline irrespective of AD pathology, and the deleterious effects of AD pathology are minimized by social engagement [27,28]. Conscientiousness in particular was associated with a slower rate of cognitive decline and a lower incidence of dementia [29]. In the MRCCFAS Study a combined Cognitive Lifestyle score (CLS) based on educational attainment, occupational complexity and social engagement was calculated. Those who maintained a high CLS throughout their lives had a 40% reduced risk of developing dementia [30].
Progress and future perspective: In the last 10-15 years the agespecific prevalence of dementia in older adults in England, Denmark, Sweden, The Netherlands and USA appears to be decreasing [31-35]. The estimates of the national prevalence of dementia in England in 2011 were revised downwards by 24% from those predicted earlier from the findings two decades earlier in the Cognitive Function and Ageing Study I and II [31]. The decreases in the age-specific prevalence of dementia in these countries were attributed to higher educational levels, lower cardiovascular risk factors and a healthier life-style in more recent generations.
In early 2012 President Obama in the USA declared a “War on Alzheimer’s disease” and in March 2012 the Prime Minister David Cameron in the UK issued a challenge to “go further and faster” making life better for people with dementia and their carers. The government authorities in both countries have voted considerable funds and advocated screening and testing for MCI and dementia of all at-risk individuals. Reservations however have been expressed about possible harms of population testing, including false-positive and false negative tests and the stigma associated with a diagnosis of dementia [36]. In January 2014 a conference of 59 experts in dementia and noncommunicable diseases (The Blackfriars Consensus on promoting brain health and reducing the risks for dementia) concluded that (a) “the evidence is now sufficient to justify policy action across the life course and for further research to reduce the modifiable risk factors and improve the populations profile for recognized protective fact, (b) these gains are likely to be greater if combined with action to protect brain health throughout life (c) the best strategy would be to work throughout the life course to bring people to the threshold of older age in good health [37,38]. These strategies may help to combat the predicted increase in the prevalence of dementia due to the increasing incidence of obesity and diabetes in many countries and to the increasing longevity and numbers of the elderly throughout the world [3]. In 2010 a seminal review on delaying the rising tide of world-wide late-life dementias concluded that delaying dementia appears feasible and that “if people can delay the onset of dementias they can lead more fulfilling lives for longer, spend less time suffering from the disease and letting their families spend less time in coping with the disease” [39]. Compression of cognitive morbidity well into old age is achievable and ageing–related cognitive impairment and dementia is not necessarily inevitable and inexorable and can be prevented, delayed or modified.
Acknowledgements
Michael Valenzuela and Translational Psychiatry for permission to publish Figure 1. Deborah Barnes and Lancet Neurology for permission to publish Table 2.ORCID number 0000-0002-0904-5462.
References
- (1907) UbereineeigenartigeErkankung der Hinrninde. AllegmineZeitschriftf?rPsychiatrie und Psychisch-GEritlicheMedizin, Alzheimer A. 64:146-8.
- Graeber MB, Kösel S, Grasbon-Frod E, Möller HJ, Mehraein P (1998) Histopathology and APOE genotype of the first Alzheimer disease patient, Auguste D.Neurogenetics 1: 223-228.
- Larson EB, Yaffe K, Langa KM (2013) New insights into the dementia epidemic. N Engl J Med 369: 2275-2277.
- Jack CR Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, et al. (2011) Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 257-262.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, et al. (2011) The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 263-269.
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, et al. (2011) The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 270-279.
- Wharton SB, Brayne C, Savva GM, Matthews FE, Forster G, et al. (2011) Epidemiological neuropathology: the MRC Cognitive Function and Aging Study experience. J Alzheimers Dis 25: 359-372.
- Kovacs GG, Milenkovic I, Wohrer A et al. (2013) Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. ActaNeuropathol.126:365-84.
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 66: 200-208.
- Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE (2011) Cognition and neuropathology in aging: multidimensional perspectives from the Rush Religious Orders Study and Rush Memory And Aging Project. Curr Alzheimer Res 8: 336-340.
- Toledo JB, Arold SE, Raibie K, Brettschneider J, Xie SX,et al. (2013) Contribution of cerebrovascular disease in an autopsy confirmed neurodegenerative disease case in the National Alzheimer’s Coordinating Centre. Brain 136:2697-706.
- Valenzuela M, Esler M, Ritchie K, Brodaty H (2012) Antihypertensives for combating dementia? A perspective on candidate molecular mechanisms and population-based prevention.Transl Psychiatry 2: e107.
- Attems J, Jellinger KA (2014) The overlap between vascular disease and Alzheimer's disease--lessons from pathology. BMC Med 12: 206.
- Kalaria RN, Akinyemi R, Ihara M (2012) Does vascular pathology contribute to Alzheimer changes? J NeurolSci 322: 141-147.
- Honjo K, Black SE, Verhoeff NP (2012) Alzheimer’s disease, cerebrovascular disease, and the β-amyloid cascade. Can J NeurolSci 39: 712-728.
- Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE (2013) Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimers Dement 9: 76-92.
- Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, Schneider JA. (2014) Cognitive and social lifestyle: links with neuropathology and cognition in late life. ActaNeuropathol.127:137-50
- Valenzuela MJ, Sachdev P (2006) Brain reserve and dementia: a systematic review. Psychol Med 36: 441-454.
- Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, et al. (2002) Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol 59: 1737-1746.
- Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 10: 819-828.
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C (2014) Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol 13: 788-794.
- Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, et al. (2014) Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 14: 643.
- O’Brien JT, Burns A(2010) Clinical practice with anti-dementia drugs: a revised (second) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol.8:997-1019.
- Davey DA (2014) Alzheimer's disease and vascular dementia: one potentially preventable and modifiable disease? Part II: Management, prevention and future perspective. Neurodegener Dis Manag 4: 261-270.
- Amieva H, Mokri H, Le Goff M, Meillon C, Jacqmin-Gadda H, et al. (2014) Compensatory mechanisms in higher-educated subjects with Alzheimer's disease: a study of 20 years of cognitive decline. Brain 137: 1167-1175.
- Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA (2007) Relation of cognitive activity to risk of developing Alzheimer disease. Neurology 69: 1911-1920.
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS (2006) The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 5: 406-412.
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, et al. (2007) Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry 64: 234-240.
- Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA (2007) Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry 64: 1204-1212.
- Valenzuela M, Brayne C, Sachdev P, Wilcox G, Matthews F (2011) Medical Research Council Cognitive Function and Ageing Study. Cognitive lifestyle and long-term risk of dementia after diagnosis in a multicenter population- based cohort. Am J Epidemiol.173:1004-12.
- Matthews FE, Arthur A, Barnes LE,Bond J, Jagger C, et al. (2013) A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet.382:1405-12.
- Christensen K, Thinggaard M, Oksuzyan A, Steenstrup T, Andersen-Ranberg K, et al. (2013) Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet 382: 1507-1513.
- Qiu C, von Strauss E, Bäckman L, Winblad B, Fratiglioni L (2013) Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology 80: 1888-1894.
- Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, et al. (2012) Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 78: 1456-1463.
- Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, et al. (2008) Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimers Dement 4: 134-144.
- Davey DA (2014) Alzheimer's disease and vascular dementia: one potentially preventable and modifiable disease. Part I: Pathology, diagnosis and screening. Neurodegener Dis Manag 4: 253-259
- Public Health England, UK Health Forum. Blackfriars consensus on promoting brain health; reducing risks for dementia May 2014.
- Lincoln P, Fenton K, Alessi C, Prince M, Brayne C, et al. (2014) The Blackfriars Consensus on brain health and dementia. Lancet 383: 1805-1806.
- Larson EB (2010) Prospects for delaying the rising tide of worldwide, late-life dementias. IntPsychogeriatr 22: 1196-1202.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15730
- [From(publication date):
March-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11070
- PDF downloads : 4660